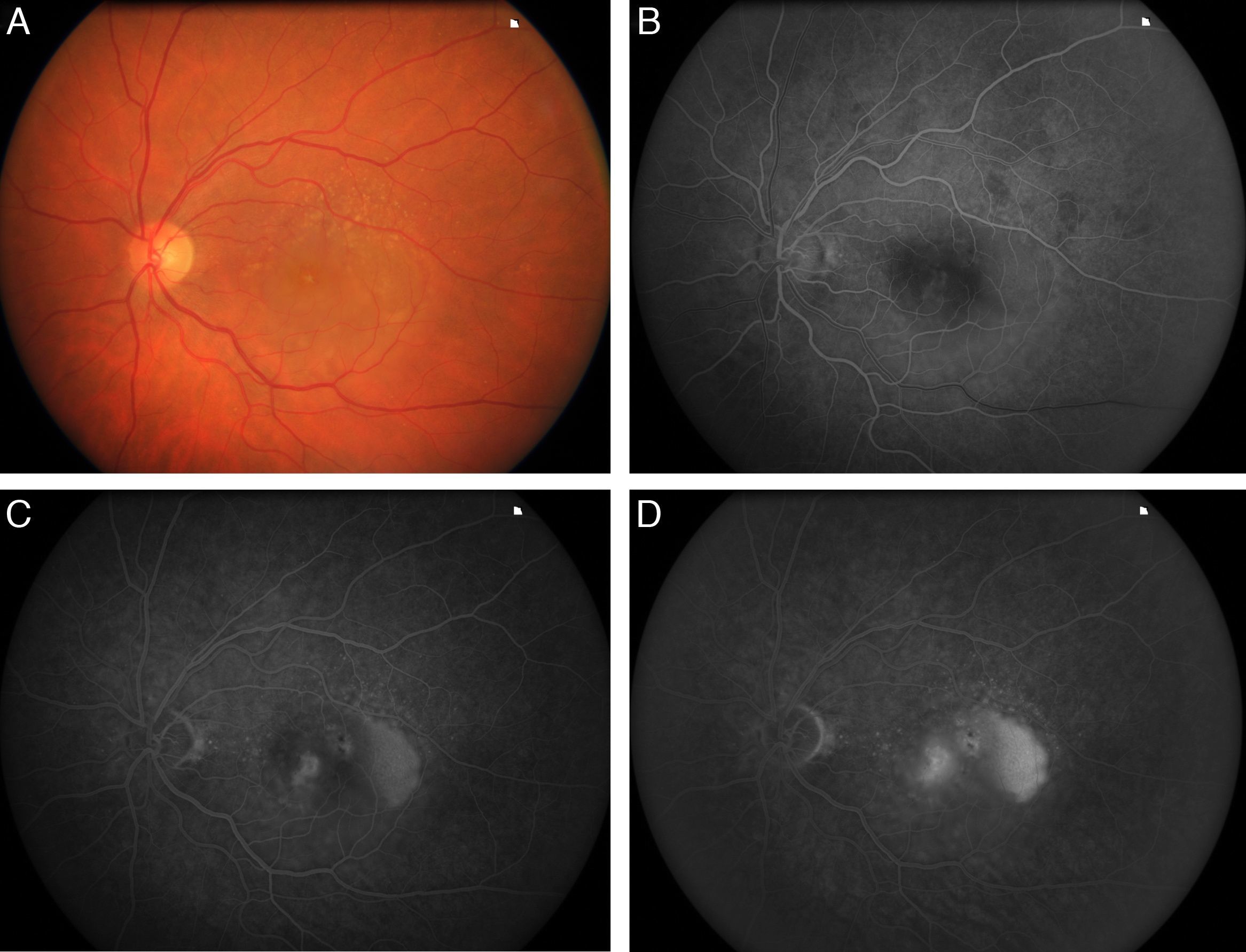
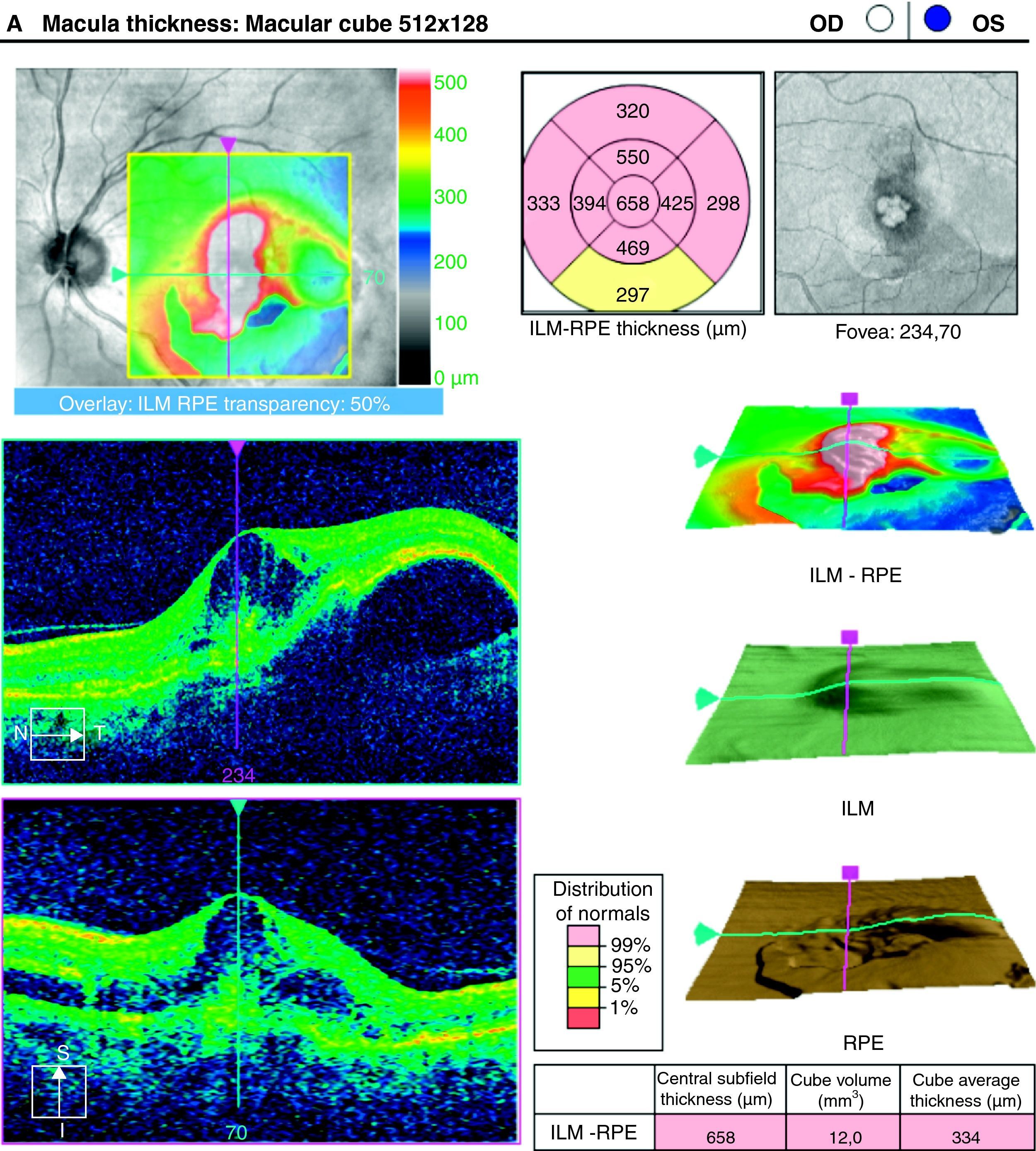
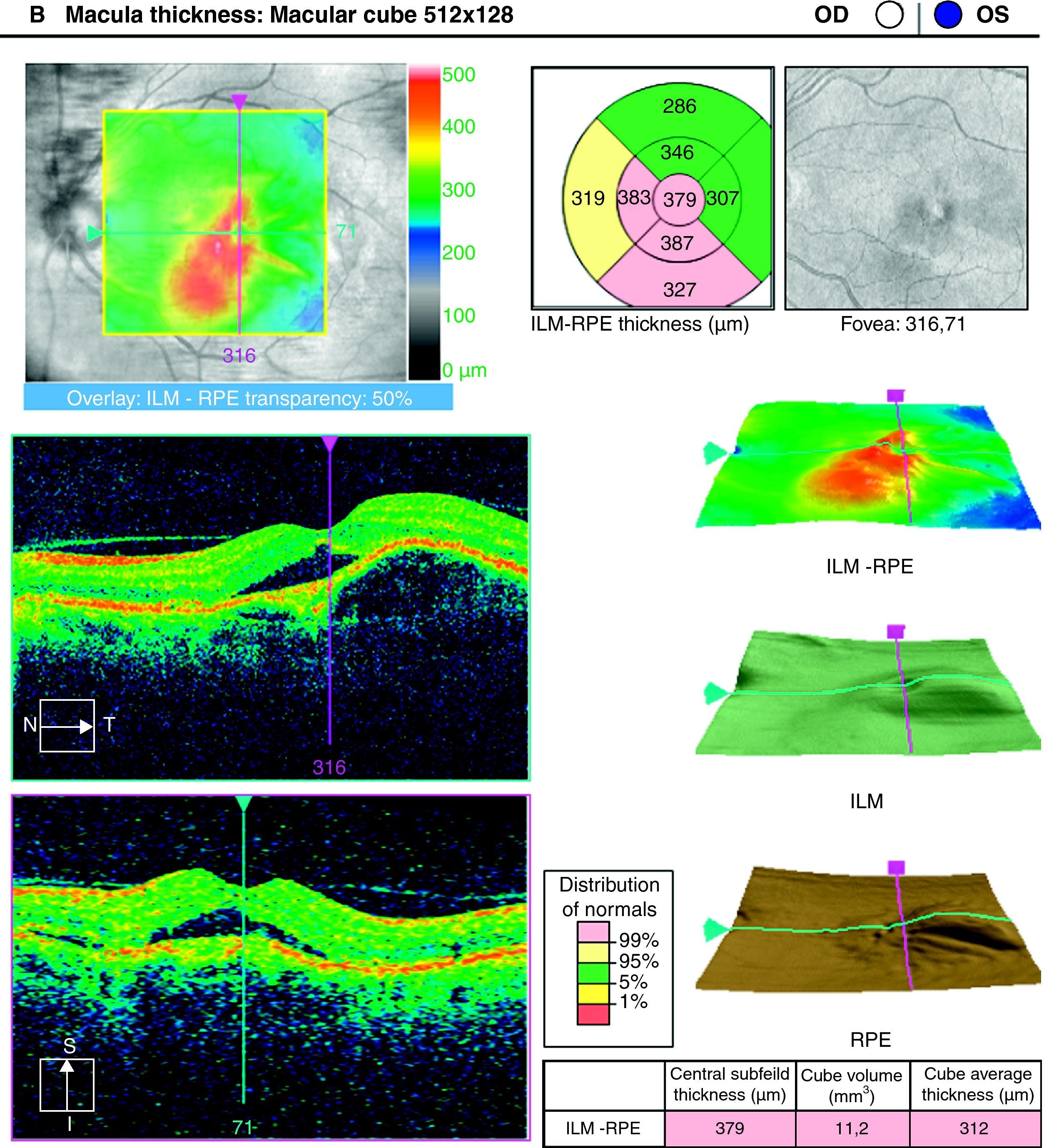
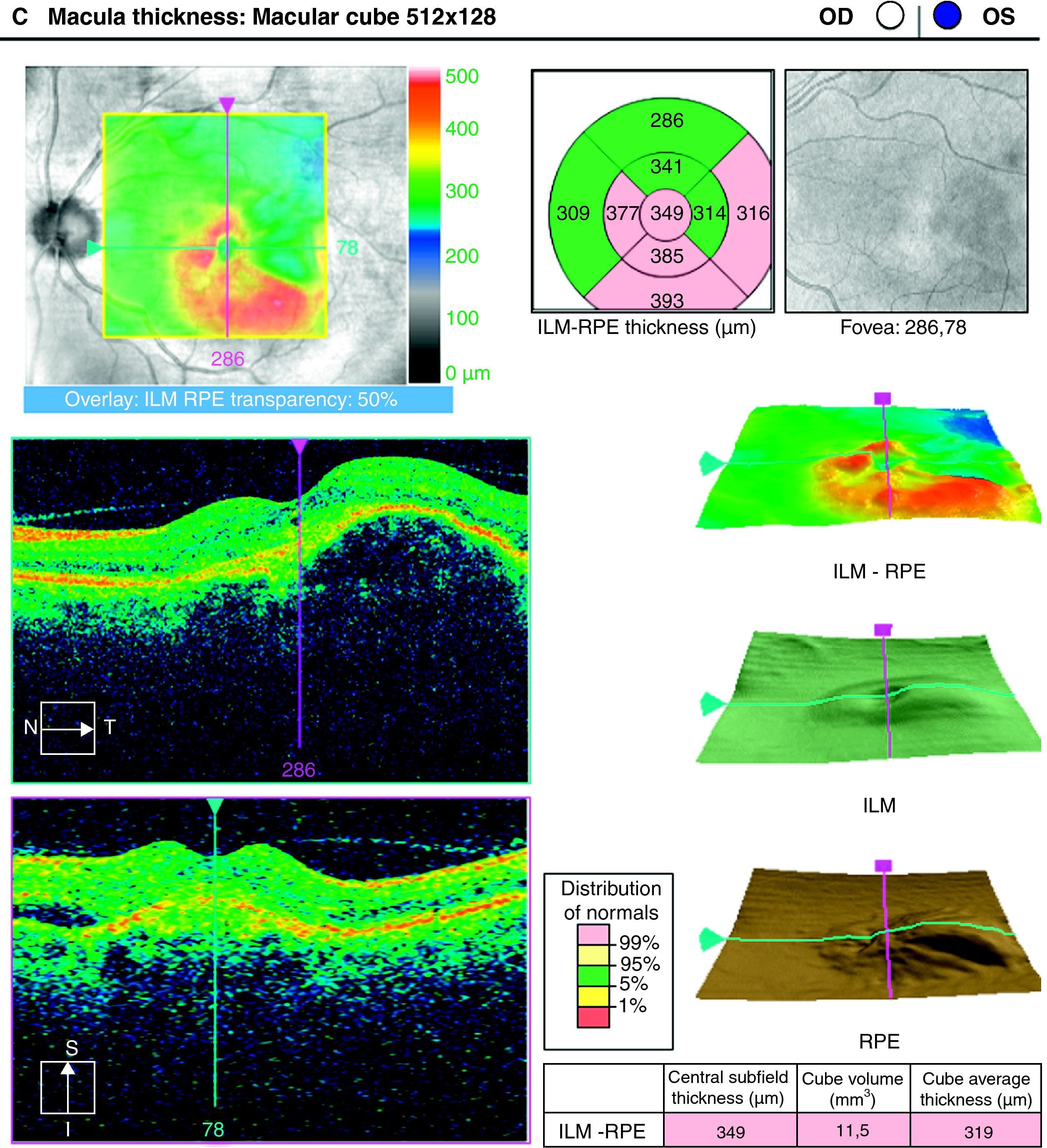
To analyse the long-term visual acuity (VA) in patients with age-related macular degeneration (ARMD) treated with ranibizumab, and who had persistent subretinal fluid after the induction therapy and/or in the successive controls.
Materials and methodsWe reviewed the medical records, optical coherence tomography (OCT) and fluorescein angiograms of 216 patients treated with ranibizumab between January 2008 and April 2010, selecting those who had persistent subretinal fluid or recurrent fluid for at least one year of follow-up.
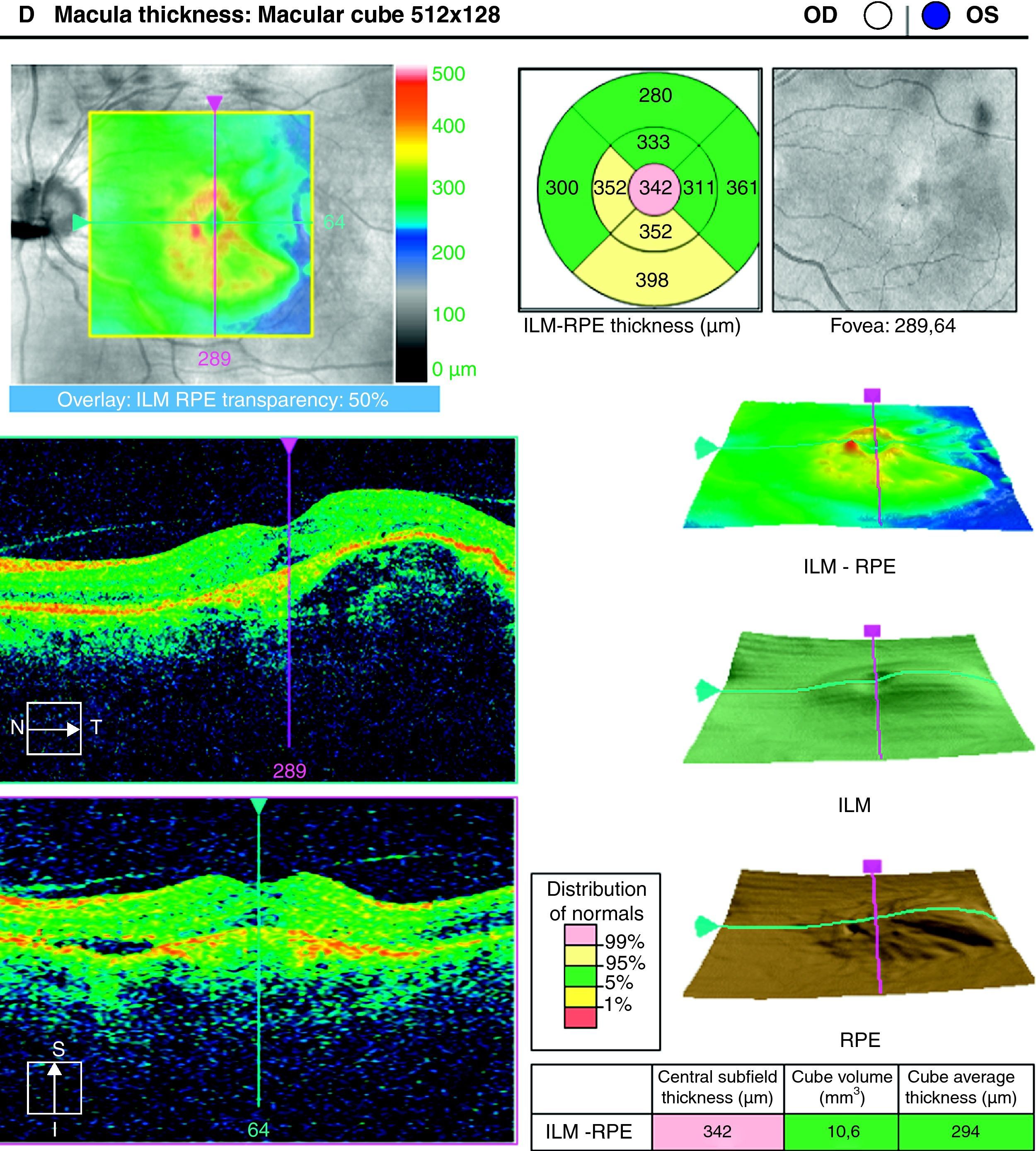
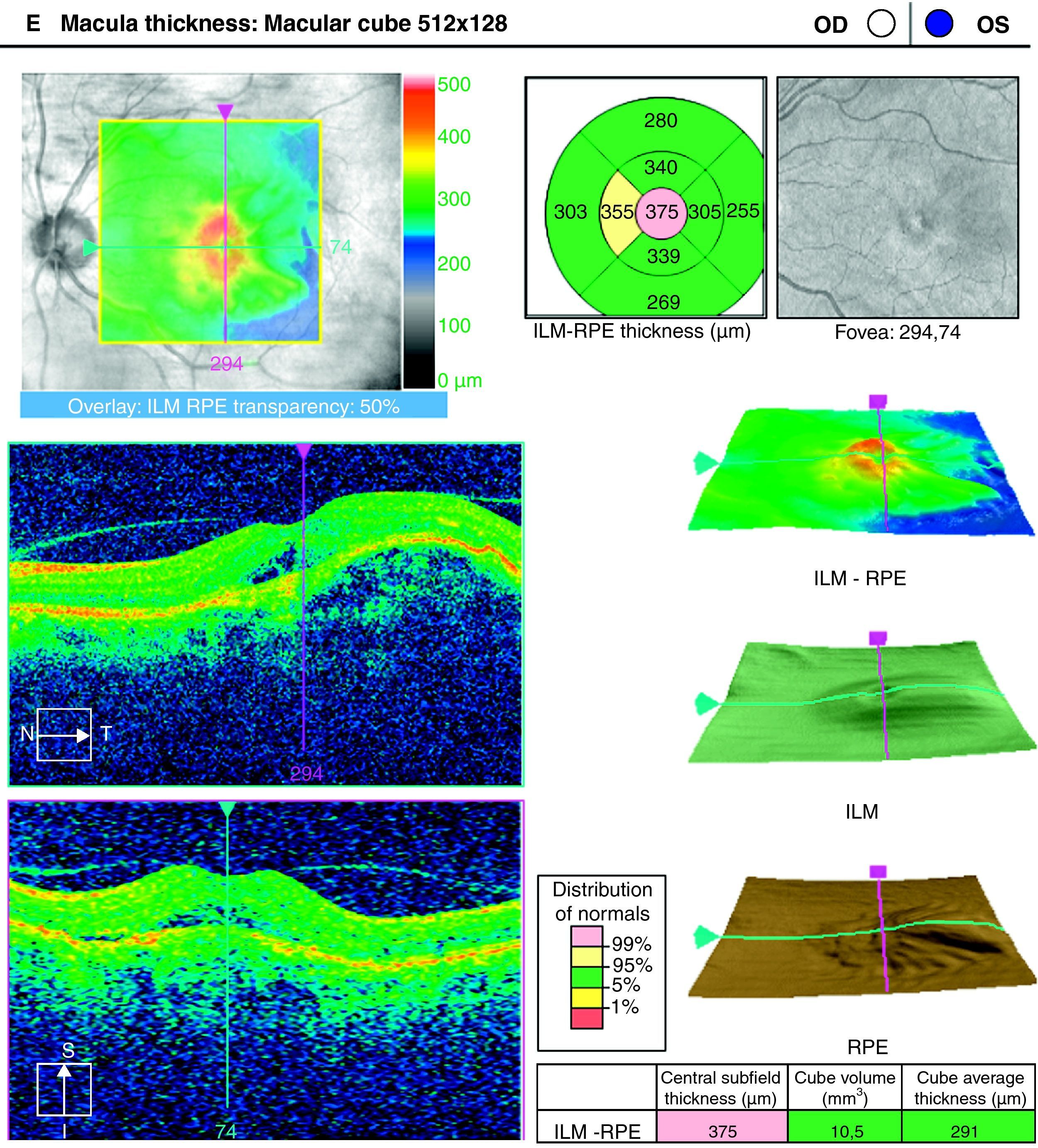
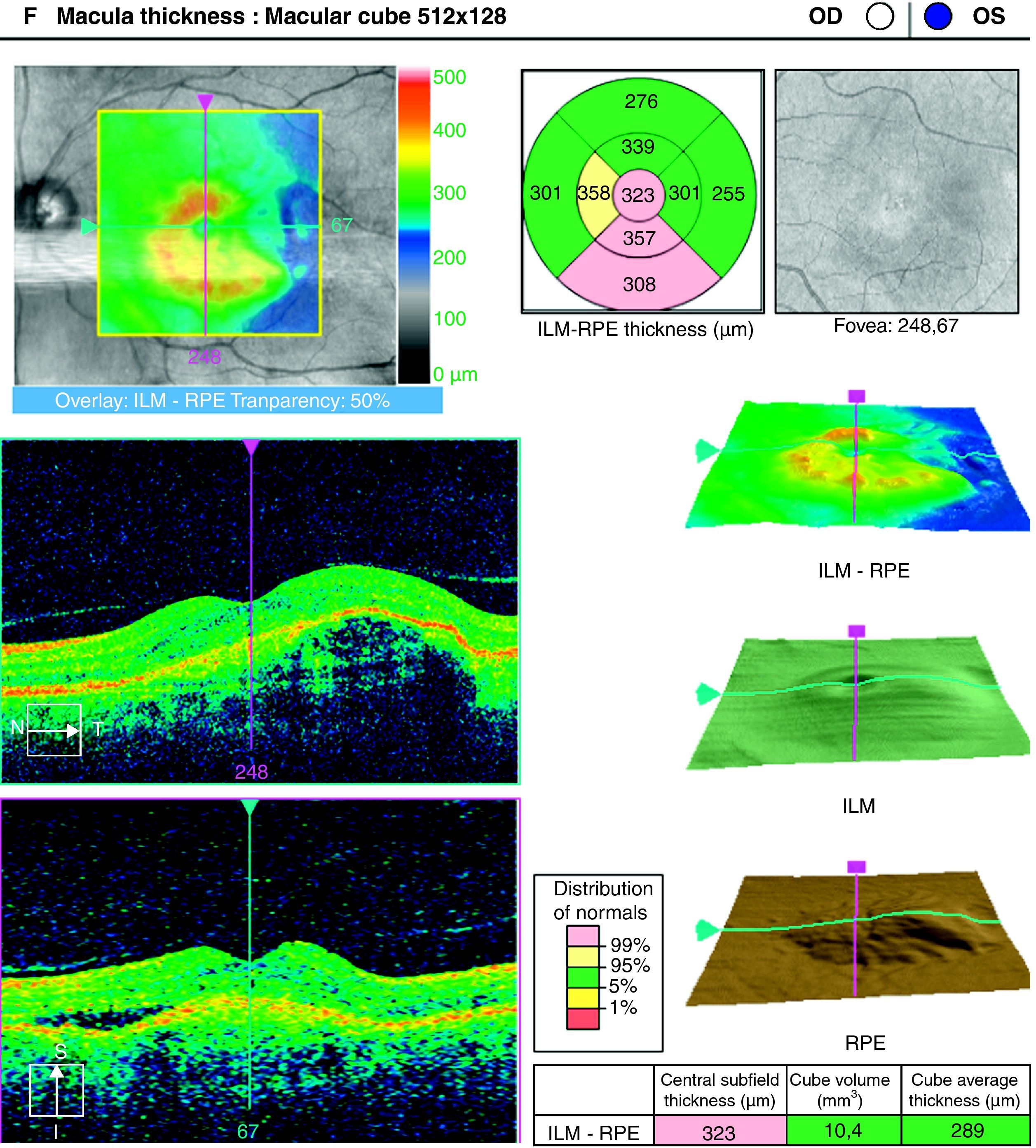
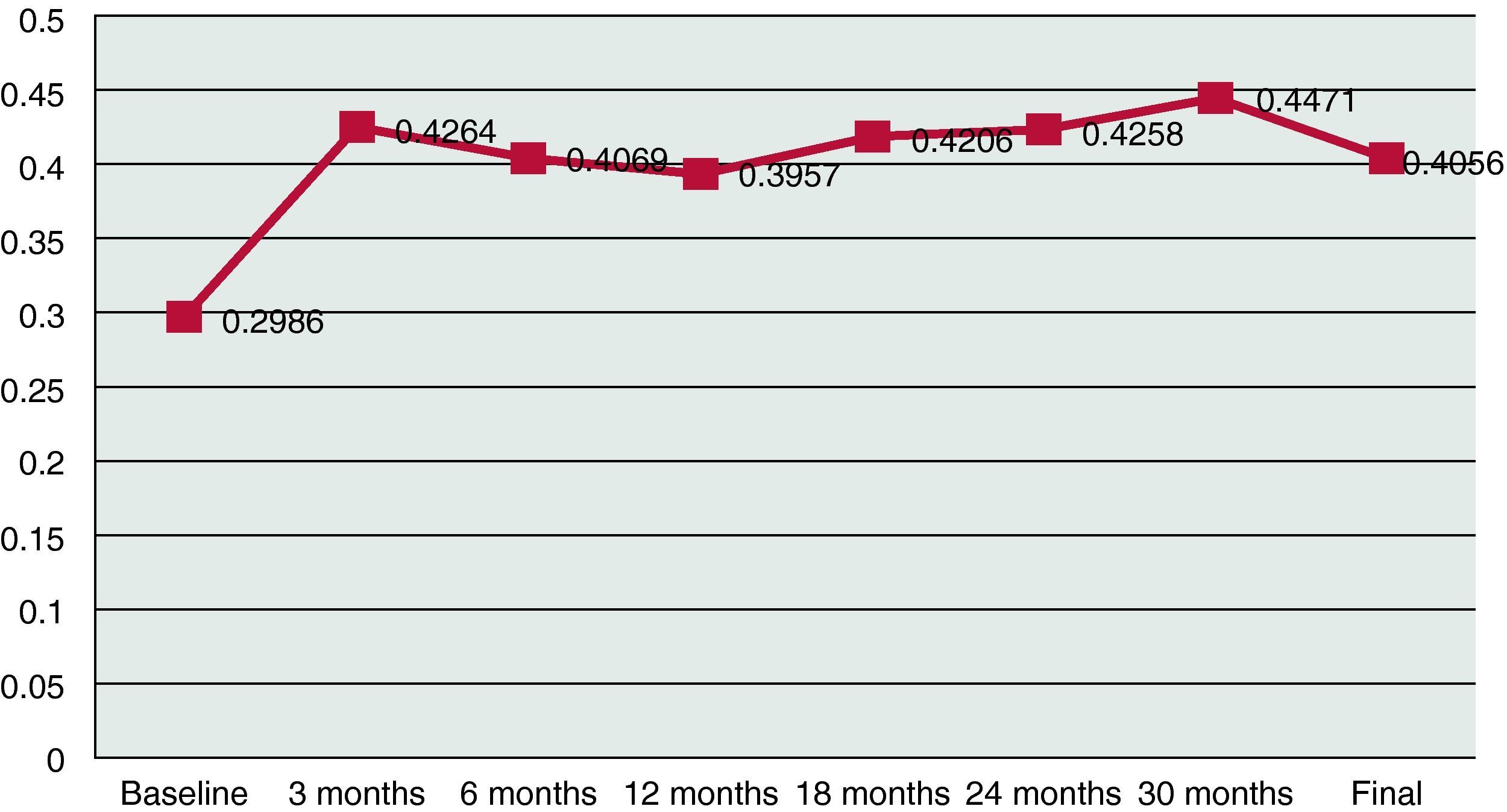
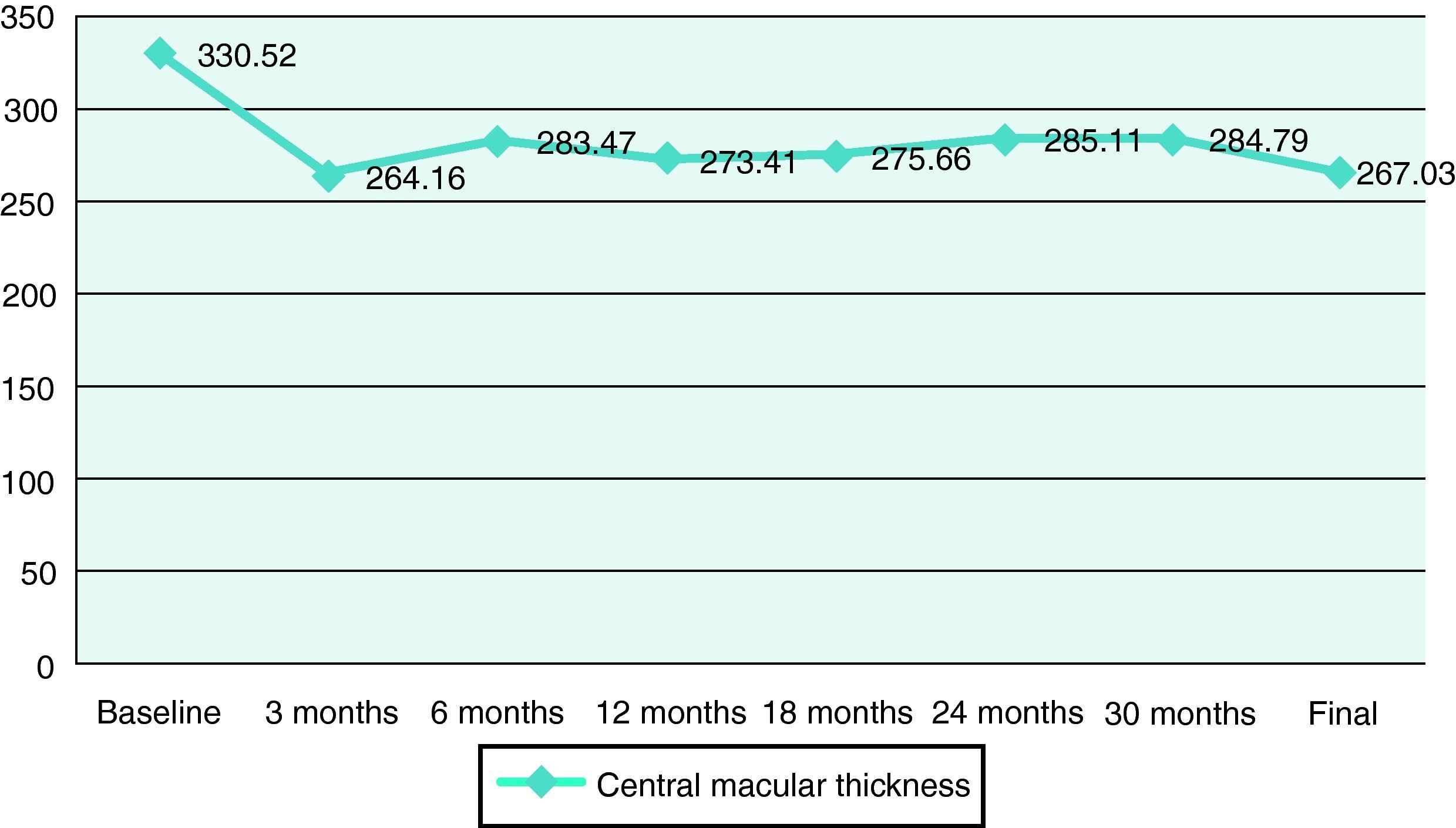
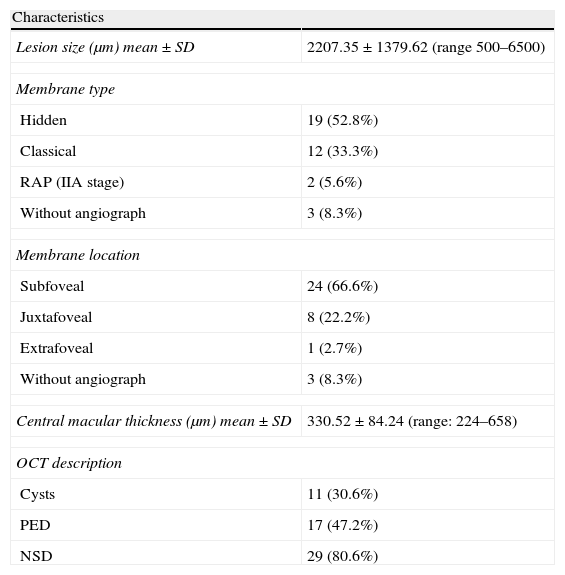
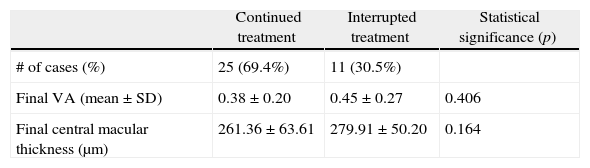
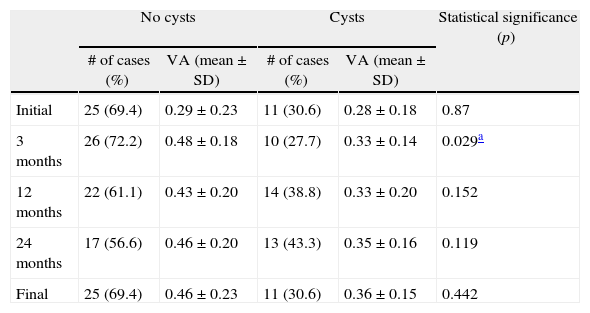
ResultsA total of 36 eyes from 34 patients were included, with 19 eyes (52.7%) having persistent, and 17 (47.2%) recurrent subretinal fluid throughout the follow-up (mean 29.06±9.28 months). The average number of injections was 7.89±3.2. The central macular thickness (CMT) at the start of follow-up was 330±84μm, at 3 months 265.2±62μm, and 294.5±37μm at the end of the follow-up. The initial mean VA was 0.3±0.2, at 3 months 0.43±0.2 (p<0.05) and at the final review, 0.41±0.22 (p<0.05). Hemorrhages in recurrences were associated with a worse final VA (p=0.004). At the end of follow-up, 18 eyes (50%) continued with ranibizumab treatment, 16 eyes (44%) were kept under observation, and 2 patients died. There were no differences between VA and CMT between the groups.
ConclusionsThe persistence or recurrence of macular subretinal fluid in patients treated with ranibizumab does not significantly reduce the visual gain obtained after induction therapy, despite discontinuation of treatment during follow-up. Haemorrhages in the recurrences were associated with a worse final VA.
Analizar la agudeza visual (AV) a largo plazo en pacientes con DMAE tratados con ranibizumab con persistencia de líquido subretiniano después del tratamiento de inducción y/o en los controles sucesivos.
MétodoHemos revisado las historias clínicas, tomografías de coherencia óptica (OCT) y angiografías fluoresceínicas de los 216 pacientes tratados con ranibizumab entre enero de 2008 y abril del 2010, seleccionando aquellos que han presentado fluido subretiniano de forma persistente o recurrente a lo largo del seguimiento mínimo de un año.
ResultadosHemos incluido 36 ojos de 34 pacientes; 19 ojos (52,7%) presentaban persistencia y 17 (47,2%) recurrencia de fluido subretiniano a lo largo del seguimiento (media 29,06±9,28 meses).nLa media de inyecciones fue de 7,89±3,2. El espesor macular central (EMC) inicial fue de 330±84μm, a los 3 meses de 265,2±62μm y de 294,5±37μm al final del seguimiento. La AV media inicial fue de 0,3±0,2, a los 3 meses 0,43±0,2 (p<0,05) y al final del seguimiento de 0,41±0,22 (p<0,05). La aparición de hemorragias en las recurrencias se asoció con peor visión final en comparación con los que no las presentaron (p=0,004). Al final del seguimiento18 ojos (50%) continúan en tratamiento con ranibizumab, 16 ojos (44%) se mantienen en observación y 2 pacientes han fallecido. No existen diferencias entre AV y EMC entre ambos grupos.
ConclusiónLa persistencia o recurrencia de fluido macular subretiniano en pacientes tratados con ranibizumab no disminuye significativamente la ganancia visual obtenida después del tratamiento de inducción, a pesar de la interrupción del mismo durante el seguimiento. La aparición de hemorragias en las recurrencias se asoció con peor AV final.