Silymarin is obtained from the Milk thistle plant Silybum marianum and has been used over the centuries to treat principally liver disease, although it has also been studied for its beneficial effects in cardioprotection, neuroprotection, immune modulation, and cancer among others. Importantly, silymarin’s active component silybin is a flavonolignan that exhibits different activities such as; scavenger, anti-oxidant, anti-inflammatory, and recently revealed, insulin-sensitizing properties which have been explored in clinical trials in patients with insulin resistance. In this review, we summarize the most relevant research of silymarin’s effect on lipid and carbohydrate metabolism, focusing the attention on insulin resistance, which is well known to play a crucial role in metabolic disease progression.
Silymarin, extracted from the fruits of the plant Silybum marianum (L.) Gaertn., contains a mixture of active flavonolignans and flavonoids. Historically, silymarin has been used to treat a range of liver diseases. The principal flavonolignans and flavonoids in silymarin have been identified as compounds that possess many pharmacological activities [1]. Biochemically, silymarin displays scavenger and antioxidant properties that act against free radicals [2], as well as anti-inflammatory effects [3]. Today, it is prescribed to help treat chronic inflammatory liver diseases such as chronic and acute hepatitis, liver steatosis, and cirrhosis [4], as well as, recently, type 2 diabetes (T2D) [5].
In the present review, we focus on recent clinical research related to liver disease and T2D, characterized by metabolic alterations in carbohydrate and lipid metabolism, placing special importance on insulin resistance (IR), as IR plays a pivotal role in metabolic disease progression. Clinically, IR is frequently found in association with metabolic syndrome (MetS) and represents the predominant mechanism that underlies this condition. Evidence suggests that IR is associated with an inflammatory state that drives IR and MetS generating a worsening vicious circle [6]. Several in vitro, in vivo, animal models, and recent clinical studies indicate that silymarin can reduce IR. Here, we present the most relevant research that demonstrates that silymarin exhibits insulin-sensitizing properties, reduces IR, and ameliorates altered carbohydrate and lipid metabolisms in liver conditions, such as cirrhosis, hepatitis C virus infection (HCV), T2D and Non-alcoholic fatty liver disease (NAFLD), recently termed Metabolic-dysfunction-associated fatty liver disease (MAFLD) [7]. The evidence presented suggests that silymarin remains a promising substance for the treatment of chronic metabolic liver and other chronic metabolic diseases such as obesity and T2D.
2SilymarinS. marianum [8–10] is originally from the mountains of the Mediterranean, Asia, and North African regions, but is grown today in several parts of the world [1,11,12]. Silymarin is usually found as a standardized extract, and although it is frequently referred to as obtained from the seeds of the commonly known Milk Thistle plant [13], botanically correct, the plant has cypselae [14] that may appear as seeds but are technically fruit [11,15] https://species.wikimedia.org/wiki/Silybum_marianum#/media/File:Thistle_April_2010-2.jpg. Silymarin (70–80%) is composed principally of the following six flavonolignans, silybin (silybin A and B), isosilybin (isosilybin A and B), silychristin, isosilychristin, silydianin, and silimonin, which represent 1.5–3% of the fruit’s dry weight, and other flavonoids such as taxifolin, quercetin, dihydrokaempferol, kaempferol, apigenin, naringin, eriodictyol, and chrysoeriol. However, the principal active component in silymarin is silybin, synonymous with silibin, and silibinin. (For this review, we will refer to the principal active component of silymarin as silybin, except when the results of the studies are summarized, at which point the substance will be referred to in the same way as it is in the study, to keep consistency with the nomenclature used.) Moreover, there is a certain ambiguity in the literature, whether it is silymarin [16], or silybin [17], or both unless specified, that is responsible for exerting its beneficial effects [18,19].
The remainder of silymarin (20–30%) is comprised of compounds that include 5,7-dihydroxy chromone, dehydroconiferyl alcohol, fixed oil (60% linoleic acid; 30%, oleic acid; 9% palmitic acid), tocopherol, sterols (cholesterol, campesterol, stigmasterol, and sitosterol), sugars (arabinose, rhamnose, xylose, and glucose), and proteins [15,20,21]. Silymarin is obtained through an extraction procedure that involves two steps. The fruits of the plant are defatted for six hours and then silymarin is extracted with methanol for five hours. Alternative methods for obtaining silymarin include pressurized liquid extraction that uses extractants at elevated pressure at temperatures above their boiling point [12].
Silymarin is well known for its anti-inflammatory properties and antioxidant activity, but it is also known to exert an array of positive biological and pharmacological activities such as stimulation of protein synthesis, cardioprotection, neuroprotection, neurotrophic and immune modulation. Silymarin also exerts anti-cancer effects in human carcinoma cell lines, and particularly in liver metabolism, cell regeneration in toxic liver damage. Silymarin displays anti-diabetic activity and has hypolipidemic and antifibrotic effects in chronic inflammatory liver disease as well [15,22]. Traditionally, it has been employed for over 2000 years to treat kidney, spleen, gall bladder, and principally liver afflictions [23]. Today, it is a popular supplement amply used to aid in the treatment of liver disease [24,25]. Several studies have been carried out to determine silymarin’s antioxidant and anti-inflammatory effects, as well as the biological and pharmacological properties of silymarin in liver treatment, and are summarized in a recent review [26].
When silymarin is administered orally, its primary active compound silybin undergoes extensive enterohepatic circulation. It is absorbed within two to four hours and has an elimination half-life of approximately six hours. Only a portion of oral silymarin (20–50%) is absorbed in the gastrointestinal tract, and even a smaller amount (3–8%) is excreted in an unchanged form in the urine. Absorption is decreased due to extensive phase II metabolism, decreased permeability in the intestine, low aqueous solubility, and rapid excretion in the bile and urine. Absorption of oral silymarin is thereby low, with poor bioavailability [15,22]. For this reason, many semisynthetic compounds have been formulated. Strategies of formulas designed to improve the bioavailability of silymarin include nanocrystals, nanosuspensions, and solid dispersions, complexes with cyclodextrins and phospholipids, lipid-based formulations (micro- and nanoemulsions, liposomes, polymer-based nanocarriers, and solid-lipid nanoparticles and nanostructured lipid carriers), polymer-based nanocarriers (polymeric matrices, dendrimers, and polymeric nanoparticles) and nanostructured materials based on inorganic compounds [27]. In particular, silybin can be combined with phosphatidylcholine provided as one part silybin and two parts phosphatidylcholine and is patented as Siliphos® (also referred to as silipide or silybin phytosome) [22]. Phytosomal silybin is more rapidly absorbed than silymarin and has been found to have 4.6 times greater bioavailability in the phytosome form than the simple extract [28]. A recent study with 23 healthy subjects assessed which of two formulations provided better bioavailability of silybin, either 45mgsilybin–phosphatidylcholine complex or 70mg of silymarin in tablet form. Peak plasma concentrations were 207.1mg/L at 1.4 (±0.5) h and 12.6mg/L at 4.6 (±5.8) h, respectively [29].
3Silymarin and lipid metabolismOne of the first studies in animal models to test silymarin’s ability to lower levels of cholesterol was performed in diet-induced hypercholesterolemic rats in 1998. Silymarin not only decreased liver cholesterol content but also increased high-density lipoprotein cholesterol (HDL) [30]. As the administration of silymarin together with other supplements has shown beneficial effects, some studies have examined the combination of silymarin and other nutraceuticals. A study in hypertriglyceridemic rats demonstrated that administration of silymarin and Prunella vulgaris decreased plasma very-low-density lipoprotein cholesterol (VLDL) levels and had a positive effect on the plasma lipid profile in the animals [31]. Another study also tested a combination of phytosome silybin with vitamin E in a silybin–phosphatidylcholine-Vitamin E complex, formulated to increase the bioavailability and lipophilicity of silymarin. The effect of the complex was tested on rat hepatic fibrosis, previously induced by dimethylnitrosamine administration and by bile duct ligation. Rats were fed 250mg/kg of silybin and results indicate that the complex reduced the degree of liver injury, hepatic stellate cell activation and proliferation, and collagen deposition [32]. In another study, silymarin and the polyphenolic fraction of silymarin alone were analyzed to see which of the two affected cholesterol absorption in rats previously fed a high cholesterol diet (HCD). Results showed that both were effective and additionally decreased VLDL, cholesterol, and triacylglycerol content in the liver. However silymarin, but not the polyphenolic fraction of silymarin, significantly increased HDL [33].
NAFLD is a growing worldwide epidemic and is related to obesity and IR [34]. Individuals with NAFLD are at risk of liver-related and cardiovascular mortality. NAFLD includes simple and progressive steatosis and may be associated with hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [35]. Nonalcoholic steatohepatitis (NASH) is a form of progressive liver disease and a severe form of NAFLD [36]. Interestingly, environmental exposure to bisphenol A (BPA), an endocrine-disrupting compound with estrogenic activity, may be involved in the development and progression of NAFLD due to its ability to induce oxidative stress [37]. The positive effect of silymarin on both NAFLD and NASH has been studied in animal models as well. Silybin (200mg/kg/d) was tested in a NASH model with rats. Animals were given a silibinin–phosphatidylcholine complex for five weeks after being fed a high-fat liquid diet. The complex improved liver steatosis and inflammation and decreased NASH-induced lipid peroxidation, plasma insulin, and Tumor necrosis factor α (TNFα) [36]. In a NAFLD model, silybin was administered (26.25mg/kg/d) to six-week-old high-fat diet (HFD) fed rats. Treatment with silybin reduced serum content of alanine aminotransferase (ALT) and hepatic malondialdehyde (MDA), enhanced gene and protein expression of adiponectin but inhibited expression of resistin. Additionally, silybin stabilized mitochondrial membrane fluidity [38]. Another animal model examined the effect of silibinin (0.5mg/kg/d) together with a HFD in rats after inducing NAFLD in the animals. Here, silibinin prevented visceral obesity and reduced visceral fat. The study also tested gene expression and found that treatment with silibinin enhanced lipolysis through the up-regulation of adipose triglyceride lipase, and inhibited gluconeogenesis through the down-regulation of Forkhead box O1, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase [39]. A similar experimental NAFLD rat model tested silibinin treatment in animals (silibinin 100mg/kg/day) for 12 weeks. Results showed that treatment reduced lipid accumulation, recovered cell viability, and alleviated steatosis in the animals [40].
An experimental model with the gerbil Psammomys obesus was used to test the effect of silybin on the lipid profile after animals ingested a high-calorie diet for 14 weeks. Silibinin was administered (100mg/kg/day) after seven weeks and was found to reduce triglyceride (Tg) levels, improve hepatic metabolism, and partially reverse liver steatosis [41]. Another study tested three separate forms of silymarin on dyslipidemia and liver fat accumulation in non-obese hereditary hypertriglyceridemic rats. A standardized extract of silymarin, micronized silymarin, and phytosome silymarin, were administered to animals for four weeks. All forms of treatment in animals significantly lowered Tg, total cholesterol (TC), and increased HDL levels in plasma, although the phytosome and micronized forms of silymarin were more effective. Protein expression of CYP7A1 and CYP4A was also increased and may be responsible for the hypolipidemic effect of silymarin [42]. Another study to show positive results on liver metabolism treated HCD fed rats with silybin (300 and 600mg/kg). Results showed a significant decrease in TC, Tg, low-density lipoprotein cholesterol (LDL), and an increase in hepatic HDL as well, in a dose-dependent manner [43]. Silymarin’s effects were analyzed in another NAFLD model in mice. Results showed once more that silymarin attenuated hepatic steatosis, increased HDL, and decreased LDL. Besides, this study found a role in the mRNA regulation of genes involved in lipid metabolism. Interestingly, silymarin showed no effect on liver transaminases [44], as had been reported by other studies. In a distinct NAFLD mouse model, animals were fed a HFD for three months to induce IR and obesity, after which silymarin was added to the diet (40mg/100g) for six more weeks. Silymarin had a positive effect on dyslipidemia and in particular enhanced Farnesyl X receptor (FXR) transactivity. These findings suggest that silymarin may exert its positive effects through stimulation of FXR signaling [45]. In another NAFLD model, oxidative stress, estrogen oxidation, and proliferation were induced in HepG2 cell cultures by adding BPA at a concentration of 0.05μM. BPA is an important risk factor in the worsening and progression of NAFLD. Treatment with 68μM of silybin extract counteracted the BPA’s harmful effects by decreasing glucose uptake and lipid peroxidation. Additionally, silybin treatment activated vitamin D3 metabolite synthesis and prevented steroid hormone oxidation. The results suggest that silybin treatment may be able to counteract these effects in patients with NAFLD [46]. In a NASH model in mice, animals were fed a methionine–choline-deficient diet and treated with silibinin simultaneously for six weeks. Results showed that treatment significantly activated CFLAR (CASP8 (Caspase 8) and FADD (Fas associated via death) like apoptosis regulator) and inhibited the phosphorylation of JNK, reduced ALT and AST (Aspartate transaminase) and hepatic Tg, TC, and MDA, by promoting the β-oxidation and efflux of fatty acids in the liver to relieve lipid accumulation [47].
Although experimental animal models indicate that silymarin ameliorates lipid metabolism there is a need to develop clinical controlled studies. In addition to these effects in lipid metabolism, important effects in carbohydrate metabolism have also been described. The next sections will summarize the results of experimental models specifically on carbohydrate metabolism.
4Silymarin in pancreatic damage recovery and carbohydrate metabolismOne of the very first animal models to test silymarin’s effects on carbohydrate metabolism was performed in rats treated with alloxan to induce T2D in the animals. Silymarin was then administered to the animals (200mg /kg body weight) for up to seven days. Results demonstrated that silymarin was able to induce recovery of pancreatic function through the expression of insulin and glucagon proteins, normoglycemia and recover insulin serum levels [48]. Another study analyzed the effect of silymarin in the Pdx1 transcription factor, central to insulin gene expression, in partially pancreatectomized rats. Silymarin treatment induced an increase in both Pdx1 and insulin gene expression and hence, serum insulin levels rose. Results suggest that silymarin may induce the proliferation of insulin-producing cells [49]. In a similar partially pancreatectomized rat model, the effect of silymarin on the Nkx61 transcription factor (key for differentiation, neogenesis, and maintenance of β-pancreatic cells) was tested. Rats were treated with silymarin (200mg/kg/day) for periods of up to 63 days. Silymarin treated groups showed an increase of Nkx61 and insulin genic expression, β-cell neogenesis, and a rise in serum insulin and glucose levels [50].
As silymarin has been shown to have various degrees of bioavailability depending on the type of formulation used, a study tested a nanoparticle design in a diabetes animal model induced through streptozotocin. After 28 days of silymarin treatment in animals, blood glucose levels returned to near normal values and serum insulin was normalized. There was a significant reduction in glycated hemoglobin (HbA1c) levels and liver glycogen was restored [51]. Another diabetes model investigated the cytoprotective activity of silymarin against diabetes-induced cardiomyocyte apoptosis. After animals were treated with silymarin (120mg/kg/day) for ten days, glucose levels returned to normal and exhibited pancreatic β-cell restoration [52]. Finally, in a study with hereditary hypertriglyceridemic rats, the micronized form of silybin significantly decreased glucose and insulin levels [42].
5Silymarin ameliorates insulin resistance (IR)Many liver pathologies are related to IR, and it is well known that IR often precedes the development of T2D and is strongly associated with overweight and obesity [53]. IR in obese states is also related to chronic inflammation. In brief, IR is characterized by the inflammatory environment generated in obesity, and obesity is a major contributor to develop IR [54]. It has been shown that when TNFα concentrations decrease in adipose tissue, other tissues become sensitized to insulin. TNFα also activates intracellular kinases such as c-Jun N-terminal kinase (JNK) and I kappa B kinase complex (IKK) that inhibit signaling of insulin receptors by phosphorylation of serine residues which in turn inhibit insulin substrate 1. Activation of the transcription factors Activator protein 1 (AP-1) and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), expressed in obese states, induce an exacerbated production of pro-inflammatory cytokines. Cytokine secretion is released from adipose tissue into circulation, thereby increasing circulating levels of pro-inflammatory molecules with endocrine effects in the muscle and liver, which in turn increases IR [55].
In non-insulin resistant states, activation of the insulin receptor substrate 1 (IRS-1)-phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) promotes the expression of glucose transporter type 4 (GLUT4) on the cell surface [56]. In all cell types, the insulin receptor begins metabolic signaling to recruit proteins that bind to phosphorylated tyrosine, and activate other downstream effectors in the signaling cascade [6]. In obese states, hyperglycemia, elevated fatty acid levels and pro-inflammatory cytokine production increase Reactive oxygen species (ROS) production and oxidative stress, which in turn activate serine and threonine kinase signaling cascades, sensitive to stress, as well as JNK and inhibitor of nuclear factor kappa-B kinase subunit beta (IKK-β). Altogether, these processes result in the development of IR [57–61]. The following studies, in addition to analyzing the effects of silymarin on lipid and/or carbohydrate metabolism, specifically report the effect of silymarin on IR.
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthase (NOS) and plays an important role in endothelial dysfunction. The liver regulates circulating ADMA levels, but a decrease in ADMA is related to the improvement of IR. In the following study, treatment with silibinin improved endothelial dysfunction in db/db mice by reducing circulating and vascular ADMA levels [62]. Another study tested silibin in a NAFLD rat model. After animals were fed a HFD for six weeks, silybin treatment was administered (silybin 26.25mg/kg/day) to the animals. Results showed improvement in IR in the silybin-treated rats [38]. The same study group later analyzed the effect of silibinin treatment (0.5mg/kg/day) in a similar experimental model on the Homeostasis model assessment-IR index (HOMA-IR), intraperitoneal glucose tolerance test and insulin tolerance test (ITT). The results demonstrated that silibinin improved IR by decreasing HOMA-IR and increasing the ITT slope [39]. In another rat model, rats were fed a HFD to develop NAFLD. Animals were treated with silibinin (silibinin 100mg/kg/day) for 12 weeks and treatment reduced IR by down-regulating resistin and restoring the IRS-1/P13K/Akt pathway [40].
By contrast, in another animal model, silymarin was found to both induce and enhance IR. In this study, rats were either fed normal or fructose-rich chow to develop IR. Animals were then administered silymarin orally (200mg/kg/d) and changes in phosphatase and tensin homolog (PTEN, a protein that regulates insulin signaling, lipid and glucose metabolism) expression in skeletal muscle and liver were compared. After IR was induced in rats, treatment with silymarin increased PTEN expression seen in the skeletal muscle and liver of animals. PTEN expression and insulin-related signals were also tested in Rat Myoblast Cell Line (L6) cells. After silymarin treatment, phosphorylation of Akt in L6 myotube cells decreased, and a high-glucose condition was seen. Altogether, these results suggest that silymarin was able to disrupt insulin signaling, through increased PTEN expression, and induce IR [63]. In a gerbil (Psammomys obesus) diabetes model, silibinin (100mg/kg per day) was administered to animals after seven weeks of a high-calorie diet for an additional seven weeks, and results showed that treatment with silibinin reduced IR [41]. Another in vitro study tested the effect of silibinin on palmitate-induced IR in C2C12 (mouse myoblast cell line) myotubes. Silibinin was found to improve IR by downregulating phosphorylation of both JNK and IKKβ, thus preventing the inhibition of the IRS-1/PI3K/Akt pathway [64].
In an obese mice study, silymarin treatment resulted in body weight loss and a reduction in epididymal fat mass. The lipid profile in the animals was also ameliorated and inflammation was decreased, evidenced by lower pro-inflammatory cytokine levels, reduced histological damage in the liver, and improvement in IR [65]. In another animal study with high-fat diet (HFD)-fed mice, silymarin treatment reduced body weight together with glucose intolerance and IR, by decreasing oxidative stress indicators in the liver, NF-kB expression, and TNFα levels. Another HFD mouse model was used to test silymarin’s effect on NF-kB and FXR transactivities after treatment with silymarin (40mg/100g) in obese and insulin-resistant mice. Treatment in animals with silymarin ameliorated IR [45]. Finally, S. marianum extract (SME) was administered to rats after they were fed a HFD for 11 weeks to induce obesity. Animals were then treated with SME for seven and 11 weeks after which results showed improvement in IR [66].
As results have shown, exploring the mechanisms through which silymarin ameliorates liver function by reducing IR may be useful in treating liver disease [25]. In the following section, we summarize clinical trials that, in addition to analyzing metabolic parameters before and after silymarin treatment, specifically measured fasting or daily glucose and insulin, and/or HOMA-IR, and thereby the impact of silymarin on IR.
6Silymarin in clinical trialsIn recent clinical studies, treatment with silymarin in patients with T2D, NAFLD, cirrhosis, and HCV, has yielded favorable results concerning IR.
One of the first clinical trials carried out in humans with silymarin treatment related to IR tested the effect of silymarin in 60 diabetic patients with cirrhosis. Patients were given 600mg of silymarin daily (Legalon®) or placebo for six months, in addition to standard therapy. Mean levels of fasting blood glucose (FBG), daily blood glucose (DBG), daily glycosuria, HbA1c, daily insulin need, and fasting blood insulin (FBI), among other parameters, were decreased in patients treated with silymarin. The results suggest that used in adjunct with exogenous insulin, silymarin can increase the sensitivity of insulin receptors [67]. A few years later, the same research group conducted a 12-month open controlled study in 60 diabetic patients with cirrhosis, treated with silymarin or placebo as in the previous study, and standard therapy. Results showed a significant decrease in FBI levels, mean DBG levels, daily glycosuria, and HbA1c after four months of treatment. At the end of the treatment period, a significant decrease was found in FBI levels, and mean exogenous insulin requirements, in addition to basal and glucagon-stimulated C-peptide levels and MDA levels used to measure fibrosis in the liver. Treatment with silymarin was able to reduce lipid peroxidation, IR, endogenous insulin overproduction, and the need for exogenous insulin [68]. Similar to the group of patients treated in these trials, a three-center, double-blind, randomized placebo study was performed in 42 diabetic patients with associated chronic liver disease. A silybin-beta-cyclodextrin (named IBI/S) formulation was used (135mg/day silybin per os) for six months. Treated patients showed a significant reduction in FBI and Tg levels, and the same trend was observed in DBG, HbA1c, and HOMA-IR, although differences were not significant. Insulin secretion was unaffected, similar to TC and HDL levels [69].
In a separate study, a different silybin complex with vitamin E and phospholipids (188mg silybin daily, RealSIL®) or placebo was given to 85 patients with NAFLD with or without HCV (the latter non-responders to treatment with interferon and ribavirin) for six months. Carbohydrate metabolism parameters and liver fibrosis were analyzed and treatment was found to improve IR. Results showed a significant decrease in HOMA-IR and insulinemia, as well as ALT, Gamma-glutamyl transferase (GGT) levels and liver fibrosis in both groups [70–72]. In another study, silymarin was administered to 96 NAFLD patients and 32 healthy controls with either silymarin (600mg daily) or GKY (traditional Chinese herbal mixture). Although there was no change in IR, there was a significant decrease in Tg, TC, and ALT levels in the NAFLD group treated with silymarin [73]. A randomized double-blind clinical trial in 51 diabetic patients received silymarin (600mg daily) or placebo for four months, plus conventional therapy. Results showed a significant decrease in HbA1c, FBG, TC, LDL, HDL, Tg, ALT, and AST levels, but no significant decrease in insulin levels after treatment [74]. Another study used the same silymarin complex and dosage as Trappoliere et al. for three months in 30 patients with chronic HCV, and ten patients with hepatic steatosis but without HCV. After treatment, patients with HCV showed a significant decrease in ALT, AST, C-reactive protein (CRP), Interleukin-2 (IL-2) and Interleukin-6 (IL-6) but no significant decrease in HOMA-IR, insulinemia or serum glucose. However, patients without HCV showed a significant decrease in ALT, AST, GGT, and alkaline phosphatase, Tg, FBG, insulinemia, HOMA-IR, CRP, Interferon-gamma (IFN-ɣ), TNFα, and IL-6 levels, after treatment. Results suggest that silymarin was able to improve liver and carbohydrate metabolism in patients with NAFLD more effectively without HCV infection, than those with HCV infection [75]. Another study in 138 patients with NAFLD and 36 patients with HCV used the same silybin complex as in the previous study, or placebo for 12 months together with recommended lifestyle modifications and individually designed diets in a multicenter, phase III, double-blind clinical trial. After treatment, results showed significant improvements in liver enzyme plasma levels, HOMA-IR, and liver histology [76].
A different silymarin formulation combined with another nutraceutical was given to 22 diabetic patients with suboptimal glycemic control despite the use of standard therapy. The formulation consists of silymarin and berberine, the latter used to treat hypercholesterolemia and diabetes. Patients were given silymarin 210mg (Berberol®) for 90 days. After treatment, levels of HbA1c, basal insulin, HOMA-IR, TC, LDL, and Tg levels were significantly reduced. Interestingly, no significant changes were seen in HDL, FBG, body mass index (BMI), weight, or waist circumference (WC) [77]. Berberol® was again used in another clinical trial with 105 overweight, euglycemic, dyslipidemic patients at low cardiovascular risk for three months in a double-blind, placebo-controlled design. Patients first underwent a six-month run-in period with diet and physical activity recommendations. After treatment, patients underwent a two-month wash-out period followed by treatment for three additional months. Treatment improved IR, with a significant decrease in FPI and HOMA-IR. Moreover, TC, LDL, and Tg levels decreased and HDL levels increased [78]. In another trial by the same research group, Berberol® (silymarin 210mg) was used once more to treat 137 euglycemic, dyslipidemic patients who did not tolerate high doses of statins in a double-blind, randomized, placebo-controlled clinical trial. Patients who were able to tolerate half the dose of statins began treatment with Berberol® (silymarin 210mg) for six months and statins with an adequate diet and physical activity. Treatment reduced IR, FBG, FPI, and HOMA-IR [79,80].
The same year, another three studies published results of silymarin’s effect on IR. The first trial assessed the effect of the silibin-phytosome complex formulation used previously by other research groups. RealSil® (silybin 188mg, daily) was given to 30 overweight patients with NAFLD for six months in addition to a personalized Mediterranean hypocaloric diet (1400–1600kcal/day). Another two groups of patients were either prescribed a diet alone or neither. Treatment plus diet improved IR and significantly reduced FBG, FPI, HOMA-IR, BMI, weight, WC, TC, and fatty liver index levels [81]. Yet a different silymarin formulation (Eurosil 85®) was used in another study with NAFLD patients who were either prescribed silymarin (540.3mg daily) vitamin E for three months, in addition to a hypocaloric diet and physical activity or only a hypocaloric diet. BMI, weight, WC, GGT levels, fatty liver index, and NAFLD fibrosis score decreased significantly, but not HOMA-IR, in the treated group [82]. A previously used silymarin formulation, but with a different dosage, was given to 40 diabetic patients with suboptimal glycemic control and IR in a prospective, randomized, placebo-controlled, single-blinded, pilot study. Patients were prescribed silymarin 420mg silymarin as Legalon® for 90 days or a placebo together with standard treatment for T2D. After treatment, IR improved, with a significant reduction in HOMA-IR, FBG, serum insulin, Hb1Ac, TC, Tg, LDL, and VLDL levels and a significant increase in HDL [83].
A previously used formulation of silymarin with berberine was tested, however, combined with fermented rice extract, in another study. Silymarin (210mg daily, Berberol® K), or placebo was given to 143 overweight normotensive patients with hypercholesterolemia in addition to an adequate diet plus physical activity in a three month randomized, double-blind trial. Results demonstrated a significant decrease in TC, LDL, Tg, high sensitive C-reactive protein, TNFα, and IL-6 levels in treated patients with no significant decrease in FBG or HOMA-IR [84]. Another trial that used silymarin in combination with berberine only, was carried out in 136 obese individuals with T2D, IR, and other altered glucose and lipid parameters. Patients were given 210mg silymarin or placebo and followed a low-calorie diet for 52 weeks. Treatment reduced IR, HOMA-IR, HbA1c, TC, LDL, Tg and uric acid levels, WC, trunk and visceral fat, and systolic and diastolic blood pressure significantly at six, and 12 months. Moreover, HDL levels were significantly increased after 12 months [85]. In another study, 420mg of silymarin (Livergol®) or placebo was given to 40 overweight or obese diabetic patients together with standard treatment for 45 days, in a paralleled, randomized, triple-blinded, placebo-controlled clinical trial. Treatment reduced IR, insulin, HOMA-IR, and Quantitative insulin sensitivity check index (QUICKI), TC, Tg, LDL levels, and increased HDL levels significantly [86]. A recent study analyzed the influence of the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene variant in patients with NAFLD on the response to treatment with 420mg of Eurosil 85® (approximately 60% silymarin and 30 IU of vitamin E) for six months in 54 patients with NAFLD. None of the patients showed improvement in IR. However, patients who did not carry the genetic variant showed significant reductions in AST, ALT, and GGT. Interestingly, patients with the variant showed a significant increase in HOMA-IR and glucose. The results of this study suggest that individuals with this variant, found in 49% of the Hispanic population, responded poorly to treatment [87]. Whether silymarin’s effects depend also on nutritional habits related to genetic background or not, is also important to define [88].
Later, a recent study with 90 NAFLD patients and 30 healthy controls (diagnosed with reflux disease), tested a new formulation similar to RealSIL®, with vitamin D, RealSIL 100D® (303mg of silymarin, vitamin E (used to stabilize the formula) and vitamin D in a silybin–phospholipid complex) twice daily for six months with follow-up at 12 months. The results showed significant improvement (normalization of the specific variable under the upper limit of the normality range level) in IR, by reducing insulin and HOMA-IR, as well as steatosis in treated patients [89]. Later, the same formulation (RealSIL 100D®) and dosage as in the previous study, were tested in a separate clinical trial in 32 male NAFLD patients exposed to BPA. Treatment improved IR by decreasing insulinemia and HOMA-IR levels, as well as several other metabolic parameters, including ALT and AST, C-reactive protein, and TNFα. Interestingly, treatment with this formulation of silymarin also reduced levels of thiobarbituric acid reactive substances, markers of lipid peroxidation, and oxidative stress that are directly proportional to BPA exposure levels. Furthermore, treatment increased concentrations of conjugated BPA and reduced concentrations of its free form, in urine. The results suggest that treatment may contribute to accelerated BPA detoxification, improve cellular antioxidant power in NAFLD patients, and importantly, reduce IR [90].
7DiscussionSilymarin preparations have been used for centuries to treat liver disease. Silymarin, extracted from the fruit of this plant, contains flavonolignans and flavonoids demonstrated to exert beneficial effects for the treatment of liver disease due to their anti-inflammatory and metabolic properties. Recently, a series of studies demonstrated that additionally, silymarin displays pancreatic protective properties and importantly improves IR in lipid and carbohydrate metabolic processes [48–50].
Overall, the studies designed to analyze silymarin’s effect on IR (although heterogeneous in pathology, patient characteristics, and formulation of silymarin, dosage, and length of treatment) demonstrated that the use of silymarin improves metabolic and IR parameters. Although generally silymarin is found as a standardized extract, the discrepancies in the results in some studies that failed to show improvement of metabolic status or IR, may be due in part to the different types of formulations given to patients and require further exploration [91]. Furthermore, the use of pure flavonolignans and flavonoids, combinations derived from the Milk Thistle plant thereof, or silymarin formulations with other nutraceuticals, should be uniform in future studies.
Some of the studies summarized in this review used silymarin in a Siliphos® complex with proven increased bioavailability, in conjunction with another supplement such as Vitamin E or Berberine. Other studies did not specify the amount of silybin used, or if a standardized form of the extract was employed. Still, other studies recommended patient lifestyle modifications, individually designed diets, and dietary recommendations, which may be confusing factors to pinpoint silymarin’s effects. In some cases, studies showed positive significant changes with placebo. However, taking into account the previously mentioned factors, patients with T2D were able to decrease glucose and insulin levels, and thus HOMA-IR, and the need for exogenous insulin. In patients with NAFLD with or without HCV and/or cirrhosis, liver metabolism levels were ameliorated.
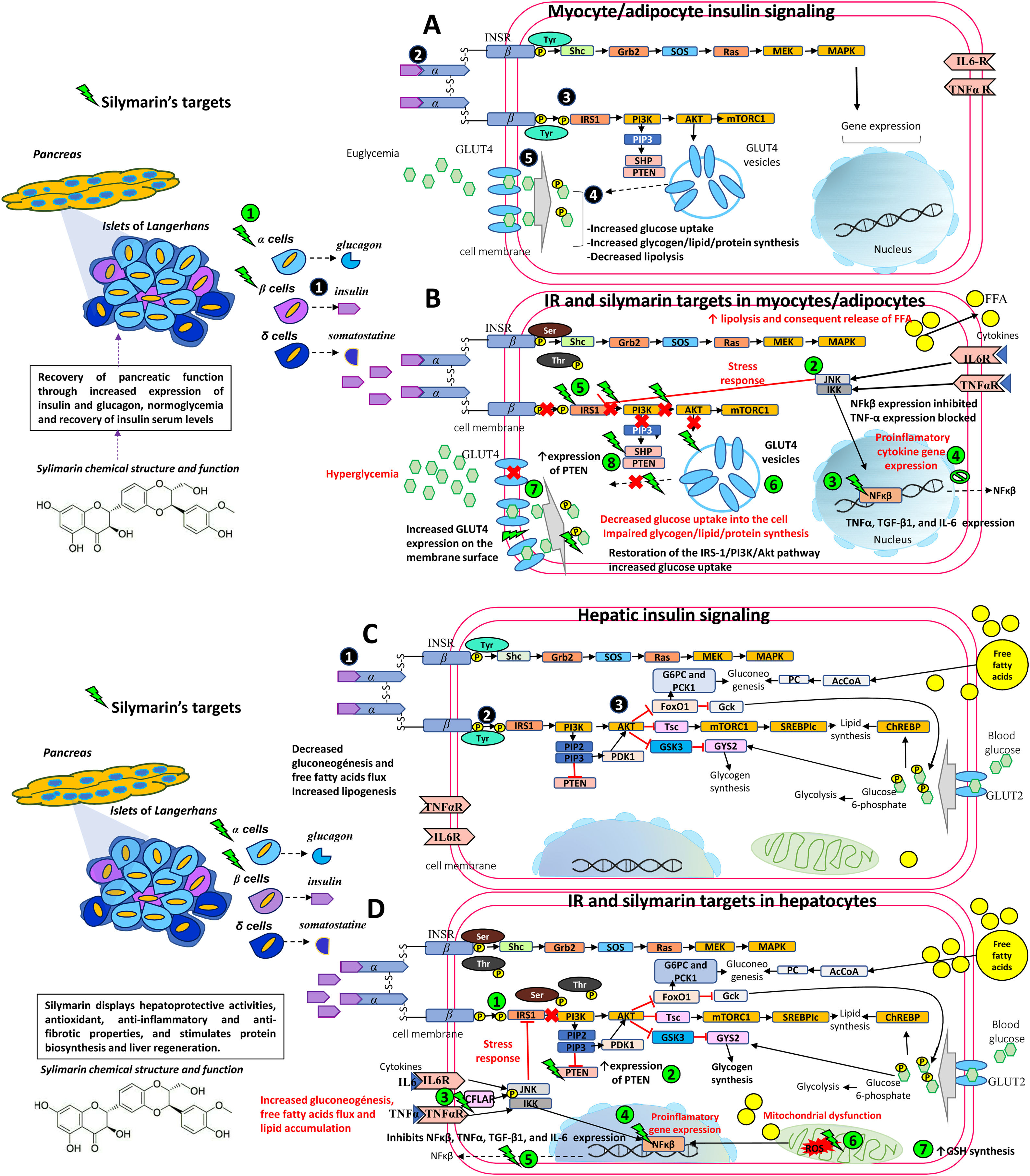
Additionally, some studies did not consider the patient’s BMI. As mentioned earlier, inflammation secondary to obesity interferes with proper insulin signaling. Therefore, it is important to take into account BMI, or at the very least visceral adiposity, lacking in many of the studies. Silymarin appears to act directly on NF-kB [5], thereby stopping the production of pro-inflammatory cytokines where Thr and Ser residues are phosphorylated and interfere with proper insulin signaling [60]. Instead, due to silymarin’s anti-inflammatory effects, lowering inflammation in obese states [65,92] allows Tyr residues to be phosphorylated and improves insulin signaling, ameliorating IR (see Fig. 1).
Therapeutic targets of silymarin in IR.
A) Insulin is released by pancreatic beta cells
in response to elevated blood glucose levels to maintain homeostatic blood glucose. Insulin binds to its receptor INSR and undergoes Tyr phosphorylation which activates multiple signaling pathways, mainly PI3K and MAPK pathways. However, insulin regulates carbohydrate and lipid metabolism principally through activation of the PI3K pathway, initiated by interaction between the active, self-phosphorylated INSR with IRS1 . Insulin promotes the translocation of GLUT4 from intracellular compartments to the plasma membrane by a pathway dependent on PI3K and Akt activation promoting the uptake and storage of glucose in muscle and adipose tissues [5,6,33,50,57].B) In an insulin-resistant state, target cells (myocytes and adipocytes) have a decreased response to insulin action and hyperinsulinemia ensues. Poor insulin signaling is attributable to defects in insulin binding with its receptor and/or post-insulin binding modifications that alter the functionality of downstream proteins, such as phosphorylation at Ser/Thr residues of INSR and IRS1. This in turn decreases PI3K and Akt activity, and GLUT4 transporter expression, function, and translocation, resulting in decreased glucose uptake of the skeletal muscle and impaired glycogen, lipid, and protein synthesis. In adipocytes, increased lipolysis increases FFA release into the liver. Silymarin aids in pancreatic function recovery through increased insulin and glucagon expression
, normoglycemia, and recovery of insulin serum levels. Silymarin also inhibits JNK and IKK phosphorylation and NfkB expression , resulting in a decreased production of pro-inflammatory cytokines (TNFα, IL6) . As inflammation is decreased, IRS-1/PI3K/Akt pathway signaling is favored , increasing GLUT4 expression , glucose uptake , and PTEN expression in muscleskeletal cells [5,6,33,38,50,57,61].C) In an insulin-sensitive state, binding of insulin to INSR
and phosphorylation or tyrosine residues leads to activation of the PI3K-Akt pathway , contributes to glycogen synthesis, decreased gluconeogenesis, protection against apoptosis, stimulation of mRNA translation, lipid and protein synthesis through the GSK3, mTOR and FoxO pathways [5,6,33,50,57].D) In an insulin-resistant state, hyperinsulinemia leads to increased gluconeogenesis, uptake of FFAs, accumulation of lipids inside the cell, and decreased glycogen synthesis. This generates ROS in the mitochondrial chain which act on the fatty acids in the cell membrane causing lipid peroxidation. ROS induces pro-inflammatory cytokine synthesis including TNF-α, TGF-β1, and IL-6. Silymarin restores IRS-1/PI3K/Akt pathway signaling
, increases PTEN expression , activates CFLAR expression and inhibits JNK and IKK phosphorylation , NfkB expression , and proinflammatory cytokine expression (TNFα, IL6) , eliminates free radicals resulting from lipid peroxidation and increases GSH cell content [5,6,33,38,44,50,57,61].Akt, Protein kinase B; CASP8, Caspase 8; CFLAR, CASP8 and FADD like apoptosis regulator; FADD, Fas associated via death; FFA, free fatty acids; FoxO, Forkhead box O; GLUT4, Glucose transporter 4; GSH, glutathione; GSK3, Glycogen synthase kinase 3; IKK, I kappa B kinase complex; IL-6, Interleukin-6; INSR, Insulin receptor; IRS1, Insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; MAPK, Mitogen-activated kinases; mTOR, Mammalian target of rapamycin; NfkB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; Ser, serine; TGF-β1, transforming growth factor beta-1; Thr, threonine; TNFα, Tumor necrosis factor α; Tyr, tyrosine.
Another possible reason for discrepancies in the studies’ results, might be due to the lack of identification of individual genetic variants in the patient populations. The results of the last clinical trial to be mentioned in this review, specifically reported a poor outcome in individuals with the PNPLA3 variant in response to treatment. To our knowledge, this was the only trial to test for variants in the population regarding silymarin’s effect on IR. This suggests that there are variants within populations that do not respond positively to silymarin regarding IR or carbohydrate metabolism. The genetic characteristics of a population must, therefore, be considered and are fundamental for the prescription of adequate treatment as has been proposed in personalized medicine [93]. Future clinical trials should test the variants within the population studied to identify possible non-responders to treatment.
Finally, as shown recently, humans and living organisms are unavoidably and unintentionally exposed to BPA [94]. BPA exposure alters metabolic parameters that may increase IR and the development of metabolic diseases such as MAFLD [37,46,90]. As such, future clinical studies in metabolic diseases should also test BPA levels, as these have been shown to play an important role in IR.
8ConclusionsAlthough the clinical trials until now and reported in this review are heterogeneous in methodology, the overall results of these studies support the fact that silymarin ameliorates IR in liver pathologies, but also in metabolic disease. The data supporting silymarin’s effect on IR warrants further investigation in clinical trials with controlled pharmaceutical preparations in patients that suffer from metabolic diseases, such as obesity and diabetes, particularly because this population is increasing and an epidemic worldwide. Additionally, the management of the disease and the high cost of treatment represents a difficulty for patients and public health. Silymarin appears to be a safe alternative to be used alone or as an adjunct with standard treatment for patients with IR. AbbreviationsADMAAsymmetric dimethylarginineAktProtein kinase BALTAlanine aminotransferaseAP-1Activator protein 1ASTAspartate transaminaseBMIBody mass indexCASP8Caspase 8CFLARCASP8 and FADD like apoptosis regulatorCRPC-reactive proteinBPABisphenol ADBGDaily blood glucoseFADDFas associated via deathFBGFasting blood glucoseFXREnhanced Farnesyl X receptorGLUT4Glucose transporter type 4HbA1cGlycated hemoglobinHCDHigh cholesterol dietHCVHepatitis C virus infectionHDLHigh-density lipoprotein cholesterolHFDHigh-fat dietHOMA-IRHomeostasis model assessment-IRIFN-ɣInterferon-gammaIKKI kappa B kinase complexIL-2Interleukin-2IL-6Interleukin-6IRInsulin resistanceIRS1Insulin receptor substrate 1ITTInsulin tolerance testJNKc-Jun N-terminal kinaseLDLLow-density lipoprotein cholesterolMAFLDMetabolic-dysfunction-associated fatty liver diseaseMDAMalondialdehydeMetSMetabolic syndromeNAFLDNon-alcoholic fatty liver diseaseNASHNonalcoholic steatohepatitisNF-KbNuclear factor kappa-light-chain-enhancer of activated B cellsPI3KPhosphatidylinositol 3-kinasePTENPhosphatase and tensin homologQUICKIQuantitative insulin sensitivity check indexROSReactive oxygen speciesSMESilybum marianum extractT2DType 2 diabetesTCTotal cholesterolTgTriglycerideTNFαTumor necrosis factor αVLDLVery-low-density lipoprotein cholesterolWCWaist circumference
AbbreviationsADMA Asymmetric dimethylarginine Protein kinase B Alanine aminotransferase Activator protein 1 Aspartate transaminase Body mass index Caspase 8 CASP8 and FADD like apoptosis regulator C-reactive protein Bisphenol A Daily blood glucose Fas associated via death Fasting blood glucose Enhanced Farnesyl X receptor Glucose transporter type 4 Glycated hemoglobin High cholesterol diet Hepatitis C virus infection High-density lipoprotein cholesterol High-fat diet Homeostasis model assessment-IR Interferon-gamma I kappa B kinase complex Interleukin-2 Interleukin-6 Insulin resistance Insulin receptor substrate 1 Insulin tolerance test c-Jun N-terminal kinase Low-density lipoprotein cholesterol Metabolic-dysfunction-associated fatty liver disease Malondialdehyde Metabolic syndrome Non-alcoholic fatty liver disease Nonalcoholic steatohepatitis Nuclear factor kappa-light-chain-enhancer of activated B cells Phosphatidylinositol 3-kinase Phosphatase and tensin homolog Quantitative insulin sensitivity check index Reactive oxygen species Silybum marianum extract Type 2 diabetes Total cholesterol Triglyceride Tumor necrosis factor α Very-low-density lipoprotein cholesterol Waist circumference
The authors have no conflicts of interest to declare.
Karla María Mac Donald Ramos is a doctoral student from the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and has received CONACyT fellowship 607933 (CVU 780099).

















![Therapeutic targets of silymarin in IR. A) Insulin is released by pancreatic beta cells in response to elevated blood glucose levels to maintain homeostatic blood glucose. Insulin binds to its receptor INSR and undergoes Tyr phosphorylation which activates multiple signaling pathways, mainly PI3K and MAPK pathways. However, insulin regulates carbohydrate and lipid metabolism principally through activation of the PI3K pathway, initiated by interaction between the active, self-phosphorylated INSR with IRS1 . Insulin promotes the translocation of GLUT4 from intracellular compartments to the plasma membrane by a pathway dependent on PI3K and Akt activation promoting the uptake and storage of glucose in muscle and adipose tissues [5,6,33,50,57]. B) In an insulin-resistant state, target cells (myocytes and adipocytes) have a decreased response to insulin action and hyperinsulinemia ensues. Poor insulin signaling is attributable to defects in insulin binding with its receptor and/or post-insulin binding modifications that alter the functionality of downstream proteins, such as phosphorylation at Ser/Thr residues of INSR and IRS1. This in turn decreases PI3K and Akt activity, and GLUT4 transporter expression, function, and translocation, resulting in decreased glucose uptake of the skeletal muscle and impaired glycogen, lipid, and protein synthesis. In adipocytes, increased lipolysis increases FFA release into the liver. Silymarin aids in pancreatic function recovery through increased insulin and glucagon expression , normoglycemia, and recovery of insulin serum levels. Silymarin also inhibits JNK and IKK phosphorylation and NfkB expression , resulting in a decreased production of pro-inflammatory cytokines (TNFα, IL6) . As inflammation is decreased, IRS-1/PI3K/Akt pathway signaling is favored , increasing GLUT4 expression , glucose uptake , and PTEN expression in muscleskeletal cells [5,6,33,38,50,57,61]. C) In an insulin-sensitive state, binding of insulin to INSR and phosphorylation or tyrosine residues leads to activation of the PI3K-Akt pathway , contributes to glycogen synthesis, decreased gluconeogenesis, protection against apoptosis, stimulation of mRNA translation, lipid and protein synthesis through the GSK3, mTOR and FoxO pathways [5,6,33,50,57]. D) In an insulin-resistant state, hyperinsulinemia leads to increased gluconeogenesis, uptake of FFAs, accumulation of lipids inside the cell, and decreased glycogen synthesis. This generates ROS in the mitochondrial chain which act on the fatty acids in the cell membrane causing lipid peroxidation. ROS induces pro-inflammatory cytokine synthesis including TNF-α, TGF-β1, and IL-6. Silymarin restores IRS-1/PI3K/Akt pathway signaling , increases PTEN expression , activates CFLAR expression and inhibits JNK and IKK phosphorylation , NfkB expression , and proinflammatory cytokine expression (TNFα, IL6) , eliminates free radicals resulting from lipid peroxidation and increases GSH cell content [5,6,33,38,44,50,57,61]. Akt, Protein kinase B; CASP8, Caspase 8; CFLAR, CASP8 and FADD like apoptosis regulator; FADD, Fas associated via death; FFA, free fatty acids; FoxO, Forkhead box O; GLUT4, Glucose transporter 4; GSH, glutathione; GSK3, Glycogen synthase kinase 3; IKK, I kappa B kinase complex; IL-6, Interleukin-6; INSR, Insulin receptor; IRS1, Insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; MAPK, Mitogen-activated kinases; mTOR, Mammalian target of rapamycin; NfkB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; Ser, serine; TGF-β1, transforming growth factor beta-1; Thr, threonine; TNFα, Tumor necrosis factor α; Tyr, tyrosine. Therapeutic targets of silymarin in IR. A) Insulin is released by pancreatic beta cells in response to elevated blood glucose levels to maintain homeostatic blood glucose. Insulin binds to its receptor INSR and undergoes Tyr phosphorylation which activates multiple signaling pathways, mainly PI3K and MAPK pathways. However, insulin regulates carbohydrate and lipid metabolism principally through activation of the PI3K pathway, initiated by interaction between the active, self-phosphorylated INSR with IRS1 . Insulin promotes the translocation of GLUT4 from intracellular compartments to the plasma membrane by a pathway dependent on PI3K and Akt activation promoting the uptake and storage of glucose in muscle and adipose tissues [5,6,33,50,57]. B) In an insulin-resistant state, target cells (myocytes and adipocytes) have a decreased response to insulin action and hyperinsulinemia ensues. Poor insulin signaling is attributable to defects in insulin binding with its receptor and/or post-insulin binding modifications that alter the functionality of downstream proteins, such as phosphorylation at Ser/Thr residues of INSR and IRS1. This in turn decreases PI3K and Akt activity, and GLUT4 transporter expression, function, and translocation, resulting in decreased glucose uptake of the skeletal muscle and impaired glycogen, lipid, and protein synthesis. In adipocytes, increased lipolysis increases FFA release into the liver. Silymarin aids in pancreatic function recovery through increased insulin and glucagon expression , normoglycemia, and recovery of insulin serum levels. Silymarin also inhibits JNK and IKK phosphorylation and NfkB expression , resulting in a decreased production of pro-inflammatory cytokines (TNFα, IL6) . As inflammation is decreased, IRS-1/PI3K/Akt pathway signaling is favored , increasing GLUT4 expression , glucose uptake , and PTEN expression in muscleskeletal cells [5,6,33,38,50,57,61]. C) In an insulin-sensitive state, binding of insulin to INSR and phosphorylation or tyrosine residues leads to activation of the PI3K-Akt pathway , contributes to glycogen synthesis, decreased gluconeogenesis, protection against apoptosis, stimulation of mRNA translation, lipid and protein synthesis through the GSK3, mTOR and FoxO pathways [5,6,33,50,57]. D) In an insulin-resistant state, hyperinsulinemia leads to increased gluconeogenesis, uptake of FFAs, accumulation of lipids inside the cell, and decreased glycogen synthesis. This generates ROS in the mitochondrial chain which act on the fatty acids in the cell membrane causing lipid peroxidation. ROS induces pro-inflammatory cytokine synthesis including TNF-α, TGF-β1, and IL-6. Silymarin restores IRS-1/PI3K/Akt pathway signaling , increases PTEN expression , activates CFLAR expression and inhibits JNK and IKK phosphorylation , NfkB expression , and proinflammatory cytokine expression (TNFα, IL6) , eliminates free radicals resulting from lipid peroxidation and increases GSH cell content [5,6,33,38,44,50,57,61]. Akt, Protein kinase B; CASP8, Caspase 8; CFLAR, CASP8 and FADD like apoptosis regulator; FADD, Fas associated via death; FFA, free fatty acids; FoxO, Forkhead box O; GLUT4, Glucose transporter 4; GSH, glutathione; GSK3, Glycogen synthase kinase 3; IKK, I kappa B kinase complex; IL-6, Interleukin-6; INSR, Insulin receptor; IRS1, Insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; MAPK, Mitogen-activated kinases; mTOR, Mammalian target of rapamycin; NfkB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; Ser, serine; TGF-β1, transforming growth factor beta-1; Thr, threonine; TNFα, Tumor necrosis factor α; Tyr, tyrosine.](https://static.elsevier.es/multimedia/16652681/000000230000000C/v3_202212060650/S1665268120301708/v3_202212060650/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


















