Collagenase II has been used to induce experimental keratoconus in animal models. However, its effect when administered by intrastromal injection has not been studied, so the purpose of this study was to study the effects of intrastromal injection of collagenase II on corneal surface and corneal morphology.
MethodsSix New Zealand rabbits were used, collagenase II was administered by intrastromal injection (5μL of 2.5mg/mL) in the right eyes and balanced salt solution in the left eyes. Keratometry was performed to evaluate curvature alteration, also at day 7 corneas were obtained and Hematoxylin-Eosin staining was performed to examine morphologic changes. Likewise, changes in type I collagen expression were investigated by Sirius Red staining and semiquantitative PCR.
ResultsK1, K2 and Km presented differences in the means with statistically significant changes. The morphological changes that were demonstrated were degradation and irregular arrangement of the corneal stroma, increase in the cellular density of keratocytes and slight cellular infiltration. Finally, it was demonstrated that there is greater expression of type I collagen fibers in the experimental group as opposed to the controls and the thickness of the fibers also increased due to the action of collagenase II, however, in terms of genetics there were no changes in the expression of type I collagen at molecular level between the control and experimental groups.
ConclusionsCollagenase II administered by intrastromal injection is able to induce changes in the corneal surface and stroma, being able to simulate a model of keratoconus.
La colagenasa II ha sido utilizado para inducir Queratocono experimental en modelos animales. Sin embargo, no ha sido estudiado su efecto cuando se administra por inyección intraestromal, por lo que el propósito de este estudio fue estudiar los efectos de la inyección intraestromal de colagenasa II sobre la superficie corneal y la morfología de la córnea.
MétodoSe trabajó con 6 conejos Nueva Zelanda, se administró colagenasa II por inyección intraestromal (5μL de 2.5mg/mL) en los ojos derechos y solución salina balanceada en los ojos izquierdos. Se realizaron queratometrías para evaluar la alteración de la curvatura, también al día 7 se obtuvieron las córneas y se realizó tinción Hematoxilina-Eosina para examinar los cambios morfológicos. Así mismo, se investigaron los cambios en la expresión de colágeno tipo I por tinción Rojo Sirio y PCR semicuantitativa.
ResultadosK1, K2 y Km presentaron diferencias en los promedios con cambios estadísticamente significativos. Los cambios morfológicos que se demostraron fueron degradación y disposición irregular del estroma corneal, incremento en la densidad celular de queratocitos y ligera infiltración celular. Finalmente se demostró que hay mayor expresión de fibras de colágeno tipo I en el grupo experimental a diferencia de los controles y el grosor de las fibras también incrementó por acción de la colagenasa II, sin embargo, en cuestión génica no hubo cambios en la expresión de colágeno tipo I a nivel molecular entre el grupo control y experimental.
ConclusionesLa colagenasa II administrada por inyección intraestromal es capaz de inducir cambios en la superficie corneal y el estroma, pudiendo simular un modelo de queratocono.
Keratoconus is a corneal ectasia characterised by the thinning and conical protrusion of the cornea with myopia and astigmatism development.1,2 It has been classified as a non-inflammatory disease; however, recent research has found proinflammatory cytokine expression and inflammatory cell infiltrate in tears of keratoconus patients.3–5 It usually occurs in adolescence, from the second decade of life onwards, possibly following refractive surgery. Worldwide prevalence is estimated at 138/100,000 people.2,6 Some of the risk factors for its development are atopy, eye rubbing, congenital diseases, connective tissue disorders and the expression of genes such as VSX1 and SOD1.1,7–12 There are several physiological alterations in the corneal stroma and epithelium, giving rise to a production imbalance of proinflammatory and anti-inflammatory molecules, proteases and their inhibitors, oxidative stress and cellular hypersensitivity.13 Recently published literature describes different proteins found in tears and corneas with keratoconus such as IL-1, IL-6, TNF-α, some metalloproteinases, TGF-β and reactive oxygen species,14–16 indicating the simultaneous activation of different signaling pathways that could establish an inflammatory profile.
Treatment depends on severity and aims to address structural corneal alterations and is not intended to directly treat the pathophysiology of the disease.14,17–19 Developing an animal model with collagenase application to induce the evolution of keratoconus in mice generates knowledge for future development of treatment mechanisms based on pathophysiological examination.20 There are models developed with type II collagenase eye drops in rabbits and several studies have demonstrated inflammation in keratoconus pathophysiology.21 The aim of this study was to develop an animal model in rabbits using intrastromal injection of collagenase type II that simulates the altered curvature, expression of inflammatory factors, oxidative stress and histopathology of keratoconus.
Material and methodsAnimals and collagenase type iiThis study used Six New Zealand rabbits of 3.0–4.0kg obtained from the Bioterio de Ciencias Básicas of the Autonomous University of Aguascalientes (Aguascalientes, Mexico). The experimental protocol was approved by the Ethics Committee for the Use of Animals in Teaching and Research of the Autonomous University of Aguascalientes (CEADI-UAA-001/003/2021). Rabbits were kept in a controlled environment of 12h light/12h dark cycles. Food and water were available ad libitum. Care was provided throughout the 7 days of the study. Animals were anaesthetized via intraperitoneal 6mL/kg of sodium pentobarbital 1:10 and for topical anesthesia 5mg/mL tetracaine hydrochloride eye drops (Lab. Sofia, Guadalajara, Mexico) were used. Animals were treated according to the ARRIVE (V 2.0) guidelines protocol (National Center for the Replacement, Refinement & and Reduction of Animals Research) for the use of animals in experimental research. Collagenase type II (Sigma-Aldrich) powder was dissolved in a dextran balanced salt solution (15%) at a concentration of 2.5mg/mL.
InterventionTwelve eyes of six rabbits were divided into two groups. The right eyes were the experimental group, the left eyes the control group. Once anaesthetized, the cornea was injected using an ultrafine needle with an inverted microscope under the corneal epithelium. In the experimental group, 5μL of collagenase type II 2.5mg/mL was injected at room temperature. Subsequently, ophthalmic chloramphenicol antibiotic was administered to prevent possible infection. The same procedure was performed on the control eyes, administering balanced salt solution with 4% dextran.
KeratometryWith a one-position keratometer (Bausch and Lomb, NY, USA), the main meridians were obtained from 3mm in the center of the cornea one day before the application of intrastromal collagenase and 7 days after the intervention. The 2 principal meridians' keratometry average, minor curve (K1), major curve (K2) and the average of the 2 meridians (Km) were recorded with results being expressed in diopters (D).
HistologyCorneas were fixed in 4% paraformaldehyde for 3 days and embedded in paraffin. Slices of 8μm thick were cut from limbus to limbus for hematoxylin-eosin and sirius red staining. Corneas stained with hematoxylin-eosin were observed in brightfield and those stained with sirius red with polarized light. In order not to induce an evaluation bias, one researcher performed the histochemistry technique until the staining phase was completed and another researcher with greater experienced with light microscopy and pathology assessment evaluated the histological sections without knowing their origin, in order to avoid influencing the examination.
RNA extraction and RT-PCRTotal RNA was extracted using a purification kit (cat. no. 12183555; Thermo Fisher, Inc., California, USA). RNA concentration and purity was quantified with a NanoDrop 2000 spectrophotometer. cDNA synthesis was performed with the iScript kit (cat. 1708891; Bio-Rad Laboratories, Hercules, California, USA) and a thermal cycler (Thermo Fisher, Inc., California, USA). Taq DNA Polymerase, Recombinant (cat. no. 11615-050; Thermo Fisher Scientific, Inc., California, USA) was used for PCR. We worked with 1μg/μL of cDNA in a final volume of 25μL. The oligonucleotides are shown in Table 1. Relative expression of collagen I mRNA was normalized against GAPDH expression. It was analyzed using ImageJ software from Fiji. Experiments were repeated in duplicate.
Statistical analysisValues were expressed as mean±standard error. Data analysis was performed using the GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA). Group results were compared using Student's t for paired samples. A p<0.05 was considered statistically significant.
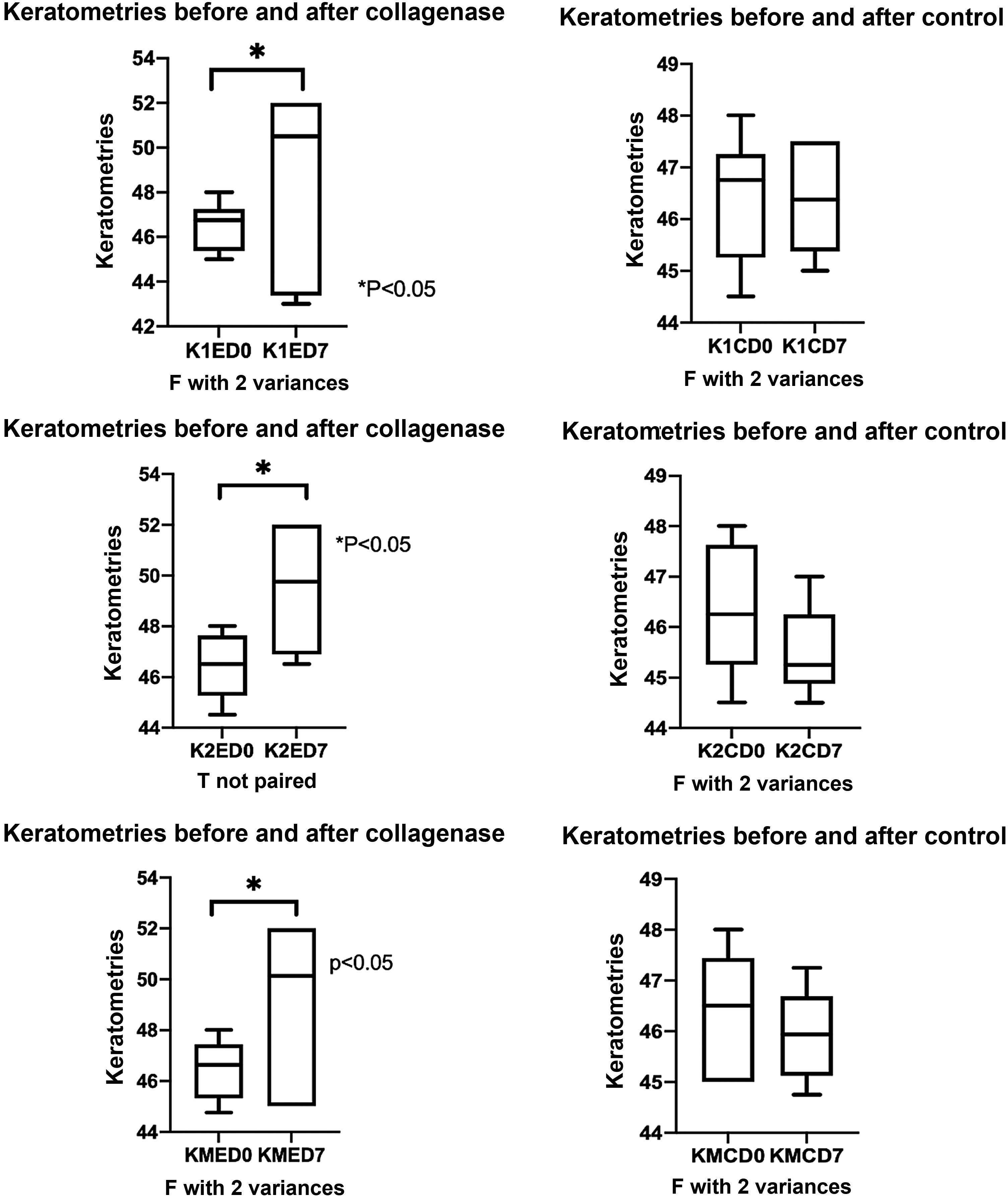
ResultsCorneal surface alteration by collagenase II actionKeratometry was measured before collagenase II administration by intrastromal injection in the experimentation group or a balanced salt solution for the control group; 7 days after application, keratometry was measured again in both meridians and in the average of the 2 meridians. The values of collagenase II corneas in K1, K2 and KM, respectively, are described below (K1ED0=46,50±1,08; K1ED7=48,58±4,29); (K2ED0=46,42±1,28; K2ED7=49,50±2,75); (KMED0=46,60±1,17; KMED7=49,04±3,45); all differences were statistically significant (p<0.05); with respect to controls, they are presented in the same order; (K1CD0=46.42±1.23; K1CD7=46,38±1,09); (K2CD0=46,33±1,29; K2CD7=45,50±0,89); (KMCD0=46,38±1,20; KMCD7=45,94±0,95), (p>0,05) (Fig. 1).
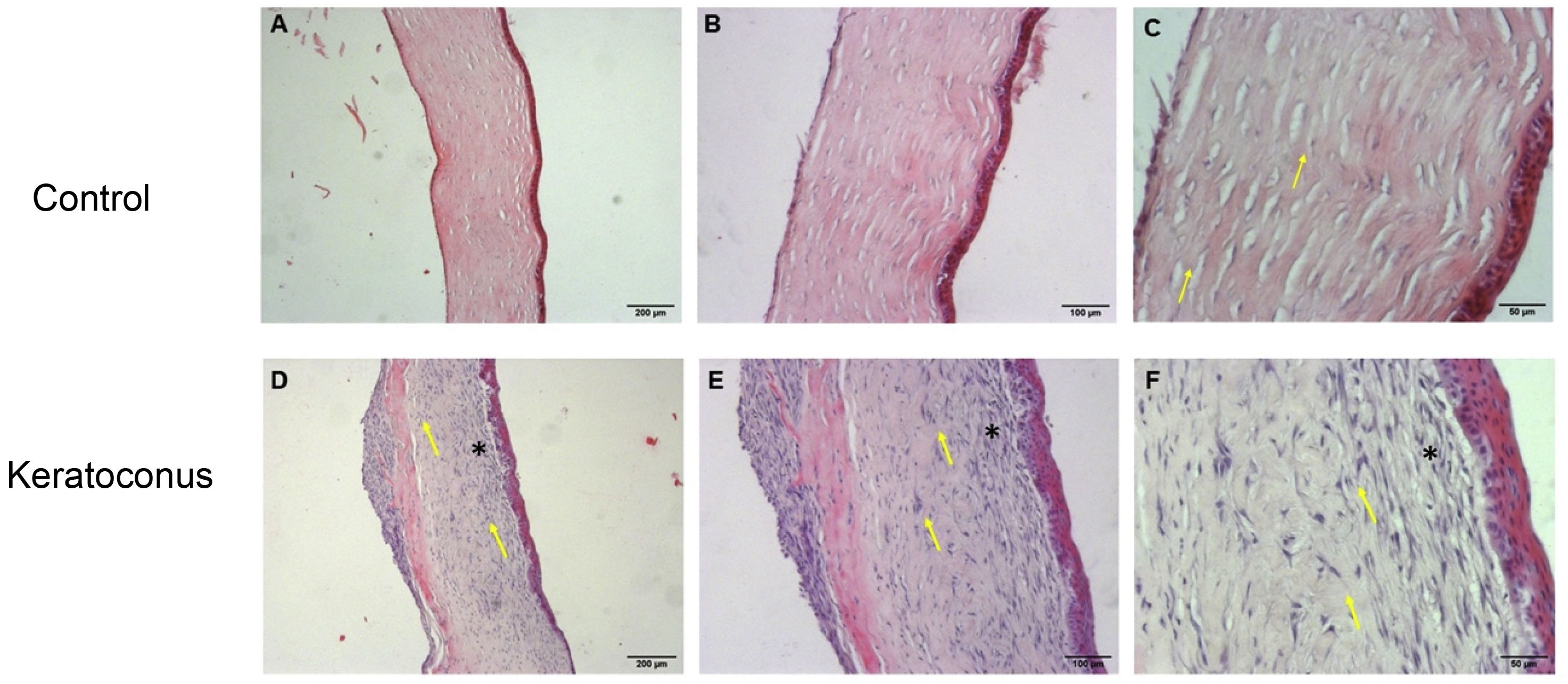
Corneal morphological changes due to collagenase II actionThe results obtained from hematoxylin-eosin staining indicate that control corneas maintained normal morphology at 7 days manifested by collagen fiber parallelism with respect to the epithelium (arrows) (Fig. 2A–C). However, in the keratoconus group, the epithelium's irregular arrangement and loss of collagen fiber parallelism (arrows) was observed in Bowman's layer. There was a mild inflammatory reaction with high keratocytes density, which led to an increase in stromal thickness due to the formation of extracellular matrix (Fig. 2D–F), indicating a process of keratocyte activation and stromal regeneration (stromal scarring).
Morphological alterations after collagenase II administration. Morphological changes of corneas exposed to collagenase II show changes characteristic of an inflammatory and regenerative process and loss of integrity in Bowman's layer, compared to controls. (A–C) Controls. (D–F) Keratoconus. Both with 5×, 10× and 20× objectives, respectively (magnification 500, 1000 and 2000, scale bar 200μm, 100μm and 50μm, respectively).
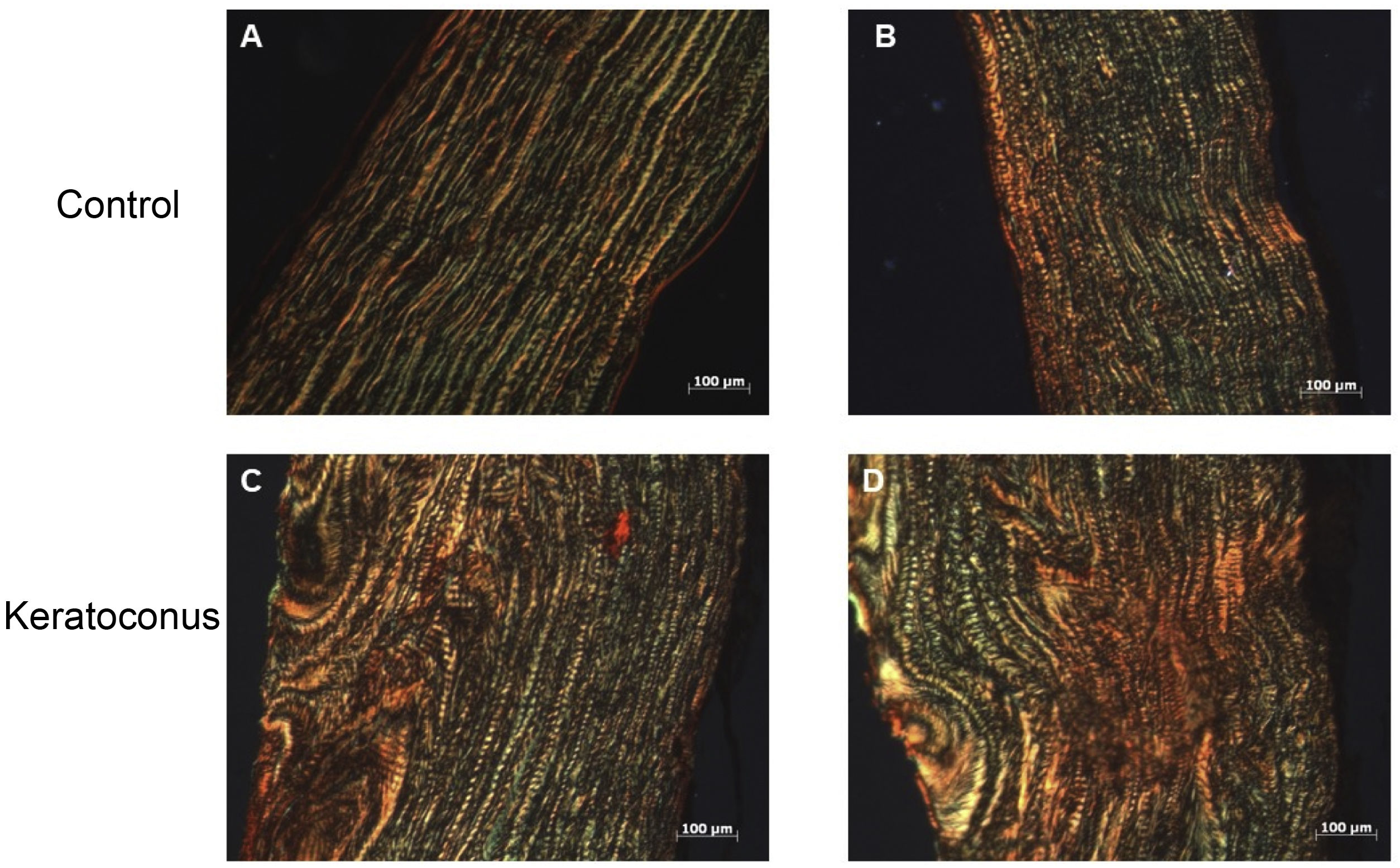
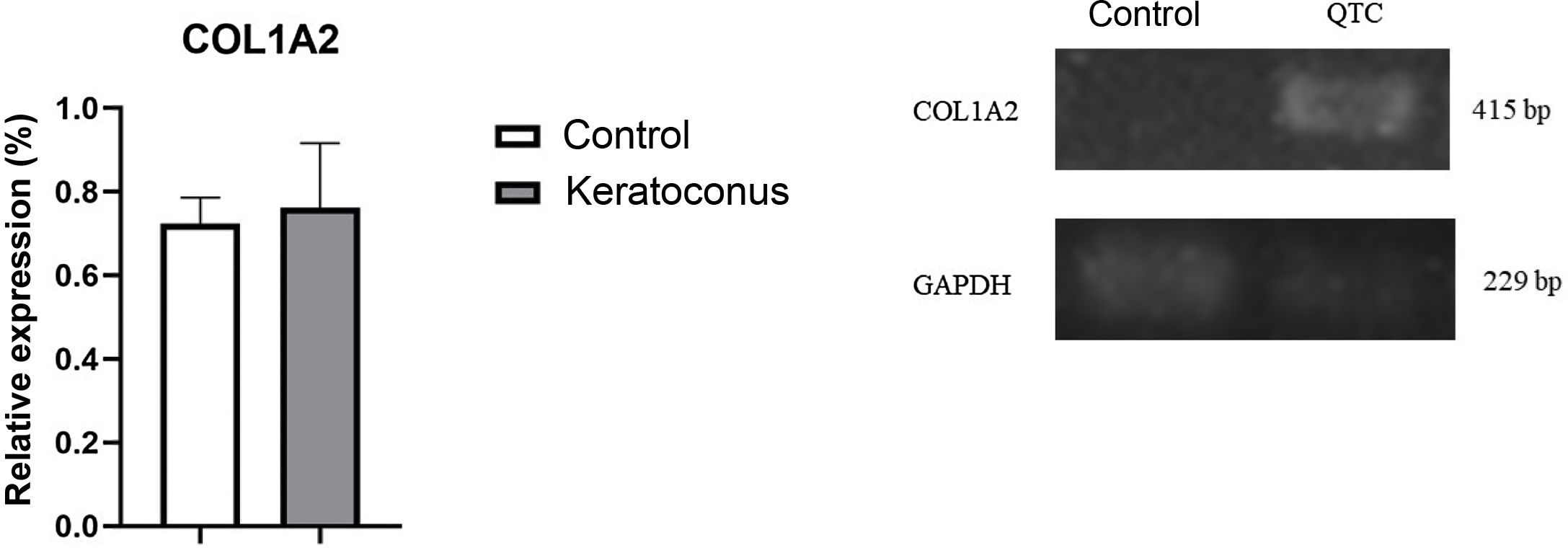
To evaluate type I collagen expression, sirius red staining of tissue and semi-quantitative PCR were performed. With sirius red staining, changes in collagen fiber arrangement were observed in the experimental group compared to the control group; and in control corneas, the presence of type I collagen was observed in the red fibers and type III collagen in the green fibers (Fig. 3A–B). In contrast, experimental corneas show a greater presence of type I and III collagen in the lesion and these fibers are thicker, indicating the development of stromal regeneration by extracellular matrix synthesis (Fig. 3C–D). Regarding type I collagen gene expression, no statistically significant changes were observed between the control and experimental groups (p=0.77) (Fig. 4). However, the trend in the graph demonstrates a slight increase in type I collagen expression in the experimental group over the control group which, with a larger corneal sample, could increase the difference between the groups.
Collagen fiber change in keratoconus animal model. The alteration in corneal collagen fibers with collagenase II indicates a process of corneal stroma regeneration by the action of collagenase II, mainly collagen type I synthesis. (A–B) Controls. (D–E) Keratoconus. Both with 10× objectives (magnification 1000, scale bar 100μm).
Collagen I gene expression in the keratoconus animal model. Although there is no significant change, a trend towards increased expression of type I collagen in the keratoconus group is evidenced. Data are presented as mean+standard error; n=6 per group. bp: base pairs; COL1A2: collagen type I alpha chain 2; QTC: keratoconus.
According to the described background of keratoconus animal models, this work was developed highlighting the following findings: (1) collagenase II has the ability to alter the cornea and corneal stroma's curvature, (2) there is a process of corneal stroma regeneration at the lesion site.
Collagenase II is a protease used in keratoconus animal models to degrade type I collagen and produce thinning and corneal surface alteration. Qiao previously reported a keratoconus animal model administrating collagenase II in eye drops at a concentration of 5mg/mL, with prior corneal debridement.21 However, although this methodology had already been published by other authors, our results did not replicate the effect they found. Qiao reported an increase in keratometry at day 7 and 14 with respect to the control, but we found no changes in the rabbits' corneas with the same eye drop procedure. In contrast, with the intrastromal injection procedure we did obtain numerical differences in the 3 keratometry values (K1, K2 and Km), being statistically significant in all 3 measurements, which is a similar result to that found in the human keratoconus model, so we attribute this result to the fact that the response we evaluated is due to the collagenase effect.8 The arrangement of collagenase entry in the direction in which it flows could be modifying the stromal lamellae, causing the meridian to weaken and become more curved, leading the biomechanical forces of the perpendicular posterior fibers to exert greater pressure on the more curved meridian.22 Regarding the decrease in collagen fibers, irregular fiber arrangement and clefts in Qiao's study, we found in contrast a different pattern, i.e., an increase in the number of keratocytes with possible activation in a stromal regeneration process with formation of a stromal scar by keratocyte activation, agreeing with Song et al.23 In Moghadam's study in rats he used collagenase by intrastromal injection, reporting corneal opacity and deformity and severe damage to collagen fibers, epithelial thinning and corneal stroma.20 We replicated this injection model and obtained similar results, mainly corneal opacity and deformity, which confirmed the surface alteration through keratometry with severe damage to the collagen fibers and corneal epithelium irregularity. These changes were attributed to the cornea's healing process by collagenase II, and after 3 days the opacity disappeared.
The cornea is made up of type I and V collagen, but type III, IV and VII collagen can be found in smaller quantities as well. During corneal regeneration, quiescent keratocytes are activated and transdifferentiate into myofibroblasts in order to restore the extracellular matrix by synthesizing collagen type I, III, IV and V.23 Collagen III is expressed in the regeneration and disorganization of type I collagen fibers, and is a marker of fibrosis.24,25 Sirius red staining results show that control corneas have regularly arranged collagen III fibers; sirius red dye binds specifically to collagen I, II and III fibers, and due to its birefringent effects it can be analyzed under a polarization microscope. This procedure allows quantitative analysis of tissue content in collagen fibers using Fiji software, which is more specific than trichrome histological techniques, that indicate that the damage caused by the needle when injecting the balanced salt solution is regenerated with new collagen fibers. Regarding corneas with keratoconus, there is a greater presence of irregularly arranged type I collagen fibers, indicating that they are in a regeneration process. Our results contrast with the research of Lorenzo-Martín et al. where stromal composition during regeneration using the sirius red technique was evaluated, and a lower presence of type III collagen in the injured cornea is demonstrated. It is important to note that there is controversy in the interpretation of results of this technique, as some authors claim that the color is due to the type of collagen and others attribute the color to the arrangement and thickness of the collagen, indicating that it is a test that only determines collagen fiber regularity. In the study by Lorenzo-Martín et al. gene overexpression of collagen I and III was found, concurring with our results of collagen II gene expression.26
In the present study the effect of various concentrations of collagenase II was not evaluated, nor was the long-term model studied, limiting our research. It is important to evaluate different concentrations in order to develop a more moderate degradation process, as well as to evaluate the long-term corneal regeneration time with immunohistochemical histological tests and longitudinal gene expression to determine the animal model duration and determine its viability. Therefore, we suggest that further research should be carried out on this model to determine the term time, as well as to further understand the damage process induced by this enzyme.
ConclusionsOur study presents a keratoconus animal model with a less invasive and faster method, additionally showing the degradative effects of collagenase II on tissue. Further research is needed to determine whether this model is viable for studying the pathophysiology and possible treatments of keratoconus.
FundingProject funded by the Autonomous University of Aguascalientes: DGIyP- PIBB18-1.
Conflict of interestNone.