Posthepatectomy liver failure (PHLF) is a serious complication after hepatectomy, and its effective methods for preoperative prediction are lacking. Here, we aim to identify predictive factors and build a nomogram to evaluate patients’ risk of developing PHLF.
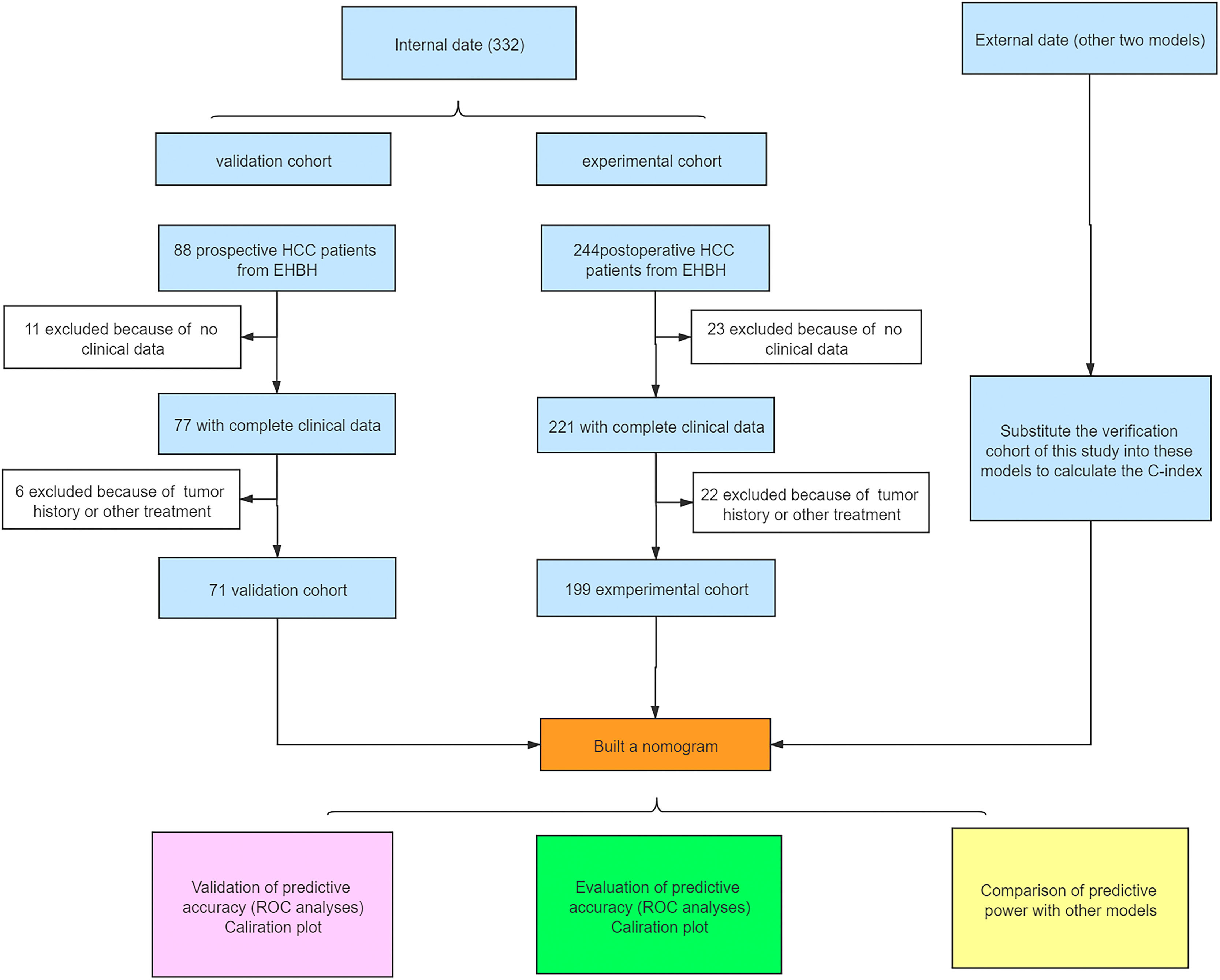
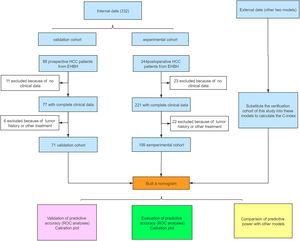
Patients and methodsA retrospective review of a training cohort, including 199 patients who underwent hepatectomy at the Shanghai Eastern Hepatobiliary Surgery Hospital, was conducted. Independent risk variables for PHLF were identified using multivariate analysis of perioperative variables, and a nomogram was used to build a predictive model. To test the predictive power, a prospective study in which a validation cohort of 71 patients was evaluated using the nomogram. The prognostic value of this nomogram was evaluated by the C-index.
ResultsIndependent risk variables for PHLF were identified from perioperative variables. In multivariate analysis of the training cohort, tumor number, Pringle maneuver, blood loss, preoperative platelet count, postoperative ascites and use of anticoagulant medications were determined to be key risk factors for the development of PHLF, and they were selected for inclusion in our nomogram. The nomogram showed a 0.911 C-index for the training cohort. In the validation cohort, the nomogram also showed good prognostic value for predicting PHLF. The validation cohort was used with similarly successful results to evaluate risk in two previously published study models with calculated C-indexes of 0.718 and 0.711.
ConclusionOur study establishes for the first time a novel nomogram that can be used to identify patients at risk of developing PHLF.
Hepatectomy has been widely used for the treatment of malignant and benign tumors, calculus of intrahepatic duct, echinococcosis, and abscesses. The safety of hepatectomy has increased significantly over the last few years, but postoperative morbidity remains relatively high, ranging from 4.09% to 47.7%, with mortality ranging from 0.24% to 9.7% [1]. The mortality of hepatocellular carcinoma (HCC), the third deadliest cancer in the world, continues to increase [2].
Post-hepatectomy liver failure (PHLF) is a serious postoperative complication that can lead to death. The incidence of PHLF worldwide is 12%, according to the International Study Group of Liver Surgery (ISGLS) [3]. PHLF currently lacks a widely accepted clinical definition. The ISGLS classifies PHLF into three grades: Grade A, Grade B and Grade C. PHLF is a postoperatively acquired deterioration of the liver's ability to maintain its synthesis, excretion and detoxification functions and is characterized by an increased international normalized ratio (INR) and concomitant hyperbilirubinemia (defined in our study population as INR > 1.14 and serum bilirubin > 28 µmol/L based on the normal ranges of our hospital) on the fifth day after the operation. Compared with previously published definitions, we feel that this definition is simpler and more reproducible and likely to be accepted by surgeons [4].
The treatment of PHLF includes replenishment with fibrinogen or the prothrombin complex and albumin, intravenous nutrition, and infusion of fresh blood. The early prevention of PHLF would be more desirable than treatment following occurrence. Some factors that correlate with the incidence of PHLF have been reported, including cirrhosis, remaining liver remnant volume, massive intraoperative hemorrhage, a model for end-stage liver disease score, hepatic venous pressure gradient, indocyanine green clearance, and liver stiffness [5]. Anticoagulant medications are widely used in various medical and surgical diseases, disorders and conditions related to thrombosis and thromboembolism. Historically, hepatic surgeons have avoided prescribing venous thromboembolism (VTE) chemoprophylaxis due to the perception of the risk of postoperative bleeding and the protective anticoagulant effects of hepatectomy [6]. Recently, studies suggested that postoperative anticoagulants can effectively reduce the incidence of portal vein thrombosis (PVT) in patients undergoing splenectomy due to liver cirrhosis [7]. However, whether the use of postoperative anticoagulants contributes to the reduction of PHLF remains unknown.
PHLF usually occurs within 5 days after surgery [8]. However, none of the current models have discussed the predictive significance of postoperative factors influencing PHLF. We speculate that factors after surgery may also play important roles in the prediction of PHLF. Thus, we developed a nomogram to predict the occurrence of PHLF based on preoperative, intraoperative and postoperative factors and validated the reliability of the model using external clinical data.
2Methods2.1Patients and methodsThis study was divided into two stages: (a) a retrospective cohort analysis that included patients who underwent elective hepatectomy from June 2018 to May 2020 at the Shanghai Eastern Hepatobiliary Surgery Hospital designated as the training cohort, and (b) a prospective analysis that collected data from a cohort of patients who underwent hepatectomy at our hospital from June 2020 to December 2020. The prospective cohort was used for the validation of our nomogram. The data of patients from the two cohorts were acquired from the digital medical records system of our hospital. The patient data was de-identified to protect patient information.
Patients were required to meet the following inclusion criteria: (a) HCC patients who received selective hepatectomy (all diagnoses were confirmed by pathological examination of the surgical specimens), (b) age 25 to 80 years, and (c) Child-Pugh Class A or B. Exclusion criteria were as follows: (a) patients with a history of other malignant tumors prior to hepatectomy; (b) patients who received preoperative antineoplastic treatment; or (c) patients with incomplete medical records of postoperative outcome.
2.2Preoperative examinationPreoperative examination of all patients included liver function tests, routine blood examination, kidney function tests, clotting function tests, hepatitis virus-related tests, computed tomography (CT) scanning and/or magnetic resonance imaging (MRI), and other investigations as necessary. The baseline hepatic and systemic hemodynamic status (hepatic venous pressures, hepatic blood flow, azygos blood flow, intrinsic hepatic clearance of indocyanine green, cardiopulmonary pressures and cardiac output) of the patients were also recorded [9].
2.3Intraoperative careThe training cohort included 63 (31.66%) patients who received major hepatectomy involving the removal of three or more hepatic segments. Partial hepatic resection is a feasible and relatively safe procedure and is now even used in living donor liver transplantation [10, 11]. Routine cholecystectomy was performed as necessary. The first hepatic hilus was blocked by the Pringle method, and the intermittent hilus was blocked optionally. The time of each portal obstruction was generally no more than 15 minutes. All cases were discussed by the multidisciplinary oncology committee, and the decision to recommend surgery was made on a case-by-case basis. All surgeries were operated upon by experienced surgeons at the Shanghai Eastern Hepatobiliary Surgery Hospital.
Intraoperative variables were recorded, including the size and numbers of the tumors and the volume of blood loss. We applied the X-tile program to determine the best cutoff point with regard to the volume of blood loss for inclusion in our nomogram used to determine the risk of PHLF in the 199 HCC cases in the training cohort. Through the log-rank method, the best cutoff points were obtained with minimum P values from lookup tables for risk. In open liver resections, sponges were weighed at the final step of the surgery.
2.4Postoperative careLiver function tests, routine blood examination, and clotting function tests were performed on postoperative days (PODs) 1, 2, and 3. All resected specimens were examined by postoperative pathological examination.
The primary endpoint was postoperative mortality, defined as patient deaths that occurred during the postoperative hospitalization or within 30 days after surgery.
2.5Establishment of a predictive nomogram for PHLFBy integrating tumor number, Pringle maneuver, the volume of blood loss, preoperative platelet count, ascites and use of anticoagulant medication into our logistic regression model, we evaluated the significance of these factors in predicting patient's risk of developing PHLF in the training cohort. Based on this data, a novel predictive nomogram was developed to offer a reliable and quantifiable method for predicting patients’ risk of developing PHLF. The risk score was calculated using the following formula: risk score = (Factor 1 × a)+(Factor 2 × b)…+ (Factor 3 × n), where letters a, b, and n represent the regression coefficients.
2.6Statistical analysisIBM SPSS statistics 23 software for Windows (SPSS Inc., Chicago, IL, USA) and R software version 4.0.3 (Institute for Statistics and Mathematics, Vienna, Austria; http://www.r-project.org/) were used for statistical analyses. Continuous variables were presented as the mean ± standard deviation or as the median. Student's t-test or the Mann–Whitney U test was used to compare continuous variables. Categorical variables were grouped based on clinical findings, presented as counts and percentages, and measured using Fisher's exact test or the chi-squared test. All variables were incorporated into a logistic regression model and variables with statistical significance (P < 0.05) were included in a multivariate logistic regression model using a forward stepwise with a proportional odds ratio (OR) methodology. The impact of variables on patient survival was assessed by calculating the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. An AUC > 0.7 was regarded as a significant variable. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant.
The C-index was calculated to assess the efficiency of the nomogram. The bootstrap self-sampling method was used for internal validation of the nomogram with 1000 resamples of the training cohort [12]. The C-index was approximately close to 1, and the prediction ability of nomograms was approximately accurate [13]. The validation cohort was used to validate our model by R software. A comparison with two other previously published data models was also performed.
2.7Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Clinical Research Ethics Committee of the Shanghai Eastern Hepatobiliary Surgery Hospital (Approval Number: EHBHKY2021-K-011).
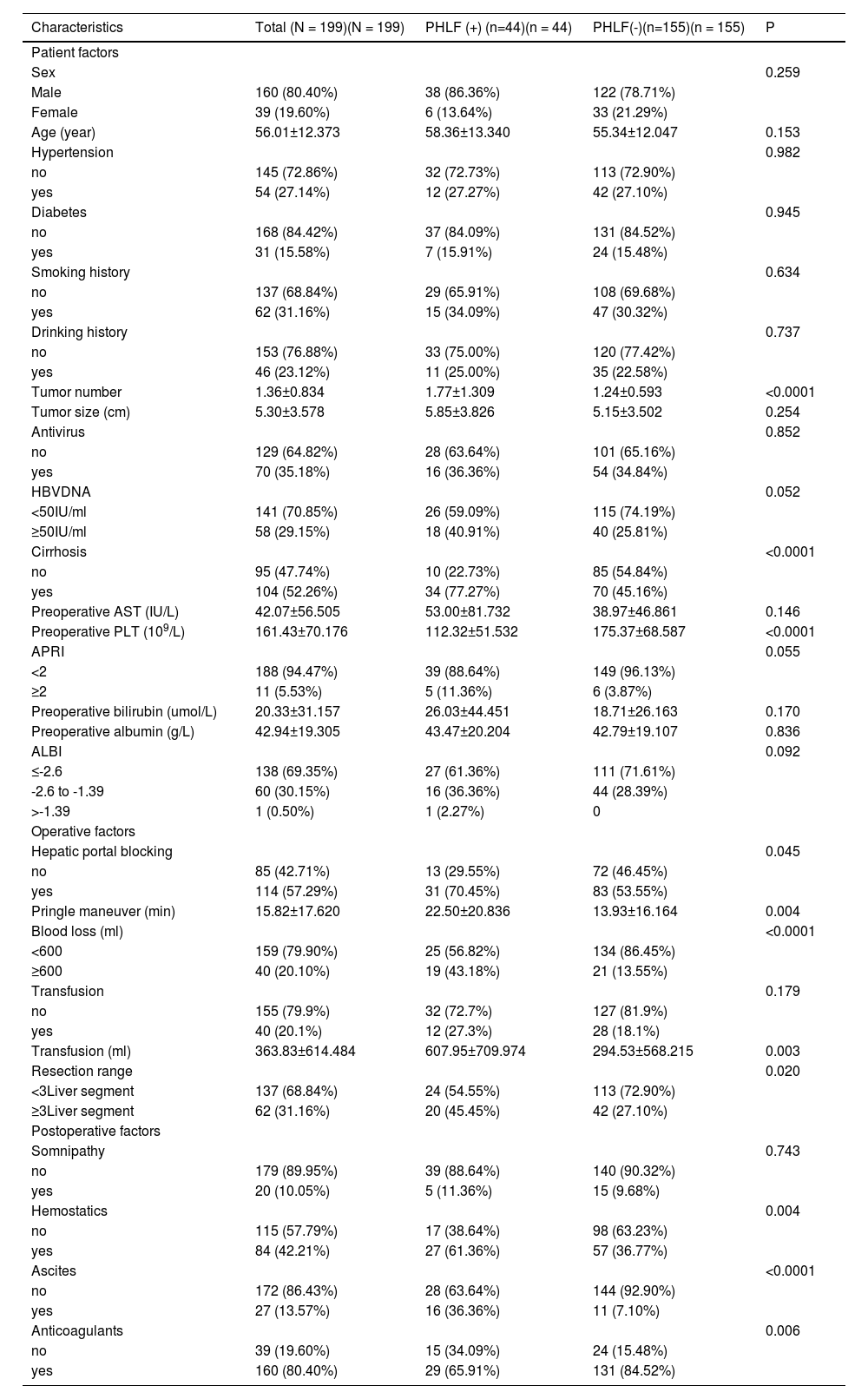
3Results3.1Clinicopathological characteristics of patientsThe flowchart of the study is shown in Fig. 1. The training cohort included 199 eligible patients who received partial hepatectomy, and the validation cohort included 71 consecutive eligible patients. The clinical pathology characteristics of the patients in the training cohort are listed in Table 1, and those of the validation cohort are listed in Supplement Table 1.
Patient characteristics in HCC patients in the validation cohort according to the development of PHLF (n=199).
HCC, Hepatocellular carcinoma; PHLF, Post-hepatectomy liver failure; HBV, Hepatitis B virus; APRI, Aspartate aminotransferase-to-platelet ratio index; ALBI, Albumin-bilirubin; PLT, Platelets; AST, Aspartate aminotransferase. P < 0.05 was defined as statistical significance.
As shown in Table 1, 104 (52.26%) patients in the training cohort had cirrhosis. The mean number of tumors was 1.36 ± 0.834. The mean tumor size was 5.30 ± 3.578 cm. With regard to intraoperative characteristics, 62 (31.16%) patients received a large range hepatectomy, blood loss was ≥ 600 mL, and blood transfusion was conducted in 40 (20.10%) patients with a mean transfusion volume of 363.83 ± 614.484 mL. 160 (80.40%) patients were prescribed postoperative anticoagulants.
Postoperative PHLF outcomes were graded using the International Study Group of Liver Surgery (ISGLS) grading. 10 patients (21.74%) had no symptomatic liver failure (ISGLS grade 0/A), 15 (32.61%) had moderate (ISGLS grade B), and 21 (45.65%) had severe (ISGLS grade C). Seventeen patients died within 30 days, and the MSD (mean ± standard deviation) of the date of death was 6.32 ± 5.55 days.
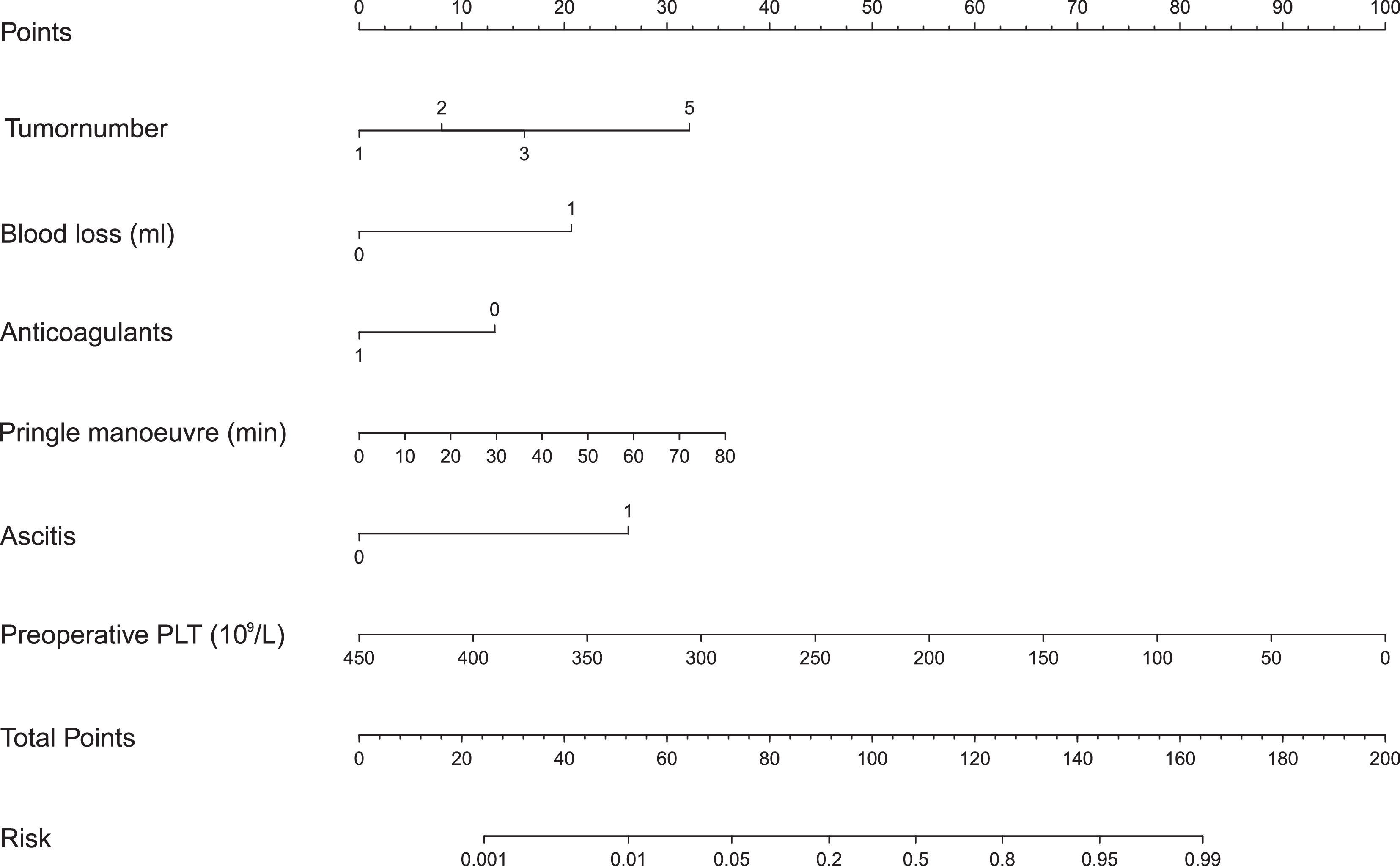
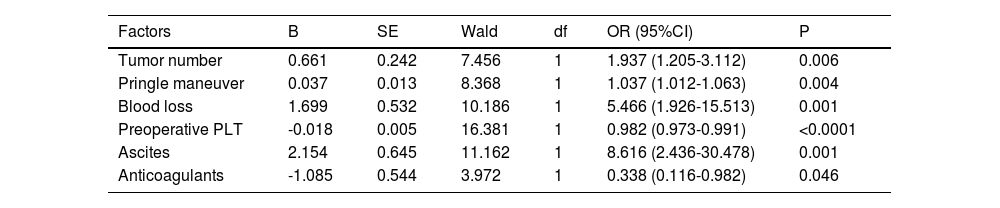
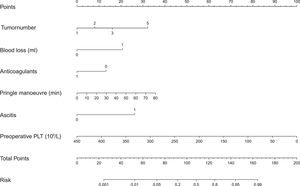
3.3PHLF-associated factorsIn the training cohort, the univariate analysis demonstrated that tumor number, hepatic portal blocking, Pringle maneuver, blood loss, blood transfusion, cirrhosis, resection range, preoperative platelet count, hemostatics, ascites, and use of postoperative anticoagulants all correlated to the development of PHLF (Supplement Table 2). Multivariate analysis showed that the following six factors were related to the development of PHLF: tumor number (P = 0.006; OR 1.937, 95% confidence interval [14,15]: 1.205-3.112), Pringle maneuver (P = 0.004; OR 1.037, CI: 1.012-1.063), blood loss (P=0.001; OR 5.466, CI: 1.926-15.513), preoperative platelet count (P < 0.0001; OR 0.982, CI: 0.973-0.991), ascites (P = 0.001; OR 8.616, CI: 2.436-30.478), and use of postoperative anticoagulants (P = 0.046; OR 0.338, CI: 0.116-0.982) (Table 2). Fig. 2 shows the univariate analyses of factors that predicted PHLF after hepatectomy. The risk score was calculated using the following formula: risk score = (tumor number × 0.661)+(Pringle maneuver × 0.037)+(blood loss × 1.699-preoperative platelet count × 0.018)+(ascites× 2.154-anticoagulants × 1.085), where letters a, b, and n represent the regression coefficients.
Multivariate analyses of factors associated with PHLF.
PHLF: Post-hepatectomy liver failure; PLT: Platelets; CI: confidence interval. P < 0.05 was defined as statistical significance.
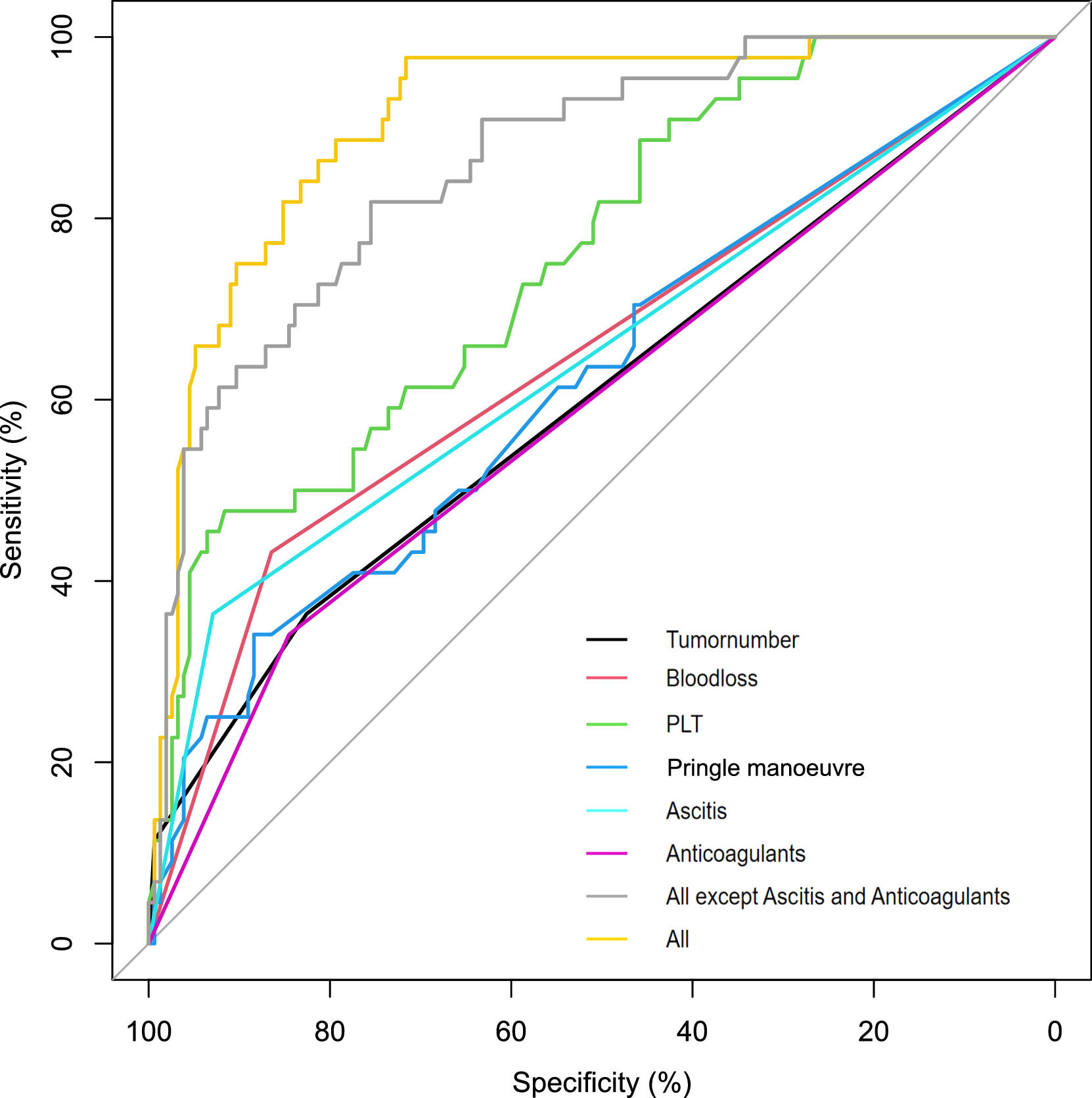
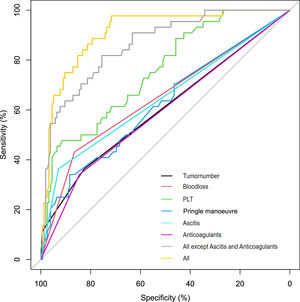
Tumor number provided an AUC of 0.571 (95%CI 0.466–0.676, P<0.05); Pringle's maneuver provided an AUC of 0.596 (95%CI 0.496–0.695, P<0.01); blood loss provided an AUC of 0.691 (95%CI 0.602–0.780, P<0.05); preoperative PLT provided an AUC of 0.739 (95%CI 0.658–0.821, P<0.01); ascites provided an AUC of 0.660 (95%CI 0.561–0.759, P<0.01); anticoagulants provided an AUC of 0.594 (95%CI 0.496–0.692, P<0.05). The model provided an AUC of 0.911.
We built a nomogram to predict the probability of PHLF using the six aforementioned independent risk factors (Fig. 3). The number of points assigned to each factor was calculated by their hazard ratios (HRs). The total score was used to predict the probability of PHLF. For instance, one HCC patient in our study had one tumor (0 points), a 30 min Pringle maneuver (10 points), 1000 mL of blood loss (20 points), no ascites (0 points), no anticoagulant usage (15 points), and a preoperative platelet count of 50 × 109/L (87.5 points). This patient's nomogram score was 132.5 in total, indicating a more than 60% probability of developing PHLF.
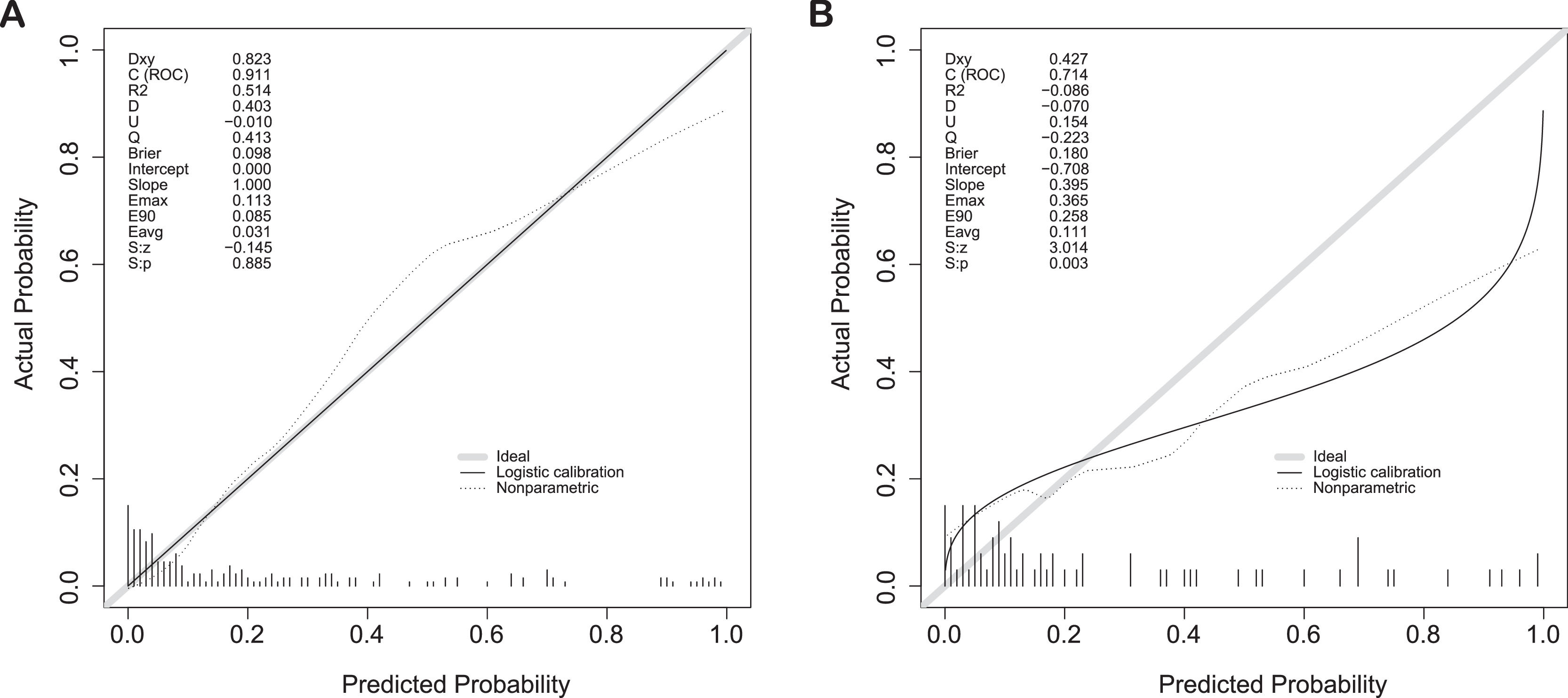
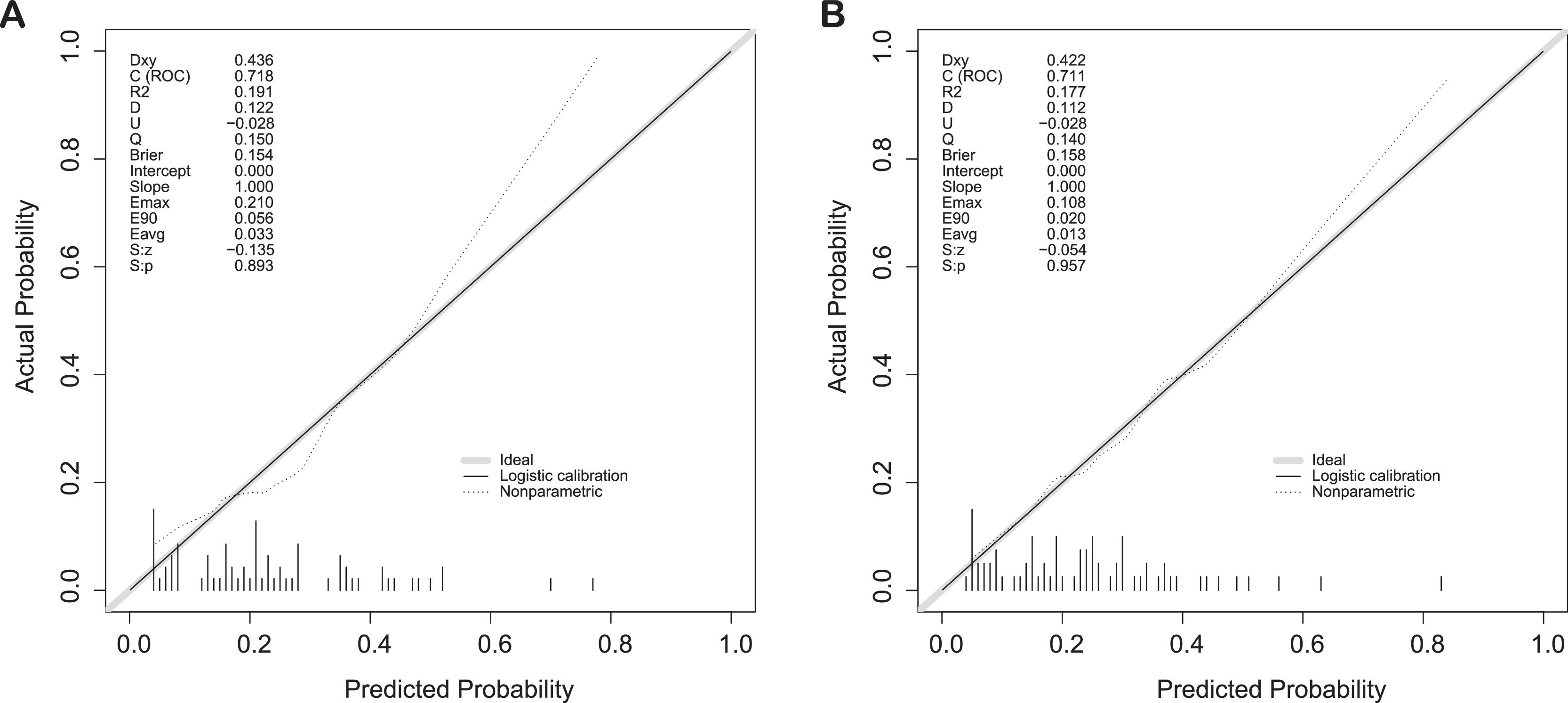
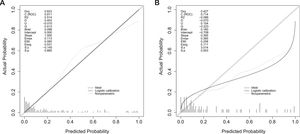
3.5Validation of the predictive accuracy of the nomogram for PHLFThe calibration curves showed a good fit between predicted and observed outcomes for the prediction model in both cohorts (Fig. 4A, B).
In addition, the AUC of the nomogram for PHLF prediction reached 0.911 (CI: 0.865-0.958) (Fig. 4A) for the training cohort, and the C-index was 0.714 (CI: 0.697-0.902) for the validation cohort (Fig. 4B), suggesting a reliable prediction effect of our modelling.
3.6Comparison with other modelsWe analyzed the validation cohort using two previously published studies to calculate the C-index[ 16, 17]. The C-indices for Dasari BVM et al. and Citterio D et al. were 0.718 and 0.711, respectively (Fig. 5). The results showed that our model had similar predictive performance for these studies.
4DiscussionAlthough hepatectomy effectively excises the primary tumor, it simultaneously causes unavoidable liver injury. Ischemia-reperfusion injury (IRI) induced by inflow occlusion during hepatectomy significantly activates local and systemic inflammatory responses, causes oxidative stress injury, and may cause multiple organ injuries, including liver, kidney, and heart injuries, which can result in dangerous postoperative complications such as PHLF [18]. The mechanisms contributing to PHLF remain unclear and difficult to predict because of the current lack of effective and validated models or biomarkers [19]. The mechanisms leading to the development of PHLF are likely related to disordered liver hemodynamics, autoimmunity, and other factors. Some risk factors leading to the development of PHLF have previously been identified, and several prediction models have been developed. We find that the clinical application of these previously reported models is limited as follows [20]: (i) some models were not based on statistical methods; (ii) some models depended on biological or hemodynamic measurements, such as indocyanine green clearance or hepatic venous pressure gradient, which are generally hard to acquire; (iii) the heterogeneous inclusion criteria of previous studies(the proportion of cirrhotic patients or the proportion of HCC) limited the general applicability of these models; and (iv) few models were externally validated in other clinical centers or study cohorts.
In our study, patients’ risk of developing PHLF could be predicted by our nomogram based on six variables. The nomogram performed well in predicting PHLF, supported by the C-index (0.911 and 0.714 for the training and validation cohorts, respectively) and the calibration curve. In comparison with previous systems, our nomogram demonstrated an accurate prediction of PHLF [20]. In our study, we used 30-day mortality as the outcome measure to avoid including deaths occurring in the 90 days postoperatively due to more complex causes secondary to early recurrence [21].
In addition to the 17 preoperative and 5 intraoperative variables analyzed, 4 postoperative variables were included in our postoperative model. We found that PHLF risk was associated not only with preoperative (tumor number, preoperative platelet) and intra-operative events (blood loss, Pringle maneuver) but also with postoperative events (postoperative ascites and use of postoperative anticoagulants). The importance of consideration of intraoperative events in the prediction of post-hepatectomy complications has been previously suggested but its role remains unclear. In addition, the role of postoperative factors has generally gone unrecognized[ 3, 22]. We believe that these postoperative factors also play a key role in the development of strategies to predict and prevent PHLF.
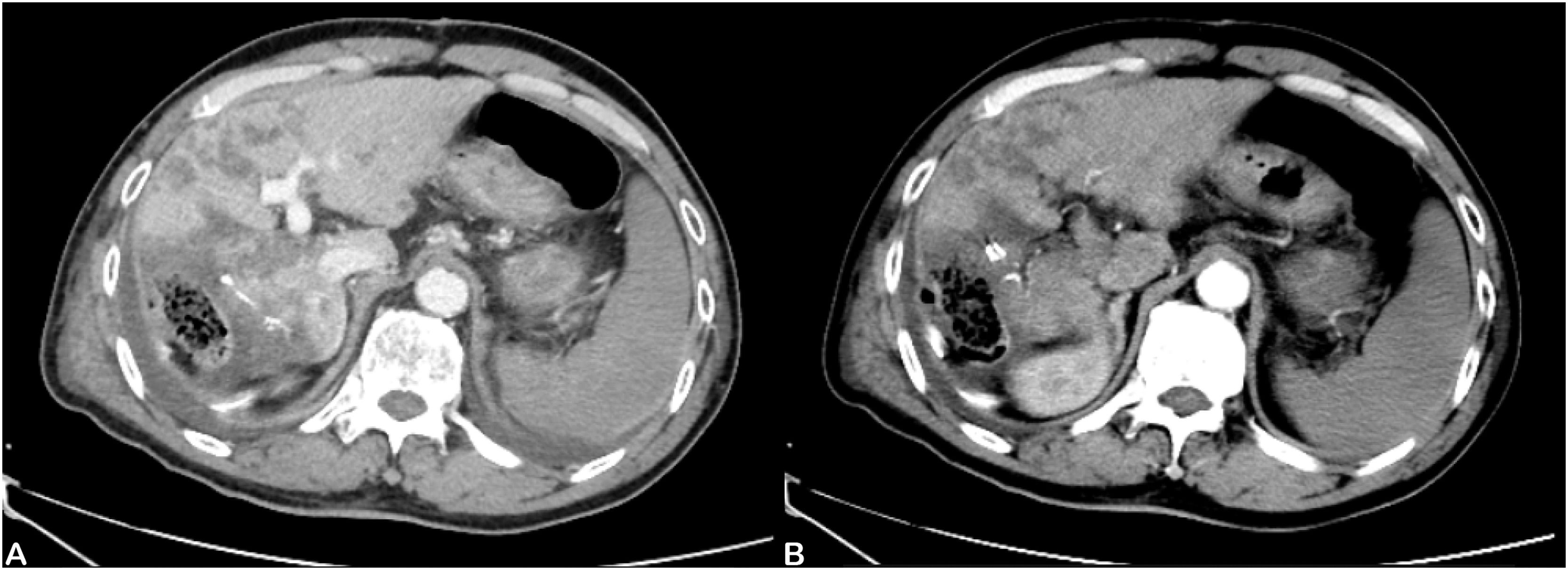
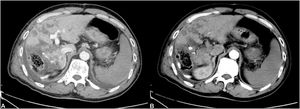
In this study, the use of postoperative anticoagulants was an independent risk factor for liver failure. Postoperative anticoagulants can greatly reduce the occurrence of liver failure. Circulatory failure during PHLF is similar to circulatory failure with sepsis [23]. The commonly observed pathophysiological changes include enhanced vascular permeability, diffuse endovascular coagulation, and peripheral vasodilatation, which are represented clinically by reduced peripheral resistance and hemodynamic instability [24]. We found many of the PHLF patients had typical CT images of PHLF, including portal vein thrombosis and obstruction, as well as large infarcts in the liver (Fig. 6). Studies have shown that postoperative hypercoagulability is caused by liver coagulation dysfunction [25]. We speculate that liver failure may be the process of liver disseminated intravascular coagulation (DIC) leading to liver infarction. Liver DIC is a complex pathological process triggered by specific events. Pathogenic factors activate the coagulation system, causing activation of platelets and deposition of fibrin, leading to DIC. These patients had prominent clinical manifestations similar to hepatic vascular embolism and microcirculation dysfunction. Liver infarction refers to partial hepatic avascular necrosis caused by severe stenosis or complete occlusion of a branch of the hepatic artery or portal vein. The Pringle maneuver, use of hemostatics, and lack of anticoagulant usage can lead to thrombosis, which aggravates stenosis or occlusion. Hemostasis is an integral and very important aspect of surgical practice [26]. Our medical team uses the following additional strategies to prevent PHLF: use of anti-infection medications, hormone therapy, intestinal probiotics, postoperative anticoagulants, and terlipressin (which plays a role in improving blood flow). Further studies on the benefits of such strategies on pathology and molecular biology are needed. We found it impossible to perform CT imaging on all the PHLF patients for the following reasons: (i) the rapid onset of PHLF; (ii) severity of illness making patients unable to be safely scanned; and (iii) in some cases, the rapid demise of the patient. Therefore in the absence of complete data, we did not include the presence of portal vein thrombosis on CT images as a statistical variable. However, in future, we plan to further explore the diagnostic and predictive value of CT imaging for portal vein thrombosis in PHLF. In terms of the standard of postoperative anticoagulant usage, we took the increase in DD-dimer level as the anticoagulant index. Some studies have shown that the earlier the anticoagulant is started, the higher the recanalization rate [27]. Postoperative anticoagulants were used in patients who demonstrated no obvious bleeding events, and in this study we performed hemostatic and small-scale resections. In this study, the patients who used anticoagulant drugs after the operation did not show an increased bleeding rate.
Ascites is the pathologic accumulation of fluid in the peritoneal cavity. It is a common manifestation of liver failure and is one of the cardinal signs of portal hypertension [28]. Ascites, an independent risk factor, is due to portal vein thrombosis, which leads to slowed portal vein blood flow, increased portal vein pressure (more than 300 mm H2O), and an insufficient amount of effective circulating blood in the liver, which may be aggravated postoperatively by increased portal resistance after large volume hepatectomy [29]. However, most researchers believe that ascites is only a minor postoperative complication and does not affect the survival of patients [30]. We postulate that the pathogenesis of ascites in liver failure may involve these events: (i) the severe degree of cirrhosis, large number of tumors, the poor compensatory function of the remaining healthy liver due to reduced liver repair capacity; and (ii) hepatic vein occlusion syndrome or portal vein thrombosis, leading to hepatic vascular disease.
We reviewed the existing literature with regard to the other variables in the postoperative model, such as platelet count, blood loss, Pringle maneuver, and tumor number. A low platelet count can be a noninvasive indicator of portal hypertension. Moreover, a low preoperative platelet count leads to increased morbidity and mortality [31]. Some rodent studies have reported that thrombocytopenia may significantly impair platelet contribution in initiating liver regeneration [32], which is consistent with our findings. However, the importance of a low preoperative platelet count in predicting postoperative prognosis in patients with hepatocellular carcinoma has not been previously reported. Excessive blood loss may result in clotting dysfunction, hypotension, and fluid displacement, which may lead to bacterial displacement [14]. Our research led to the development of a nomogram that may be useful as part of the presurgical evaluation, helping to identify patients at risk before surgery. We believe that preoperative evaluation can play a role in the prevention of PHLF. Next, we explored the value of some preoperative factors in the diagnosis and prediction of PHLF.
We explored factors that predict operative mortality associated with PHLF. As shown in Supplement Table 3, age, cirrhosis, preoperative bilirubin, and the use of hemostatics were independent predictors of death in multivariate analysis, including variables otherwise significant in univariate analysis (namely, blood loss, preoperative PLT, aspartate aminotransferase-to-platelet ratio index, albumin-bilirubin, ascites, and anticoagulants). Moreover, the use of hemostatic drugs is an independent risk factor for increased mortality in patients with liver failure. This is also consistent with our hypothesis concerning the pathogenesis of liver DIC leading to liver infarction and the postoperative use of anticoagulant medications.
5ConclusionIn this research study, we developed a model based on 6 simple and readily identifiable variables (tumor number, Pringle maneuver, blood loss, preoperative platelet count, ascites, and use of anticoagulants) that can predict the risk of development of PHLF. This model can be a valuable tool for identifying those patients at risk for the development of PHLF when considering the risks of surgical resection.
FundingThis study was supported by the National Natural Science Foundation of China, 81972574 (Guang-Zhi Jin) and by the Shanghai Key Medical Specialty Project (Grant No.ZK2019B14) and Shanghai Municipal Commission of science and technology (Grant NO. 20ZR1451900).
Authors’ contributionsStudy concept and design: Guang-Zhi Jin, Yi-Ran Li, Yu-Ming Sun and Jin-Dong Chen. Drafting of the manuscript: Yi-Ran Li, Jin-Dong Chen and Guang-Zhi Jin. Acquisition of data, analysis and interpretation of data: Yi-Ran Li and Jin-Dong Chen. Critical revision of the manuscript: Guang-Zhi Jin and Yi-Ran Li. Statistical analysis: Guang-Zhi Jin and Yi-Ran Li. All authors read and approved the final manuscript.
None.