Introduction. Although it is standard procedure in the evaluation of liver diseases, biopsy is an invasive method subject to sampling error and intra or inter-observer variability. Thus, surrogate markers of liver fibrosis have been proposed, with variable availability and accuracy.
Aim. Validate and compare the performance of APRI and FIB-4 as predictors of liver fibrosis in HCV patients.
Material and methods. Cross-sectional study including patients with HCV-RNA (+) who underwent liver biopsy. Significant fibrosis was defined as METAVIR stage ≥ 2. The diagnostic performance of the models in predicting significant fibrosis were evaluated and compared by ROC curves.
Results. The study included 119 patients, mean age 43.7 ± 10.6 years and 62% males. Significant fibrosis was identified in 41 patients. The AUROCs observed were: APRI = 0.793 ± 0.047, FIB-4 = 0.811 ± 0.045 and AST/ALT = 0.661 ± 0.055 (P = 0.054 for APRI vs. AST/ALT, and P = 0.014 for FIB-4 vs. AST/ALT). Considering classic cutoffs, the PPV and NPV for APRI and FIB-4 were, respectively, 77% and 92% and 83% and 81%. Thirteen (19%) patients were misdiagnosed by APRI and 16 (18%) by FIB-4. By restricting the indication of liver biopsy to patients with intermediate values, it could have been correctly avoided in 47% and 63% of the patients with APRI and FIB-4, respectively.
Conclusion. The models APRI and FIB-4 were superior to AST/ALT ratio in the diagnosis of significant fibrosis in chronic HCV infection. Even though the overall performance of APRI and FIB-4 was similar, a higher proportion of patients may be correctly classified by FIB-4.
Chronic hepatitis C virus (HCV) infection is emerging as an increasing burden to health and an important cause of morbidity and mortality worldwide,1-2 with an estimated global prevalence ranging between 2 to 3%, representing up to 170 million of chronic carries.3-4 In Brazil, although few studies have evaluated the prevalence of HCV infection, a recent population-based work conducted by the Ministry of Health including 19,634 individuals showed 1.38% of anti-HCV prevalence.5
HCV infection is associated with varying degrees of liver inflammation and progressive fibrosis, which may result in cirrhosis and hepatocellular carcinoma.6 During the course of infection, about one third of chronic hepatitis C patients will develop cirrhosis and 18% of those will ultimately progress to decompensated liver disease within five years.7-8 There is also an increased risk of developing hepatocellular carcinoma in patients with HCV-related cirrhosis, with an estimated annual incidence of 1% to 5%.9 Overall, more than 350,000 annual deaths are related to HCV infection worldwide.1
In chronic HCV infection, liver fibrosis is related to a process of continuous regeneration in response to constant liver tissue injury. The advanced and disordered fibrogenesis may result in progressive architectural distortion, scar tissue and, subsequently, liver cirrhosis.10 Currently, liver biopsy is the most important method for the diagnosis of liver fibrosis. Histological analysis of liver tissue has the advantage of the availability of validated classification systems to estimate the necroinflammatory activity and fibrosis, and the ability to indicate a differential diagnosis. Nevertheless, it is an invasive procedure with associated morbidity and subject to inter-and intraobserver variability in the assessment of fibrosis.11-13 For these reasons, noninvasive methods estimating HCV-related liver fibrosis were proposed over the last few years. Indirect or simple serum tests are hematological and biochemical parameters measured in peripheral blood that, when used solely or combined, may reflect the established liver fibrosis. Among those tests, the most important are the AST/ALT ratio, the FIB-4 and the AST to platelet ratio index (APRI).14-16 These models are based on routine and low cost tests, with techniques that can be easily performed, which increases the possibility of its use in daily clinical practice. The main purpose of this study was to validate and compare the performance of simple blood tests as noninvasive markers of significant liver fibrosis in patients with chronic HCV infection.
MATERIALS AND METHODSPatientsThis retrospective cross sectional study included consecutive adult patients with HCV infection who underwent percutaneous liver biopsy at our institution, between January 2001 and January 2010, after giving their written informed consent. Liver biopsies were performed using a 16-gauge suction needle. HCV infection was defined as a positive HCV-RNA by PCR (> 50 IU/ mL). Patients with the following conditions were excluded: prior interferon therapy, HBV and/or HIV co-infection, insufficient liver tissue for fibrosis staging, and incomplete data on blood counts and/or liver panel.
The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration and was approved by our institutional review board.
MethodsInformation about all HCV-infected subjects who underwent liver biopsy in our institution was reviewed and demographics, laboratory and other clinical variables were extracted from medical records.
The following laboratory variables were studied (expressed in absolute values): aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), platelet count, total protein, albumin, prothrombin activity. Only laboratory tests performed within 6 months from the date of the liver biopsy were used for this study.
The following models for estimating HCV-related fibrosis were evaluated:
Histological analysisAll patients had a liver biopsy irrespective of ALT levels. Histological features were analyzed using the METAVIR group scoring system. Fibrosis was staged on a scale of F0 to F4, as follows:
- •
F0 = no fibrosis.
- •
F1 = portal fibrosis without septa.
- •
F2 = few septa.
- •
F3 = numerous septa without cirrhosis.
- •
F4 = cirrhosis.
Significant fibrosis was defined by the presence of F2, F3 or F4 METAVIR stages.
Statistical analysisContinuous variables were compared using the Student’s t test or the Mann-Whitney test, when appropriate. Categorical variables were compared using the χ2 test. A P value of less than 0.05 was considered statistically significant. The predictive accuracies of the models were tested by measuring the areas under the receiver operating characteristic curves (AUROC) and by calculating the sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively). All tests were two-tailed and performed by SPSS, version 17.0 (SPSS Inc., Chicago, IL). ROC curve comparisons were performed using the MedCalc software package, version 9.3 (MedCalc Software, Mariakerke, Belgium), which employs calculation of the AUROC and 95% confidence intervals by the technique described by Hanley & McNeil.18
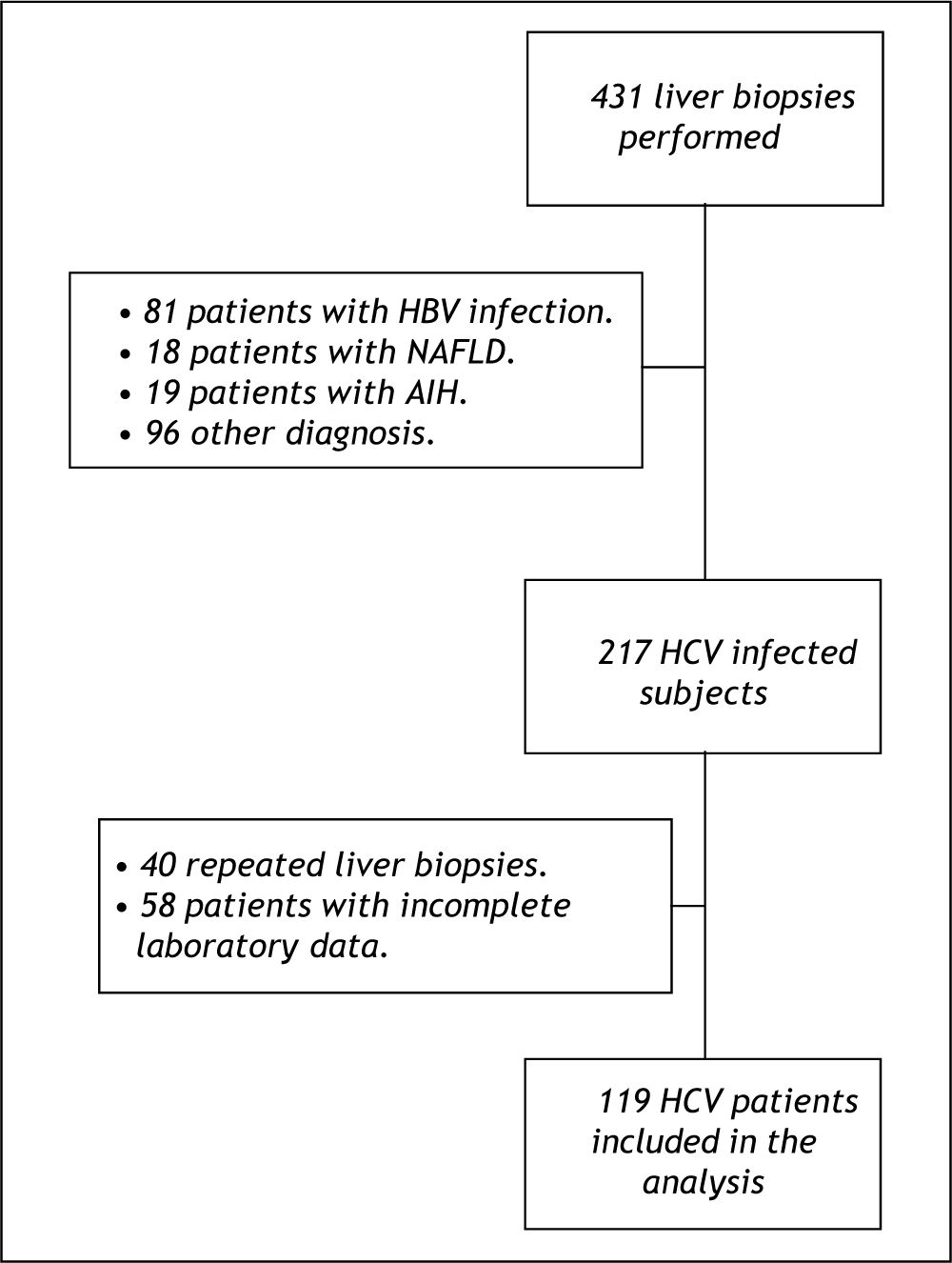
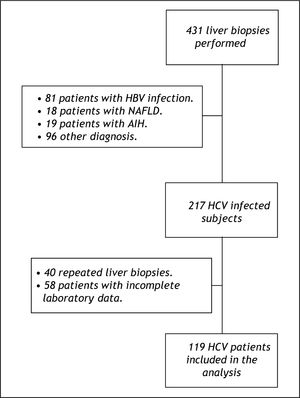
ResultsPatient characteristicsFrom January 2001 to January 2010, 431 liver biopsies were performed at our institution. Among these, 217 patients had HCV infection, of whom 40 were repeated biopsies and 58 had incomplete laboratory data and for this reason were not included in the study. Other 214 patients were not considered for the study for the following reasons: 81 were HBV-infected, 18 had non-alcoholic fatty liver disease, 19 had autoimmune hepatitis and 96 had other diagnoses. The final sample was composed by 119 HCV-infected subjects (Figure 1). No significant differences were observed when the patients who were excluded due to incomplete data were compared to the included ones regarding clinical, demographic, laboratory and histological variables available (data not shown).
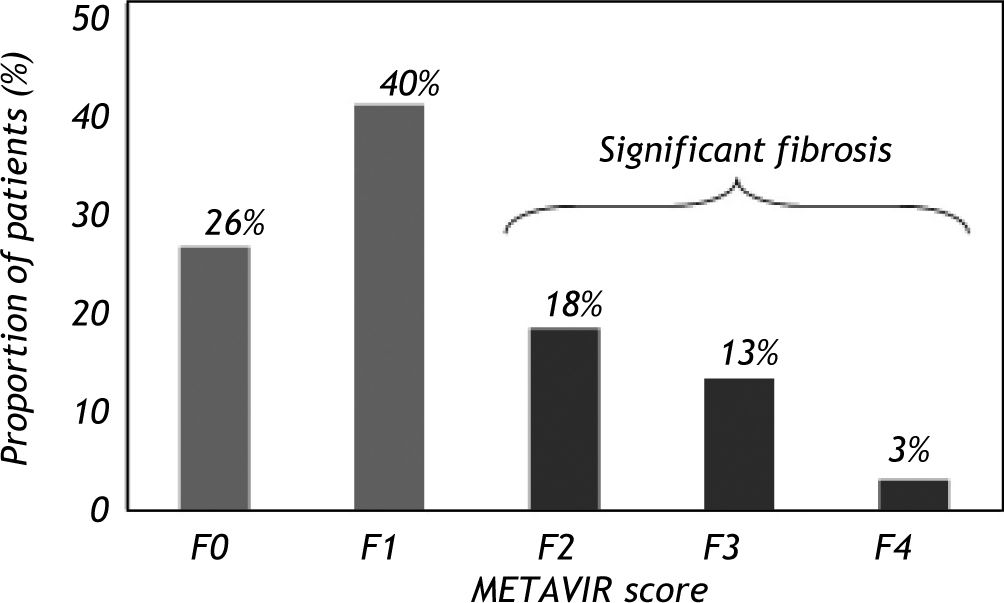
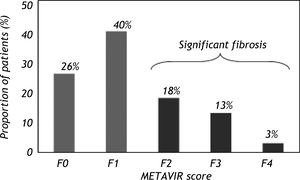
The mean age at biopsy was 43.7 ± 10.6 years and a male predominance was observed (62%). Significant alcohol consumption (> 50 g/day) was noted in 18% of the patients. The median levels of ALT were 78 IU/L, AST 48 IU/L and GGT 77 IU/L. The mean prothrombin activity was 86.4 ± 12.7% and the platelet count was 200.20 ± 54.59 109/L. Hepatic steatosis was observed in 54% of patients and significant fibrosis (F2-F3-F4) was identified in 41 patients (35%) (Figure 2). None of the included patients showed any signs or symptoms of hepatic decompensation.
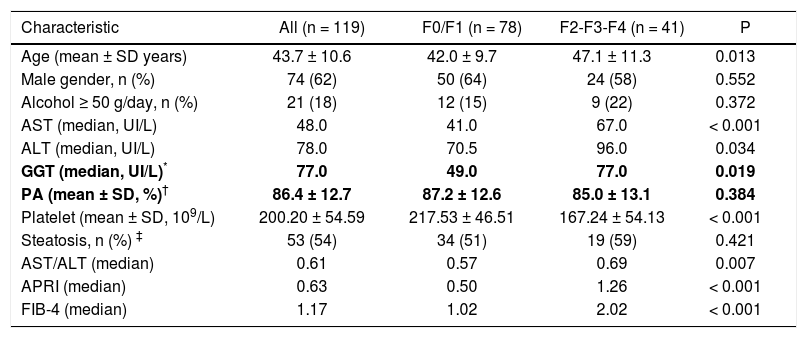
Factors associated with fibrosisAs shown in table 1, when we compared patients with and without significant fibrosis, univariate analysis revealed no differences regarding gender and history of significant alcohol consumption. Similarly, the mean prothrombin activity and the proportion of individuals with steatosis at biopsy were similar in both groups. Patients with significant fibrosis were older and presented with significantly higher median levels of AST, ALT and GGT. In addition, F2-F3-F4 patients exhibited lower platelet counts as compared to F0-F1 subjects. The median values of AST/ALT ratio (P = 0.007), APRI (P < 0.001) and FIB-4 (P < 0.001) were significantly higher in those with significant fibrosis.
Demographic, clinical and biochemical features of included patients.
| Characteristic | All (n = 119) | F0/F1 (n = 78) | F2-F3-F4 (n = 41) | P |
|---|---|---|---|---|
| Age (mean ± SD years) | 43.7 ± 10.6 | 42.0 ± 9.7 | 47.1 ± 11.3 | 0.013 |
| Male gender, n (%) | 74 (62) | 50 (64) | 24 (58) | 0.552 |
| Alcohol ≥ 50 g/day, n (%) | 21 (18) | 12 (15) | 9 (22) | 0.372 |
| AST (median, UI/L) | 48.0 | 41.0 | 67.0 | < 0.001 |
| ALT (median, UI/L) | 78.0 | 70.5 | 96.0 | 0.034 |
| GGT (median, UI/L)* | 77.0 | 49.0 | 77.0 | 0.019 |
| PA (mean ± SD, %)† | 86.4 ± 12.7 | 87.2 ± 12.6 | 85.0 ± 13.1 | 0.384 |
| Platelet (mean ± SD, 109/L) | 200.20 ± 54.59 | 217.53 ± 46.51 | 167.24 ± 54.13 | < 0.001 |
| Steatosis, n (%) ‡ | 53 (54) | 34 (51) | 19 (59) | 0.421 |
| AST/ALT (median) | 0.61 | 0.57 | 0.69 | 0.007 |
| APRI (median) | 0.63 | 0.50 | 1.26 | < 0.001 |
| FIB-4 (median) | 1.17 | 1.02 | 2.02 | < 0.001 |
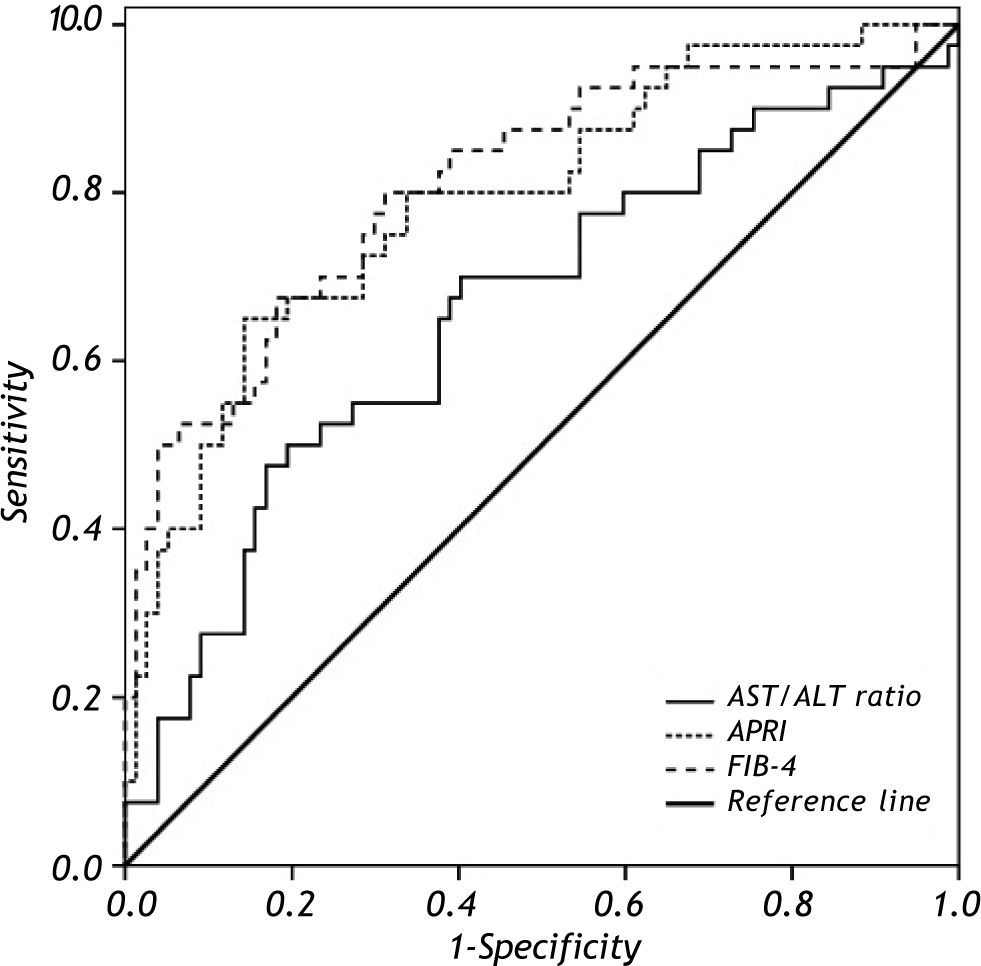
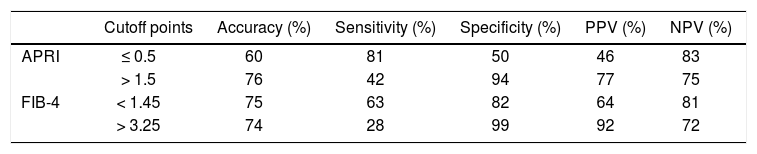
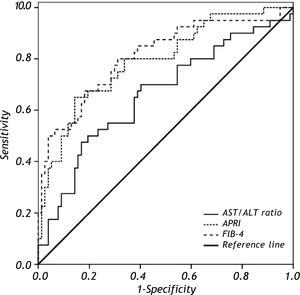
To discriminate subjects with significant fibrosis, the observed AUROC for AST/ALT ratio, APRI and FIB-4 were 0.661 ± 0.055, 0.793 ± 0.047 and 0.811 ± 0.045, respectively (Figure 3). In the comparison of the AUROCs, no differences were observed for APRI vs. FIB-4 (P = 0.575). FIB-4 exhibited a significantly higher AUROC as compared to AST/ALT ratio (P = 0.014) and there was a trend toward higher AUROC for APRI when compared to the AST/ALT ratio (P = 0.054). Table 2 depicts the diagnostic performance of the APRI and FIB-4 models for the evaluation of significant liver fibrosis by using the classic cutoffs. When compared to liver biopsy, APRI values > 1.5 showed a PPV of 77% for the diagnosis of significant fibrosis, while APRI ≤ 0.5 excluded significant fibrosis with a NPV of 83%. Among the 119 included patients, 69 (58%) were classified by APRI (scores ≤ 0.5 or > 1.5), and 13 of them (19%) were misdiagnosed by this model. Similar results were observed by applying the FIB-4 original cutoffs. FIB-4 > 3.25 showed a PPV of 92% for the presence of significant fibrosis, and scores < 1.45 excluded significant fibrosis with a NPV of 81%. Ninety patients (77%) were classified by FIB-4 and 16 (18%) of these were misdiagnosed. If biopsy indication was based only on those models and restricted to scores in the intermediate range, 47% of liver biopsies could have been correctly avoided by using the APRI and 63% with FIB-4.
ROC curves of AST/ALT ratio, APRI and FIB-4 in distinguishing significant liver fibrosis (F2-F3-F4) from nonsignificant liver fibrosis (F0-F1). Comparison of AUROCs showed superior diagnostic accuracy of FIB-4 (P = 0.014) and a trend toward higher AUROC for APRI (P = 0.054) when compared to the AST/ALT ratio.
Diagnostic accuracy of APRI and FIB-4 models in predicting significant fibrosis (METAVIR F2-F3-F4).
| Cutoff points | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| APRI | ≤ 0.5 | 60 | 81 | 50 | 46 | 83 |
| > 1.5 | 76 | 42 | 94 | 77 | 75 | |
| FIB-4 | < 1.45 | 75 | 63 | 82 | 64 | 81 |
| > 3.25 | 74 | 28 | 99 | 92 | 72 |
PPV: positive predictive value. NPV: negative predictive value.
The use of a quick, safe and accurate tool for estimating the stage of liver fibrosis in patients with chronic HCV infection is now considered essential in the clinical evaluation and follow-up of these patients.19-20 Determining the stage of fibrosis and understanding the possible factors associated with the rate of liver fibrosis progression (such as gender, age at infection, obesity, diabetes mellitus, daily alcohol intake, hepatic iron content), it is possible to obtain relevant data on the prognosis of the disease, which can also guide future therapeutic decisions.
Although widely performed and acknowledged as the gold standard procedure for the staging of hepatic fibrosis, liver biopsy is an invasive technique with associated morbidity and some important limitations.11-13 Hence, several noninvasive tests such as APRI, FIB-4 and AST/ALT ratio have been developed, compared and validated as markers of liver fibrosis in patients with chronic liver diseases, especially those infected by HCV.
APRI is a simple noninvasive method to identify significant fibrosis or cirrhosis, based on simple and widely available blood tests. It was initially proposed and validated for patients with chronic Hepatitis C and then it was subsequently validated for several liver diseases.15,21-24 To discriminate subjects with significant fibrosis (F2-F3-F4), the AUROC of APRI in the present study was 0.793 ± 0.047, with a PPV of 77% for values > 1.5, and NPV of 83% for APRI ≤ 0.5. In addition, a cutoff of 0.5 showed 81% of sensitivity and 50% of specificity, while a cutoff of 1.5 was more specific (94%) and less sensitive (42%). When the APRI model was initially described in the original study of Wai, et al.,15 the AUROC for significant fibrosis was 0.88 in the validation cohort, with PPV of 91% for values of APRI > 1.5 and NPV of 90% for values ≤ 0.5. These results were superior to those observed in the present study. However, two systematic reviews that investigated the performance of APRI in HCV-infected patients showed similar results to the data presented here. The first one, which included 19 studies that analyzed APRI as a predictor of significant fibrosis in a total of 3,788 patients,25 showed a grouped AUROC of 0.76. Considering the mean prevalence for significant fibrosis of 47% observed in the reviewed studies, the estimated PPV and NPV for the cutoff point of ≤ 0.5 were 59% and 75% respectively, while for APRI values > 1.5, the PPV was 77% and NPV was 61%. The second meta-analysis performed by Lin, et al.26 showed similar APRI performance for predicting significant fibrosis related to HCV monoinfection when compared to the prior review by Shaheen and Myers.25 Analyzing 33 studies that included a total of 6.259 patients, the AUROC was 0.77, with sensitivity of 74% and specificity of 49% for APRI ≤ 0.5. The cutoff value of > 1.5 was more specific (93%), but less sensitive (37%). The mean prevalence of significant fibrosis was 46%, which corresponded to a PPV of 55% and a NPV of 69% to APRI values ≤ 0.5, and estimated PPV and NPV of 82% and 63%, respectively, for values > 1.5. Some factors such as the use of different histological classifications, limitations inherent to liver biopsy and different prevalences of significant fibrosis may explain the disparity of the performance of noninvasive markers across distinct studies.
The FIB-4 is also a noninvasive method for the evaluation of liver fibrosis, based on simple variables such as age, AST, ALT and platelet count. It was initially proposed by researchers of the APRICOT study (AIDS Pegasys Ribavirin International Coinfection Trial) to evaluate the presence of liver fibrosis in HIV/HCV coinfected patients16 and was subsequently validated in HCV monoinfected patients.27 In the present study, the AUROC of FIB-4 to detect significant fibrosis was 0.811 ± 0.045, with a sensitivity of 63% and 28% and a specificity of 82% and 99% for cutoffs of < 1.45 and > 3.25, respectively. The NPV was 81% for FIB-4 values < 1.45, whereas the PPV for a cutoff > 3.25 was 92%. Similarly, Vallet-Pichard, et al.27 observed an AUROC of 0.85 for identifying fibrosis (F3-F4), in HCV monoinfected patients. The cutoff point < 1.45 showed a NPV of 94.7%, with sensitivity and specificity of 74.3% and 80.1%, respectively. Fib-4 values > 3.25 have a PPV of 82.1%, with lower sensitivity (37.6%) and higher specificity (98.2%). Similar results were obtained by other authors who evaluated the performance of FIB-4 in HCV monoinfected patients, with the AUROCs ranging between 0.732 and 0.799.28-32
When the diagnostic performance of APRI was compared to the FIB-4, there were no significant differences between the AUROCs (0.793 vs. 0.811, P = 0.575). These results are consistent with the findings of most studies in which the performances of the APRI and FIB-4 models were assessed by AUROC (28, 31-34). In a recent study, Hsieh, et al. reported an AUROC of 0.651 for the APRI and 0.785 for FIB-4 in the detection of significant fibrosis. Although the AUROC for APRI was unexpectedly low, no statistically significant differences were observed for comparison between the models.31 Similar findings were described by Ahmad, et al. in a study that included 157 HCV-infected patients where APRI exhibited an AUROC of 0.715 for significant fibrosis and FIB-4 showed an AUROC of 0.732 for advanced fibrosis (F3-F4).32 Even though the ARUOCs were somewhat lower than those observed in the present study, no differences for comparison of the AUROCs were observed in the Pakistani study.
The proportion of biopsies that could have been correctly avoided in the present study was substantially higher with FIB-4 than with APRI (63% vs. 47%). Although there were few data specifically concerning the proportion of correct classifications, similar results were demonstrated in a recent study that included 340 HCV-infected in which a higher proportion of individuals were correctly classified by FIB-4 when compared with APRI (59% vs. 48%), despite the similar AUROC for both tests (0.85 vs. 0.83).34 These findings suggest that, despite having a more complex calculation, taking into account the greater proportion of correct classifications FIB-4 is probably a more useful tool for incorporation into daily practice.
The AST/ALT ratio has been used for several years as a noninvasive method for assessing the severity of chronic liver diseases, including chronic HCV infection.35-37 Although some studies have found promising results, its performance as a noninvasive marker of fibrosis is generally low, especially in the diagnosis of less advanced stages of fibrosis.38-39 These findings are corroborated by the results of this study, in which the AUROC for the AST/ALT ratio in the diagnosis of significant fibrosis was 0.661 ± 0.055. When comparing the AUROC of all the biomarkers assessed, we can observe a superiority of APRI and FIB-4 in relation to the AST/ ALT ratio. Similar results were described by Lackner, et al.40 who showed a higher diagnostic accuracy for APRI as compared to the AST/ALT ratio for the diagnosis of significant fibrosis (AUROC 0.80 vs. 0.57, P < 0.05).
We acknowledge some limitations to the present study. The inclusion of retrospectively collected data and the small proportion of patients with cirrhosis could indicate selection bias. However, when HCV carriers who were excluded from the analysis were compared to those included, there were no significant differences either in clinical or histological variables. Moreover, the prevalence of different stages of fibrosis is variable between studies and it is possible that the findings of this sample reflect local peculiarities. Another potential limitation is the usage of liver biopsy as the gold standard for evaluation of fibrosis staging, as this method has several limitations as discussed above. However, this problem is common to all studies that evaluate noninvasive markers of liver fibrosis and, up to the present, biopsy remains the main tool for quantification of liver fibrosis.
This study demonstrated that APRI and FIB-4 models were superior than the AST/ALT ratio in the diagnosis of significant fibrosis in patients with chronic HCV infection. Although the overall performance of the APRI and FIB-4 was similar, the gain observed in the proportion of patients classified by FIB-4 may represent a greater number of biopsies correctly avoided in clinical practice, which is the ultimate goal of the search for noninvasive markers of liver fibrosis.
Conflicts of InterestNothing to report
Abbreviations- •
ALP: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
APRI: AST to platelet ratio index.
- •
AST: aspartate aminotransferase.
- •
GGT: gamma-glutamyltransferase.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
NPV: negative predictive value.
- •
PPV: positive predictive value.