Vanishing bile duct syndrome is a rare acquired condition, characterized by progressive loss of intrahepatic bile ducts leading to ductopenia and cholestasis. It can be associated with infections, ischemia, drug adverse reactions, neoplasms, autoimmune disease, and allograft rejection. Prognosis is variable and depends on the etiology of bile duct injury. We report the case of a 25-year-old female with cholestatic hepatitis and concomitant intakes of hepatotoxic substances, such as garcinia, field horsetail, and ketoprofen. On suspicion of a drug-induced liver injury, the drugs were promptly withdrawn and ursodeoxycholic acid was started with initial clinical and laboratory improvement, and the patient was discharged from the hospital. One month later, she had a new increase in bilirubin levels and canalicular enzymes, requiring a liver biopsy that showed significant loss of intrahepatic bile ducts, which was compatible with vanishing bile duct syndrome. This was confirmed by using cytokeratin 19 on immunohistochemistry. There was subsequent lymph node enlargement in several chains, and relevant weight loss. Histological analysis of a cervical lymph node revealed nodular sclerosis-subtype classic Hodgkin lymphoma. In this setting, vanishing bile duct syndrome was related to Hodgkin lymphoma and a drug-induced liver injury overlap, leading to progressive cholestasis with a worse prognosis. The patient's response to chemotherapy was poor, requiring biological therapy with brentuximab vedotin. It is crucial for physicians to create a broad differential diagnosis in suspected vanishing bile duct syndrome patients, especially to rule out malignancies.
Vanishing bile duct syndrome (VBDS) is a rare acquired condition, characterized by progressive loss of intrahepatic bile ducts, which is evidenced in liver biopsy, and leads to ductopenia and cholestasis [1,2]. It represents 0.5% of the small biliary ducts diseases [3], and is associated with infections, ischemia, drug adverse reactions, neoplasms, autoimmune disease, and allograft rejection. The prognosis is variable—from complete recovery to biliary cirrhosis, liver failure, and death [2,4,5]. We describe an unusual case of VBDS related to Hodgkin lymphoma (HL) and a drug-induced liver injury (DILI) overlap.
2Case reportA 25-year-old previously healthy Brazilian female was referred to our center after a hematemesis episode and a complaint of jaundice and intense pruritus. She had no recent trips or family history of liver diseases. The patient had been using manipulated drugs for 45 days, prescribed by an endocrinologist for weight loss and ketoprofen to relieve a dorsal pain. Many substances were in the formulation, such as southern field horsetail (Equisetum arvense), garcinia (Garcinia cambogia), bupropion, sertraline, safflower oil, green mate, sene, bearberry (Arctostaphylos uva-ursi), metformin, orlistat, common bean (Phaseolus vulgaris), devil's tongue (Amorphophallus konjac), dyed broccoli extract, yerba mate (Ilex paraguariensis) and ginger (Kaempferia galanga). She denied the use of alcoholic beverages or illicit drugs.
The initial physical examination revealed jaundice, reactive cervical lymph nodes (measuring nearly 1cm) and tenderness on right hypochondrium palpation, without signs of peritonitis or organomegaly. Laboratory tests showed an increase in liver enzymes, such as aspartate aminotransferase 146U/L (normal value [NV]<35) and alanine aminotransferase 199U/L (NV<35), gamma-GT 1150U/L (NV<64), alkaline phosphatase 1395U/L (NV<120), total bilirubin 19.6mg/dL (NV<1.2), direct bilirubin 14.0 (NV<0.2) and international normalized ratio 1.34 (NV<1.25). The hemogram showed normochromic and normocytic anemia (hemoglobin=9.3g/dL), with decreased serum iron (25μg/dL, [NV=60–180]) and a high platelet count (570,000/mm3). Viral hepatitis serologies, plus anti-nuclear, anti-mitochondrial and anti-smooth muscle antibodies were negative. Ceruloplasmin was normal and protein electrophoresis only showed low serum albumin (2.46g/dL [NV=3.5–5.2]). Other infectious disease panels – including HIV, toxoplasmosis, mononucleosis, and blastomycosis – were negative.
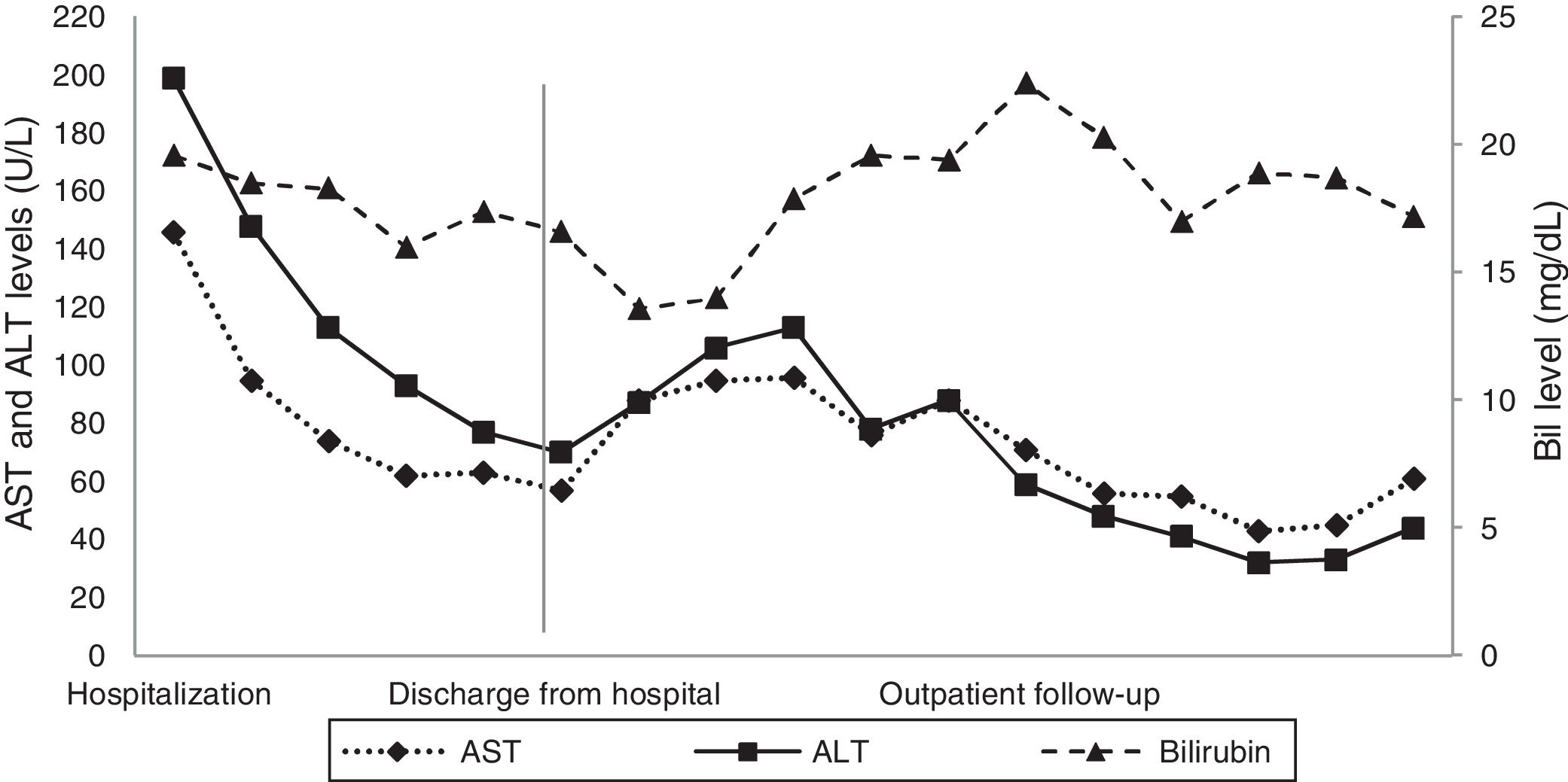
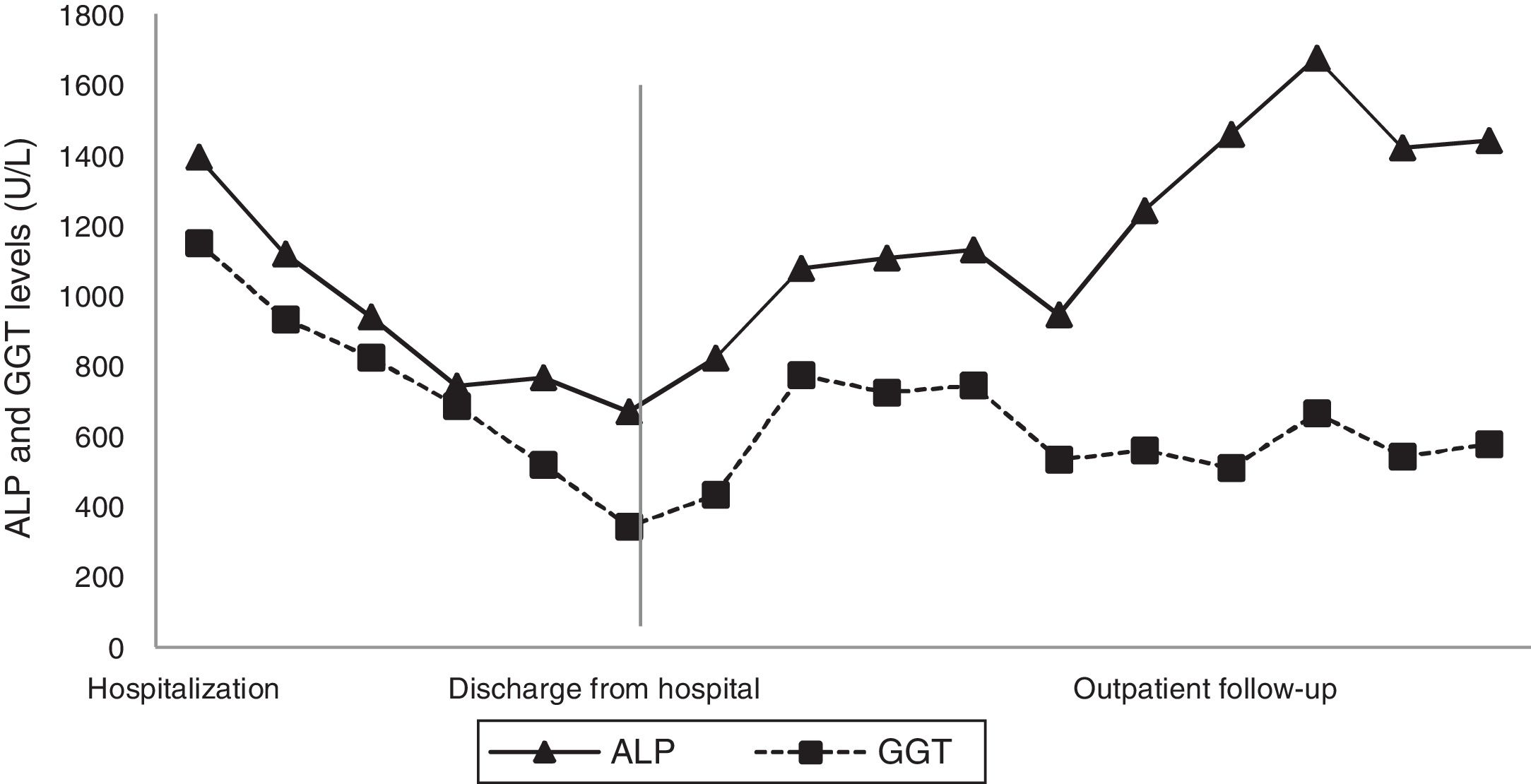
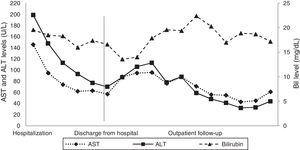
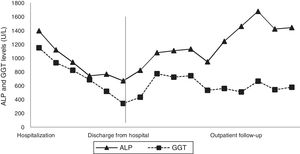
A digestive endoscopy revealed erosive and hemorrhagic pangastritis; an abdominal ultrasonography was unremarkable; and magnetic resonance cholangiography showed hepatomegaly with a transient perfusional disorder of the liver, reactive lymph node enlargement in the hepatic hilum (larger measuring 2.3cm×1.1cm), without intra- and extrahepatic bile ducts dilatation. On suspicion of DILI, all potentially harmful substances were discontinued, and ursodeoxycholic acid was commenced. The patient's abdominal pain and pruritus had resolved, and there was a partial biochemical improvement (Figs. 1 and 2), so the patient was discharged from the hospital.
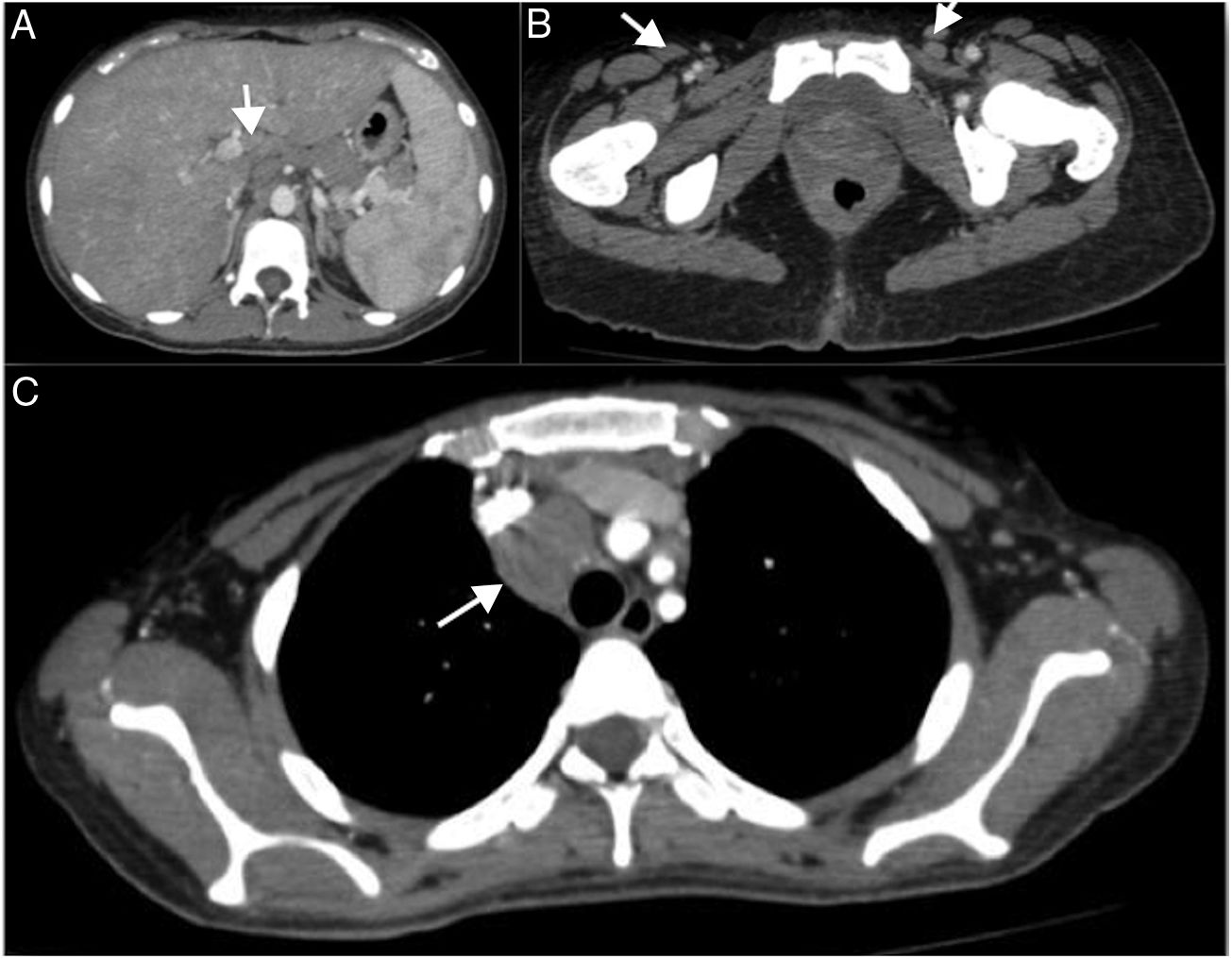
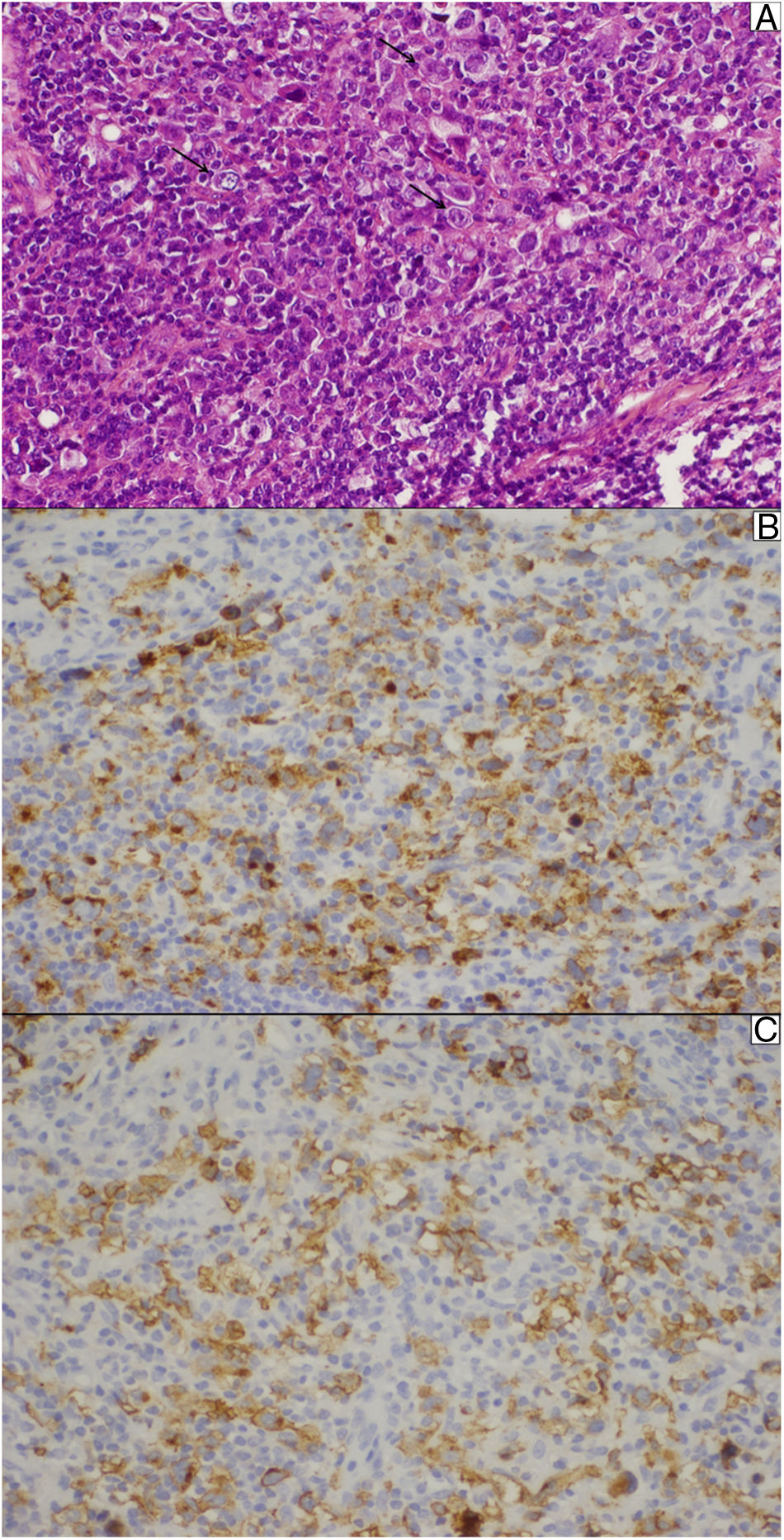
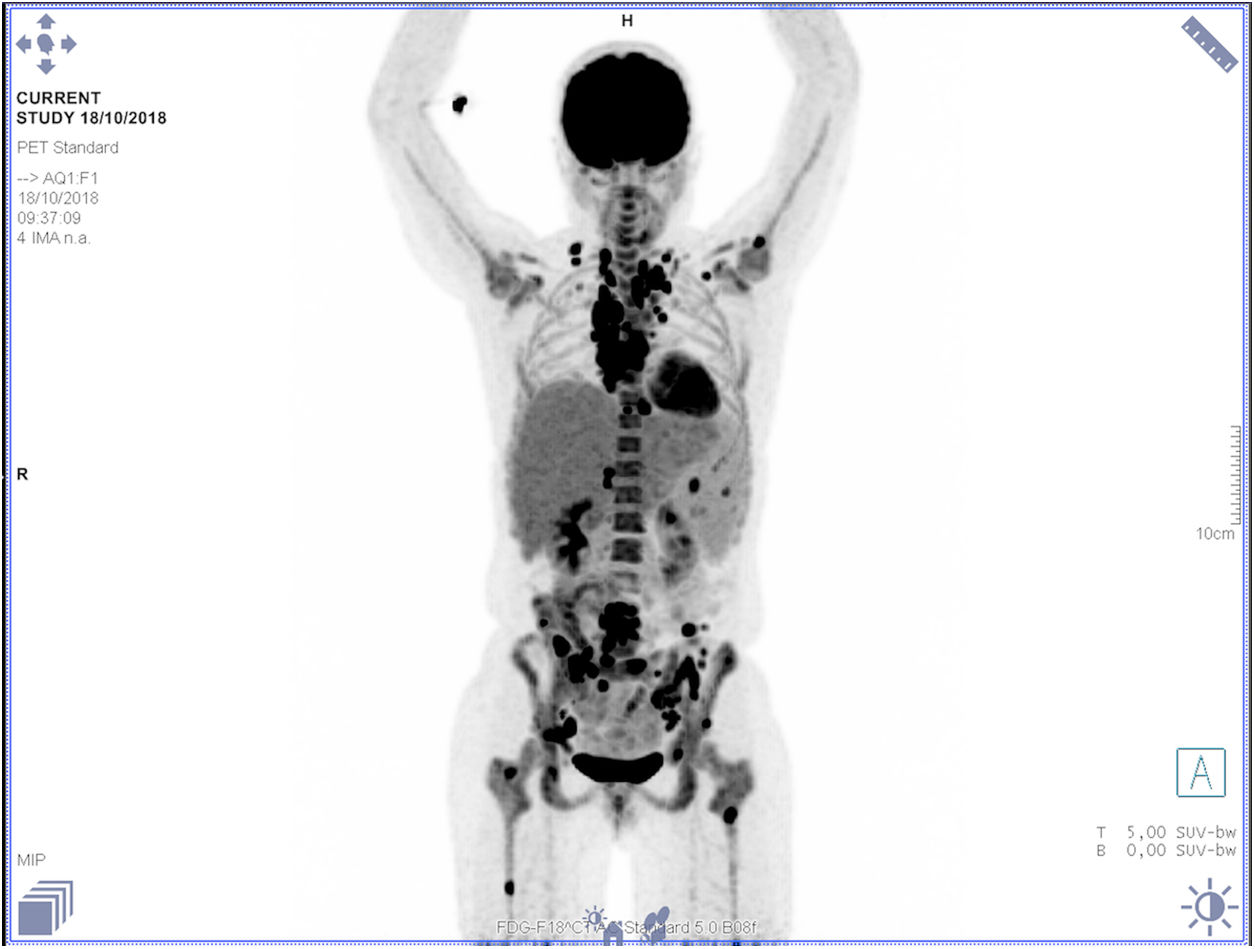
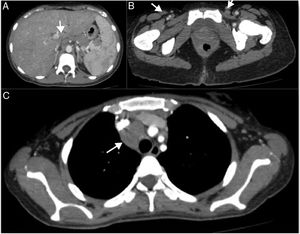
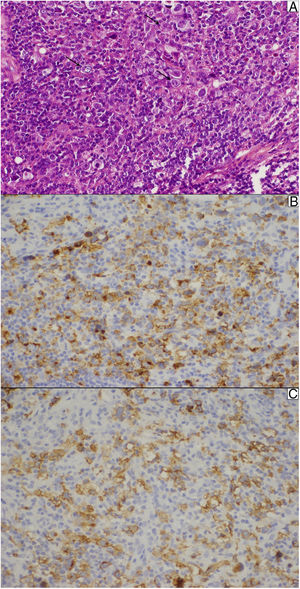
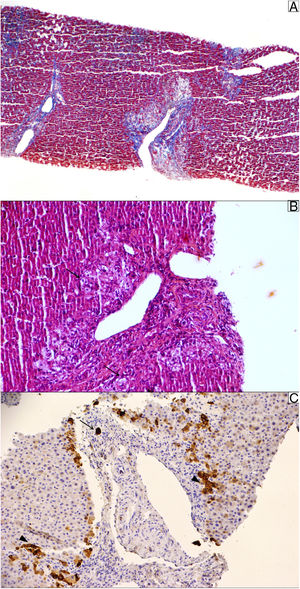
At the outpatient follow-up, there was a new increase in bilirubin levels and canalicular enzymes. One month after hospital discharge, a percutaneous liver biopsy showed mild portal fibrosis, significant loss of intrahepatic bile ducts, and moderate to intense ductular reaction. Over the next 4 months, the patient presented weight loss (20kg), night sweats, and intense pruritus. An unsuspected factor had worsened the cholestasis, and the cervical lymph nodes were enlarged. The thorax and abdomen computed tomography evidenced splenomegaly and wide lymph node conglomerates, which were more evident in the aortic, caval, colic, and iliac chains, and in the hepatic hilum (Fig. 3). Exeresis of the left cervical lymph node was performed and the histological analysis revealed a partially nodular growth pattern neoplasm, with fibrous bands, and an inflammatory background with lymphocytes, histiocytes, and some eosinophils, plus large multinucleated neoplastic cells with two mirror-image nuclei (Reed–Sternberg cells), which were positive for CD30 and CD15 on immunohistochemistry stains. This confirmed the diagnosis of nodular sclerosis—a subtype of classic HL (Fig. 4). The bone marrow biopsy was negative for lymphoma involvement. The liver biopsy was reviewed, and the finding of loss of intrahepatic bile ducts (previously described) was compatible with VBDS, which was confirmed by using cytokeratin 19 on immunohistochemistry (Fig. 5). There was no lymphoma spread in the liver fragment.
Lymph node biopsy. (A) Reed–Sternberg cells (arrows) are seen in a cellular background rich in lymphocytes, histiocytes, and some eosinophils. (B) The typical membrane and paranuclear dot-like staining of Reed–Sternberg cells for CD15 on immunohistochemistry. (C) These cells were also positive for CD30.
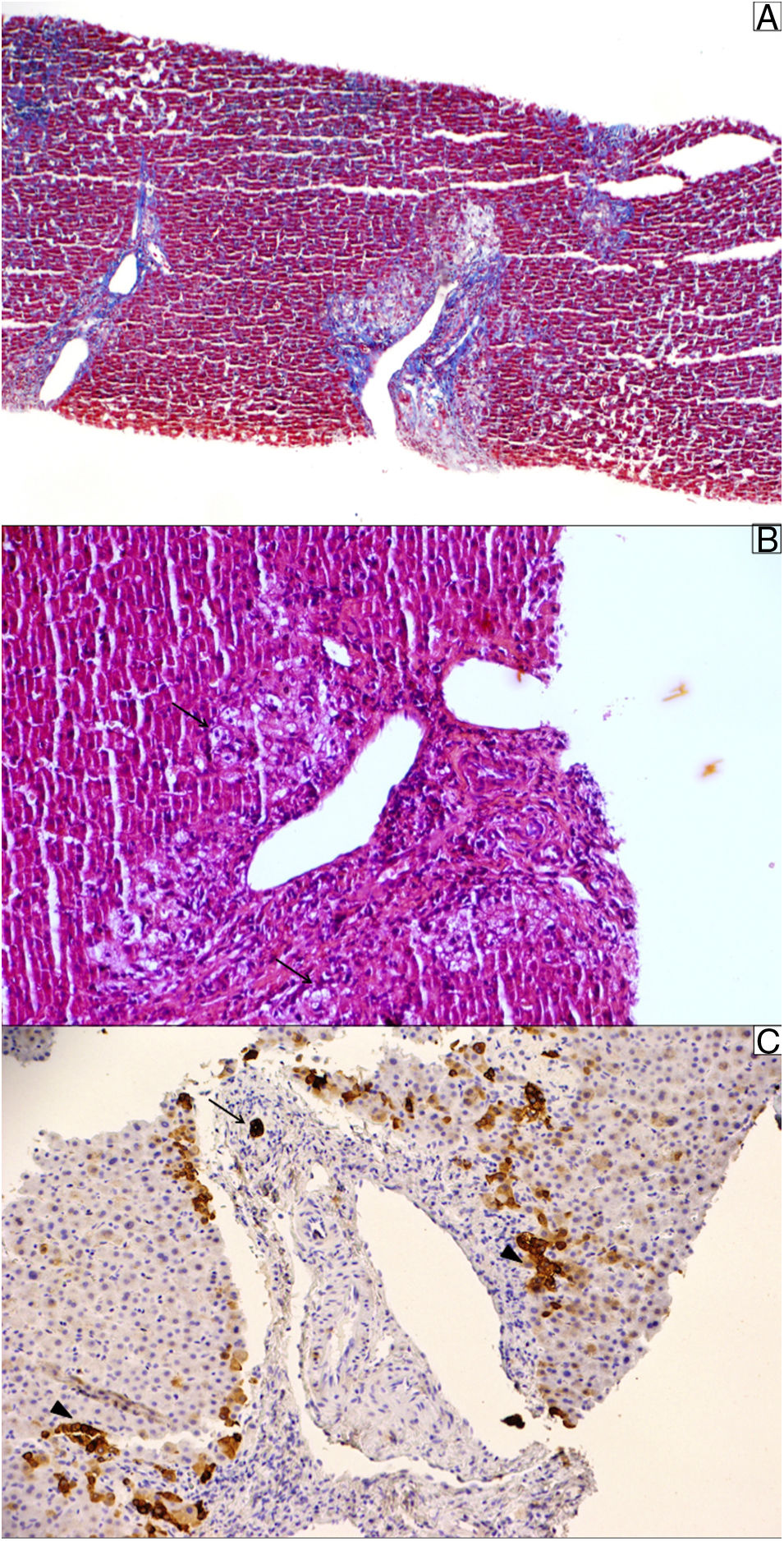
Liver biopsy. (A) Masson trichrome stain shows moderate portal fibrosis without septa. (B) The portal areas show a relatively mild lymphocytic infiltrate with prominent ductular reaction and cholestasis (pseudoxanthomatous change, arrow). (C) Cytokeratin 19 stain was used to identify the remaining ducts (arrow); they were positive in ductular reaction (arrowhead). The sample showed 10 portal tracts, of which 6 did not exhibit bile ducts, corroborating the diagnosis of loss of intrahepatic original ducts.
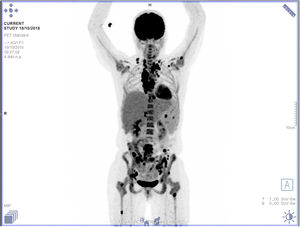
The patient was started on prednisone 40mg/day and completed six cycles of chemotherapy with Adriamycin/doxorubicin, bleomycin, vinblastine, and dacarbazine. The first cycle was infused without doxorubicin and vinblastine; the remaining doses were full because of better liver function. The patient presented a slight clinical improvement and weight gain, but a poor response to chemotherapy (Fig. 6), and is currently awaiting biological therapy with brentuximab, an anti-CD30 antibody drug.
3DiscussionVBDS is a rare group of disorders related to progressive destruction and disappearance of intrahepatic bile ducts leading to cholestasis [5]. The pathogenesis is poorly understood, but it has been associated with many conditions, including drug reactions, ischemia, infection, autoimmune disease, allograft rejection, and neoplasms [6]. The diagnosis is confirmed when less than 50% of bile ducts are seen on liver biopsy. This exam is fundamental and should not be postponed [5].
The disorder has been associated with more than 40 drugs, including chlorpromazine, amoxicillin, carbamazepine, clindamycin, meropenem, ajmaline, phenytoin, trimethoprim–sulfamethoxazole, arsenic derivatives, and tetracyclines [3,7,8]. Even though the pathogenesis of VBDS secondary to DILI has not been fully elucidated, multiple mechanisms have been proposed, including tumor necrosis factor-α-activated apoptosis, the inhibition of mitochondrial function, and neoantigen formation [7]. It is possible that an immune response, mainly mediated by T cells, leads to antigen recognition on biliary epithelial cells, resulting in immune cell infiltration into the intraepithelial layer of the bile ducts, apoptosis, and T-cell cytotoxicity [3,9]. Sundaram and Björnsson described a prospective report from the DILI network, where 26 of 363 (7%) patients were reported to experience VBDS secondary to drug injury. The most frequently implicated agents were amoxicillin/clavulanate, temozolomide, herbal products, and azithromycin [3].
Unfortunately, a major limitation in DILI diagnosis is the current lack of specific biomarkers [3,10]. Several promising DILI biomarker candidates have been discovered, such as glutamate dehydrogenase, high-mobility group box 1 protein, and keratin-18. Furthermore, microRNAs have lately received attention as potential non-invasive DILI biomarkers, in particular miR-122 [10]. A thorough history is necessary regarding a timing relation between the beginning of the use of these medications (or vitamins and herbal supplements) and the appearance of the disease [3]. In fact, among all the medications ingested by our patient, field horsetail (E. arvense) [11], garcinia (G. cambogia) [12], bupropion [13], sertraline [14,15], orlistat [16], and ketoprofen [17] have been associated with DILI. And only sertraline has been associated with VBDS as well [15].
The association of VBDS with HL is rare and poorly understood [1]. Hubscher et al. first described it in 1993 in three patients with intrahepatic cholestasis and ductopenia [18]. It may be a paraneoplastic phenomenon associated with HL, which is typically presented as jaundice, pruritus, and weight loss; this can even precede the diagnosis of lymphadenopathy [2,5], as seen in our patient. Despite the uncertain pathophysiology, the most common hypothesis to explain HL-related VBDS is an immune-mediated lesion of the bile epithelium, due to autoantibodies produced by the lymphoma or a T cell-mediated toxicity (or both), and direct damage to the bile duct by cytokines [4,5].
VBDS treatment is based on the intervention of the underlying cause. The first step is to discontinue the offending agent (when present) as soon as possible, as it is critical to spontaneous recovery and is generally followed by clinical improvement [8]. Alternative causes of acute liver injury should be excluded, including alcohol consumption; viral, autoimmune, and ischemic hepatitis; and other metabolic liver diseases. All patients with jaundice should undergo abdominal ultrasound as a means of identifying evidence of extrahepatic biliary obstruction, with subsequent cross-sectional imaging (computed tomography or magnetic resonance imaging) as appropriate [7].
Treatment of cholestasis and pruritus is necessary. The use of ursodeoxycholic acid and cholestyramine may benefit the patient's symptoms [2,3]. Ursodeoxycholic acid protects the epithelium against cytotoxicity caused by toxic bile salts. It stimulates hepatobiliary secretion, has antioxidant activity, enhances glutathione levels, and inhibits liver cell apoptosis [3]. Other drugs that manage pruritus secondary to severe cholestasis include antihistamines, rifampicin, phenobarbital, and opioid analogs [3,5].
When HL is the factor, treatment is challenging; however, the need for aggressive chemotherapy – which is limited by the degree of hepatic dysfunction and worsening cholestasis – should be balanced. The most commonly used regimen is Adriamycin/doxorubicin, bleomycin, vinblastine, and dacarbazine; however, there is no consensus whether the dose of chemotherapy should be total or reduced [5,19,20]. Radiotherapy has shown to improve liver-failure-free survival, and chemoradiation can be performed [2].
Up to 30% of patients with HL in stage III or IV harbor refractory disease or relapse after frontline treatment with Adriamycin/doxorubicin, bleomycin, vinblastine, and dacarbazine [20]. Autologous hematopoietic cell transplantation is an option for treatment, as described by Wong et al., where a 38-year-old man with HL-related VBDS achieved complete remission with normalization of serum bilirubin after eight cycles of Adriamycin/doxorubicin, bleomycin, vinblastine, and dacarbazine, followed by autologous hematopoietic cell transplantation. However, its long-term benefit requires further observation [4]. Brentuximab vedotin is an anti-CD30 monoclonal antibody with direct action to Reed–Sternberg cells in classic HL. It has been approved for treatment after autologous stem-cell transplantation failure, or after two or more chemotherapy regimens in patients who are not candidates for transplantation. The drug has also been approved as a post-transplantation consolidation therapy in patients with increased risk for relapse or progression of the neoplasm [20].
While hepatic failure is a major cause of mortality among patients with HL-related VBDS, many reported subjects had complete remission of the disease and improvement of hepatic function after lymphoma treatment. Some patients have significant liver dysfunction requiring liver transplantation; however, this remains controversial given the limited data and rarity of this syndrome [5].
The prognosis of VBDS is variable and depends on the etiological trigger of biliary epithelium apoptosis and the capacity for biliary regeneration. Various factors can influence the prognosis, including the severity of hepatic dysfunction, disease stage, performance status, comorbidities, history of ethanol consumption, pre-existing liver disease, and histologic features on the bile ducts [19]. Ballonoff et al. reported that one-third of patients could have a reversible disease course [21], although it is often progressive, leading to prolonged bile duct loss, biliary cirrhosis, liver failure, and death [2,3,19]. In a study from the DILI network cohort, approximately 19% of liver-related mortality was observed among patients with bile duct loss, either as the primary or the contributing cause of death [3]. This mortality rate is numerically greater than the 6.2% overall mortality seen in a recently published report of 899 DILI patients, in which half of the deaths were ultimately liver related, thus underscoring the seriousness of VBDS compared to other presentations of DILI [3].
To our knowledge, this is the first case reporting VBDS related to HL and DILI overlap. It is not possible to define whether both conditions were involved or if one of them was the trigger and the other was just a confounding factor. There may have been a higher susceptibility to DILI in the setting of lymphoma, and the large number of toxic drugs found a proper scenario to induce VBDS. It is crucial for physicians to create a broad differential diagnosis in suspected VBDS patients, especially to rule out malignancies.AbbreviationsVBDS vanishing bile duct syndrome drug-induced liver injury Hodgkin lymphoma aspartate aminotransferase normal value alkaline phosphatase gamma-glutamyl transferase international normalized ratio human immunodeficiency virus cluster of differentiation Adriamycin/doxorubicin, bleomycin vinblastine and dacarbazine tumor necrosis factor ribonucleic acid Reed–Sternberg positron emission tomography/computed tomography
R.D.G., M.C.S. and J.G.C. contributed substantially to the conception and design of the study, and drafting of manuscript. L.B.E.C. performed the histological examination of the liver and was a major contributor in writing the manuscript. D.F.C.M.; T.S.P. and J.R.S.A. provided critical revision of the article and final approval of the version to be published. M.M.C.N.; I.E.P.; F.C.O.; G.A.S.F. and F.L.P.N. contributed substantially to the acquisition, analysis and interpretation of data.
Ethical approvalThe study was approved by the hospital ethics committee at the University of Campinas and written informed consent was obtained from the patient.
FundingThe authors received no specific funding for this work.
Conflict of interestThe authors have declared that no competing interests exist.