To assess the mean best-corrected visual acuity (BCVA) change in patients with exudative-hemorrhagic age-related macular degeneration (EH-ARMD) after 12-month period of treatment with ranibizumab.
MethodsA retrospective, multicentre and national study of intravitreal administered ranibizumab was conducted on two groups of EH-ARMD patients: only one eye affected (group 1) vs second eye affected (group 2), having the first one affected.
Eligible subjects were ≥50 years old with primary or secondary active subfoveal EH-ARMD-related choroidal neovascularisation (CNV).
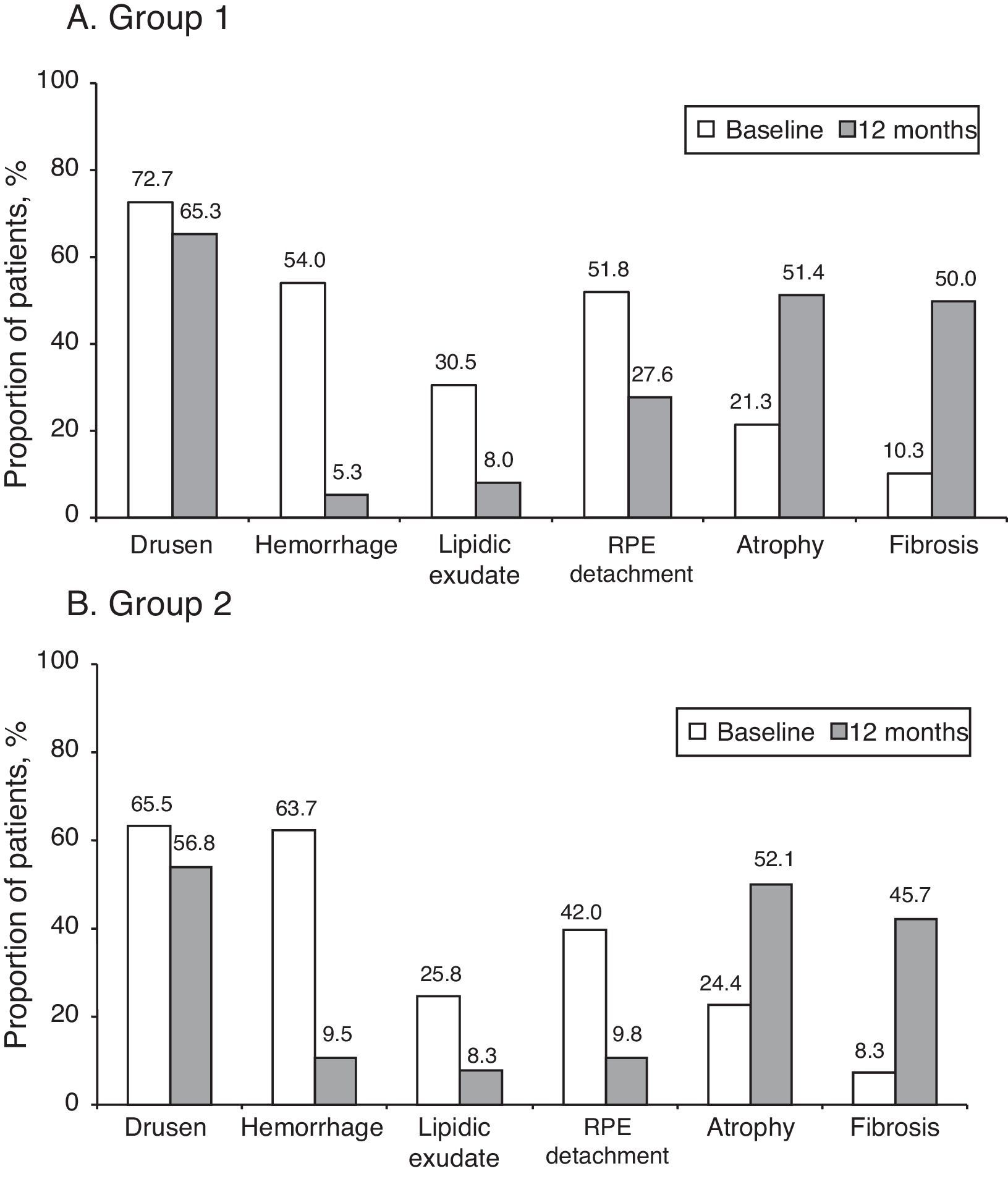
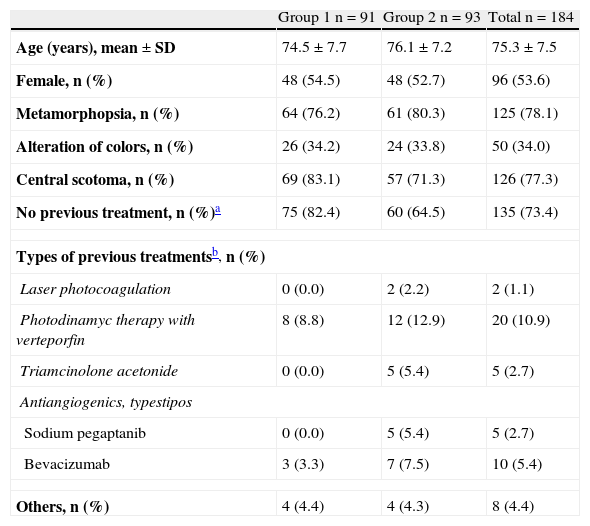
ResultsA total of 184 patients (91 group 1 and 93 group 2) were included. Mean age (SD) was 75.3 (7.5) years, and 53.6% were women. The BCVA showed a VA improvement at 12 months of 9.3 (18.0) number of letters in group 1 and 5.1 (16.8) number of letters in group 2 (p<0.0001 and p=0.0042, respectively). No statistical differences between groups were observed. Lesion characteristics in the total population (baseline vs 12-month) were: drusen (69.1% vs 61.1%), macular hemorrhages (59.0% vs 7.3%), lipid exudates (28.1% vs 8.2%), and retinal pigment epithelium detachment (46.8% vs 19.0%). The optical coherence tomography (OCT) in the total population (baseline vs 12-month) showed a reduction in macular edema (73.6% vs 20.9%), subretinal fluids (71.3% vs 14.7%), and intraretinal cysts (38.5% vs 19.7%), as well as a reduction of the mean foveal thickness 377.4±109.8μm vs 249.1±67.8μm in group 1 and 354.1±123.2μm vs 254.6±67.4μm in group 2, p<0.0001, both groups, with no significant differences between groups.
ConclusionsIntravitreal administration of ranibizumab for a minimum of 12-months significantly improved the BCVA, decreased lesion characteristics, and reduced the initial mean foveal thickness in patients with CNV primary or secondary to EH-ARMD, both in patients with only one eye affected and in patients with a second eye affected, having the first one affected.
Evaluar el cambio medio de la mejor agudeza visual corregida (MAVC) en pacientes con DMAE exudativa-hemorrágica (DMAE-EH) a los 12-meses de tratamiento con ranibizumab.
MétodosEstudio observacional, retrospectivo, multicéntrico y nacional, en dos grupos de pacientes con DMAE-EH: primer ojo afectado (grupo 1) y segundo ojo afectado (grupo 2), teniendo afectado el primer ojo.
Se incluyeron pacientes ≥50 años, diagnosticados de neovascularización coroidea subfoveal activa principal o recurrente secundaria a DMAE-EH.
ResultadosSe incluyeron 184 pacientes (91 grupo 1 y 93 grupo 2), edad media±DE de 75,3±7,5 años y 53,6% mujeres. La MAVC mostró una mejoría de la AV a los 12-meses: 9,3±18,0 número de letras grupo 1 y 5,1±16,8 número de letras grupo 2 (p<0,0001 y p=0,0042, respectivamente), sin observarse diferencias significativas (NS) entre ambos grupos. Las lesiones retinianas en la población total (basal vs 12-meses) fueron: drusas (69,1% vs 61,1%), hemorragias maculares (59,0% vs 7,3%), exudados lipídicos (28,1% vs 8,2%) y desprendimiento del epitelio pigmentario (46,8% vs 19,0%). Los resultados de la OCT en la población total (basal vs 12-meses) mostraron una reducción del edema macular (73,6% vs 20,9%), del fluido subrretiniano (71,3% vs 14,7%) y de quistes intrarretinianos (38,5% vs 19,7%), así como una reducción del grosor foveal medio de 377,4±109,8μm vs 249,1±67,8μm grupo 1 y 354,1±123,2μm vs 254,6±67,4μm grupo 2, p<0,0001, ambos grupos, sin observarse diferencias significativas entre ambos grupos.
ConclusionesLa administración de ranibizumab intravítreo durante un mínimo de 12-meses conllevó una mejoría significativa en la MAVC, una disminución de las lesiones retinianas y una disminución del grosor retiniano medio inicial en pacientes con NVC principal o secundaria a DMAE E-H, tanto en los pacientes con primer ojo afectado como en los pacientes con segundo ojo afectado, teniendo afectado el primero.