Introduction and aim. It is well known that development of acute kidney injury (AKI) increases mortality in hospitalized cirrhotic patients; therefore many novel markers have been studied for early detection, differential diagnosis and prognosis in cirrhotic patients with AKI. The aim of the current work is to evaluate urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL) as a diagnostic biomarker for different causes of acute kidney injury in liver cirrhosis and to assess it as a prognostic marker.
Material and meth-ods. Out of 83 cirrhotic patients with AKI admitted between October 2015 and June 2016; 70 patients were included in this prospective study. Routine laboratory tests, uNGAL and fractional excretion of Na were obtained on admission. End points were death or improvement of kidney function and discharge.
Results. The patients included in our study were 41 males and 29 females with mean age 54.27 ± 6.08 years. HCV was the etiology of cirrhosis in 69 cases while one had combined HBV and HCV infection. More than 50% of patients were classified as Child C. Causes of kidney injury were prerenal, hepatorenal syndrome (HRS) and intrinsic tubular injury (iAKI) in 39 patients (55.7%), 17 patients (24.3%) and 14 patients (20%) respectively. mean value of uNGAL in prerenal, HRS and iAKI was 21.70 ± 7.31, 115.53 ± 68.19 and 240.83 ± 116.94 ng/mg creatinine respectively. MELD above 20 and uNGL above 32 were predictors of mortality.
Conclusion. A single baseline measurement of uNGAL level has the ability to determine type of kidney dysfunction in cirrhotic patients, perhaps accelerating management decisions and improving outcomes.
Liver cirrhosis is a common disease in Egypt which has the highest prevalence of HCV infection worldwide.1
Acute kidney injury (AKI) in patients with cirrhosis is a serious and common complication due to hemodynamic changes associated with cirrhosis. It is worth noting that about one-fifth of hospitalized cirrhotic patients will develop AKI and once AKI occurs, 4 folds increase in the risk of mortality is reported.2
In cirrhosis, differential diagnosis of AKI includes prerenal azotemia, hepatorenal syndrome (HRS), and intrinsic acute kidney injury (iAKI).3
Recent advances in understanding the early stress response of kidney tubule cells to ischemic injury have provided several novel biomarkers for AKI.4
Many novel urinary biomarkers of kidney injury have been studied for early detection, differential diagnosis and prognosis of AKI. These urinary markers have led to a revolution in the study of AKI cases.5 Neutrophil gelatinase-associated lipocalin (NGAL) is a novel and promising biomarker for diagnosing acute kidney injury (AKI). NGAL showed potential both to detect AKI and to diagnose HRS.6 Several studies have demonstrated the utility of early NGAL measurements for predicting the severity and clinical outcomes of AKI.7
This study aimed to evaluate (uNGAL) as a diagnostic biomarker for different etiologies of acute kidney injury in liver cirrhosis. Secondary aim was to investigate if there is a correlation between uNGAL level and inpatient mortality in such cases.
Material and MethodsWithin period of October 2015 and June 2016, 83 cirrhotic patients with AKI who admitted to TBRI were assessed. Diagnosis of cirrhosis was based on clinical, biochemical and ultra-sonographic data while acute kidney injury network (AKIN) criteria was used to define AKI.8
Patients with urinary obstruction, proteinuria > 500 mg/day, anuria and urinary tract infection (urinary WBCs > 10 per high power field or positive urinary culture) were excluded. Those on renal replacement therapy and patients who underwent liver or kidney transplantation were also excluded.
After obtaining the approval of the Research and Ethics Committee of Ain Shams University, in accordance with local research governance requirements and after signing informed consent patients were included.
Clinical and biochemical data including CBC, liver profile and kidney function tests were collected at the time of admission with reference to previous patients’ data done in outpatient clinics. Child-Pugh and MELD scores were calculated based on laboratory data obtained on admission. Estimated GFR was calculated using Modification of Diet in Renal Disease (MDRD) formula. Fresh urine samples were taken to measure the urinary levels of sodium, creatinine, and NGAL. Urine sodium was measured by ion-selective electrode assay and used to determine fractional excretion of sodium (FENa). Urine sample was immediately centrifuged, separated, and stored at-80 C and urinary NGAL was measured using a NGAL ELISA kit (BioVendor GmbH, Germany) in relation to urinary creatinine.
Ten patients with cirrhosis with no AKI (7 were Child C and 3 were Child B with mean age 55.12 ± 5.89) were examined for uNGAL for standardization of results. Urine analysis and abdominal ultrasound were performed for all participants. All the patients were followed up until death or discharge.
Definitions used for different types of AKIHRS: Defined according to criteria published by International club of ascites9
- •
Cirrhosis with ascites.
- •
Serum creatinine > 133 µmol/l (1.5 mg/dL).
- •
No improvement of serum creatinine (decrease to a level of < 133 µmol/l) after at least 2 days with diuretic withdrawal and volume expansion with albumin. The recommended dose of albumin is 1 g/kg of body weight per day up to a maximum of 100 g/day.
- •
Absence of shock.
- •
No current or recent treatment with nephrotoxic drugs.
- •
Absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/day, microhaematuria (> 50 red blood cells per high power field) and/or abnormal renal ultrasonography.
A transient increase in Scr to > 1.5 mg/dL and 0.3 mg/dL above baseline, with subsequent, decreased in sCr to < 1.5 mg/dL or to mean baseline creatinine within 48 h of treatment with diuretic withdrawal and intravenous hydration.7
Intrinsic acute kidney injuryDefined as acute elevation in Scr to > 1.5 mg/dL and 0.3 mg/dL above baseline, not responding with 48 h of volume resuscitation and not meeting the criteria for HRS.7
Statistical AnalysisAll data were collected, tabulated and statistically analyzed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous Quantitative variables were expressed as the mean ± SD & median (range), and categorical qualitative variables were expressed as absolute frequencies “number” & relative frequencies (percentage). Continuous data were checked for normality by using Shapiro Walk test. Student’s t-test was used to compare two groups of normally distributed data while MannWhitney U test was used for non-normally distributed data. One way ANOVA test was used to compare more than two groups of normally distributed data while Kraskall Wallis H test was used for non-normally distributed data. Categorical data were compared using the Chi-square (χ2) test or Fisher’s exact test when appropriate. Receiver operating characteristic (ROC) curve analysis was used to identify optimal cut-off values of uNAGAL with maximum sensitivity and specificity for diagnosis of AKI and prediction of mortality. Overall Survival (OS) was calculated as the time from diagnosis to death or to discharge from hospital (censored). Stratification OS was done according to kidney function groups and uNAGAL groups. These time-to-death distributions were estimated using the method of Kaplan-Meier plot and compared using two-sided exact log-rank test. p < 0.05 was considered statistically significant (S) and p > 0.05 was considered non statistically significant (NS).
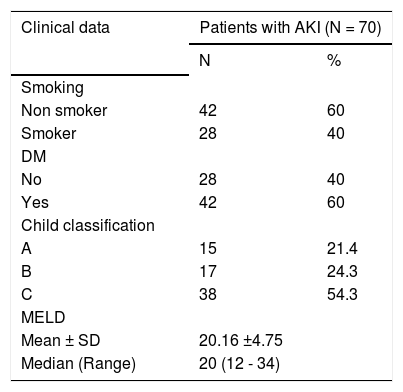
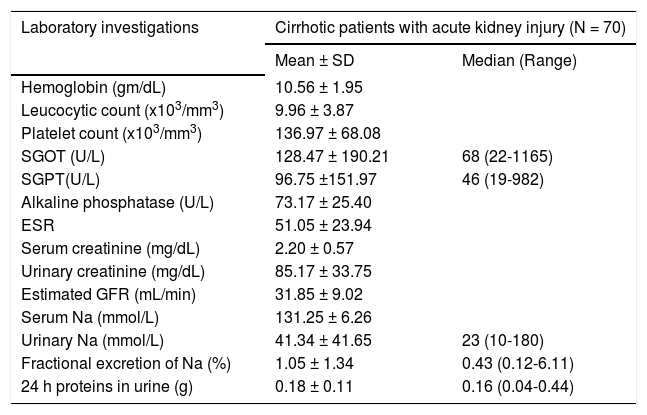
Results70 cirrhotic patients (41 of them were males (58.6%) and 29 were females (41.4%) with Mean age 54.27 ± 6.08 years old) fulfilling inclusion criteria were enrolled. In 69 patients (98.6%) HCV was the etiology of liver cirrhosis and only one patient (1.4%) had combined HCV and HBV infection. Most of the studied patients had decompensated liver disease with 54.3% of included patients classified as Child C and baseline mean MELD score was 20.16 ± 4.75. Clinical and laboratory characteristics of included patients are shown in tables 1 and 2.
Clinical features of the studied patients.
| Clinical data | Patients with AKI (N = 70) | |
|---|---|---|
| N | % | |
| Smoking | ||
| Non smoker | 42 | 60 |
| Smoker | 28 | 40 |
| DM | ||
| No | 28 | 40 |
| Yes | 42 | 60 |
| Child classification | ||
| A | 15 | 21.4 |
| B | 17 | 24.3 |
| C | 38 | 54.3 |
| MELD | ||
| Mean ± SD | 20.16 ±4.75 | |
| Median (Range) | 20 (12 - 34) | |
DM: diabetes mellitus. MELD: model for end stage liver disease.
Laboratory characteristics of studied patients.
| Laboratory investigations | Cirrhotic patients with acute kidney injury (N = 70) | |
|---|---|---|
| Mean ± SD | Median (Range) | |
| Hemoglobin (gm/dL) | 10.56 ± 1.95 | |
| Leucocytic count (x103/mm3) | 9.96 ± 3.87 | |
| Platelet count (x103/mm3) | 136.97 ± 68.08 | |
| SGOT (U/L) | 128.47 ± 190.21 | 68 (22-1165) |
| SGPT(U/L) | 96.75 ±151.97 | 46 (19-982) |
| Alkaline phosphatase (U/L) | 73.17 ± 25.40 | |
| ESR | 51.05 ± 23.94 | |
| Serum creatinine (mg/dL) | 2.20 ± 0.57 | |
| Urinary creatinine (mg/dL) | 85.17 ± 33.75 | |
| Estimated GFR (mL/min) | 31.85 ± 9.02 | |
| Serum Na (mmol/L) | 131.25 ± 6.26 | |
| Urinary Na (mmol/L) | 41.34 ± 41.65 | 23 (10-180) |
| Fractional excretion of Na (%) | 1.05 ± 1.34 | 0.43 (0.12-6.11) |
| 24 h proteins in urine (g) | 0.18 ± 0.11 | 0.16 (0.04-0.44) |
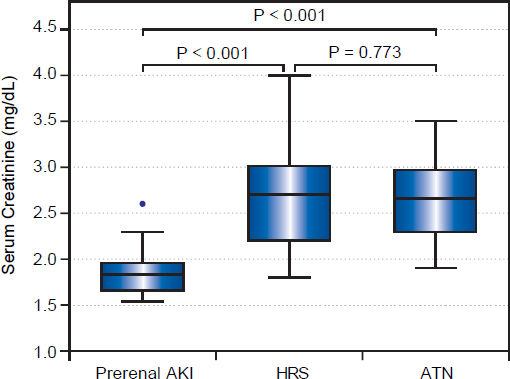
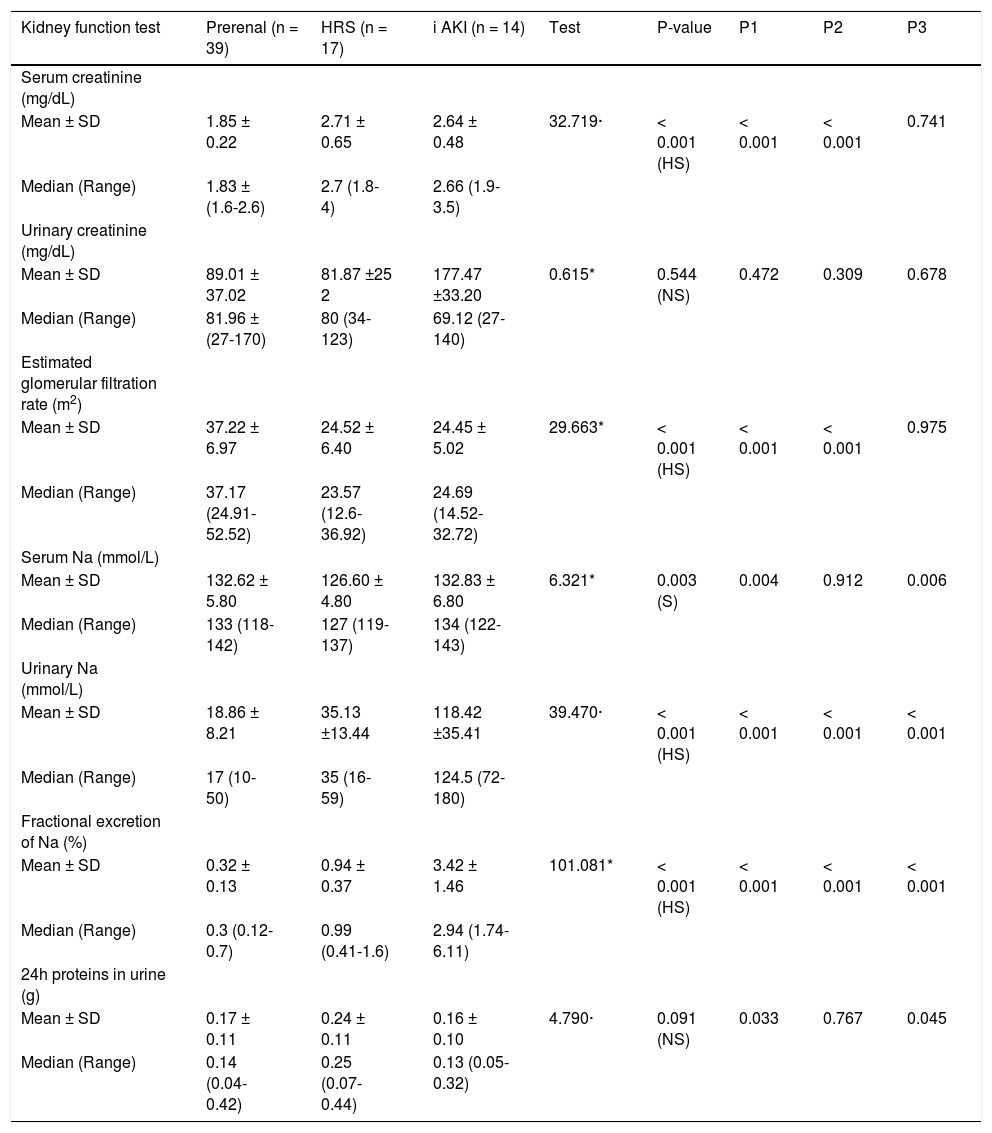
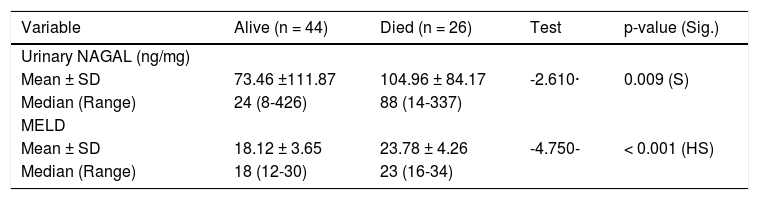
Among included patients, 39 patients (55.7%) turned out to have prerenal AKI, while 17 patients (24.3%) and 14 patients (20%) were diagnosed as HRS and iAKI respectively. Although serum creatinine and eGFR were significantly higher in patients with HRS and iAKI, they were not able to differentiate between both of them as shown in table 3 and figure 1.
Kidney function tests in different groups.
| Kidney function test | Prerenal (n = 39) | HRS (n = 17) | i AKI (n = 14) | Test | P-value | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|---|
| Serum creatinine (mg/dL) | ||||||||
| Mean ± SD | 1.85 ± 0.22 | 2.71 ± 0.65 | 2.64 ± 0.48 | 32.719· | < 0.001 (HS) | < 0.001 | < 0.001 | 0.741 |
| Median (Range) | 1.83 ± (1.6-2.6) | 2.7 (1.8-4) | 2.66 (1.9-3.5) | |||||
| Urinary creatinine (mg/dL) | ||||||||
| Mean ± SD | 89.01 ± 37.02 | 81.87 ±25 2 | 177.47 ±33.20 | 0.615* | 0.544 (NS) | 0.472 | 0.309 | 0.678 |
| Median (Range) | 81.96 ± (27-170) | 80 (34-123) | 69.12 (27-140) | |||||
| Estimated glomerular filtration rate (m2) | ||||||||
| Mean ± SD | 37.22 ± 6.97 | 24.52 ± 6.40 | 24.45 ± 5.02 | 29.663* | < 0.001 (HS) | < 0.001 | < 0.001 | 0.975 |
| Median (Range) | 37.17 (24.91-52.52) | 23.57 (12.6-36.92) | 24.69 (14.52-32.72) | |||||
| Serum Na (mmol/L) | ||||||||
| Mean ± SD | 132.62 ± 5.80 | 126.60 ± 4.80 | 132.83 ± 6.80 | 6.321* | 0.003 (S) | 0.004 | 0.912 | 0.006 |
| Median (Range) | 133 (118-142) | 127 (119-137) | 134 (122-143) | |||||
| Urinary Na (mmol/L) | ||||||||
| Mean ± SD | 18.86 ± 8.21 | 35.13 ±13.44 | 118.42 ±35.41 | 39.470· | < 0.001 (HS) | < 0.001 | < 0.001 | < 0.001 |
| Median (Range) | 17 (10-50) | 35 (16-59) | 124.5 (72-180) | |||||
| Fractional excretion of Na (%) | ||||||||
| Mean ± SD | 0.32 ± 0.13 | 0.94 ± 0.37 | 3.42 ± 1.46 | 101.081* | < 0.001 (HS) | < 0.001 | < 0.001 | < 0.001 |
| Median (Range) | 0.3 (0.12-0.7) | 0.99 (0.41-1.6) | 2.94 (1.74-6.11) | |||||
| 24h proteins in urine (g) | ||||||||
| Mean ± SD | 0.17 ± 0.11 | 0.24 ± 0.11 | 0.16 ± 0.10 | 4.790· | 0.091 (NS) | 0.033 | 0.767 | 0.045 |
| Median (Range) | 0.14 (0.04-0.42) | 0.25 (0.07-0.44) | 0.13 (0.05-0.32) |
HRS: hepatorenal syndrome. ¡AKI: acute tubular necrosis. uNGAL: urinary neutrophil gelatinase associated lipocalin. Pi: prerenal against HRS. P2: prerenal against iAKI. P3: HRS against iAKI.
Urinary sodium and FeNa showed significant mean values difference among different groups, however, there was considerable overlap in ranges between prerenal group and HRS group as shown in table 3.
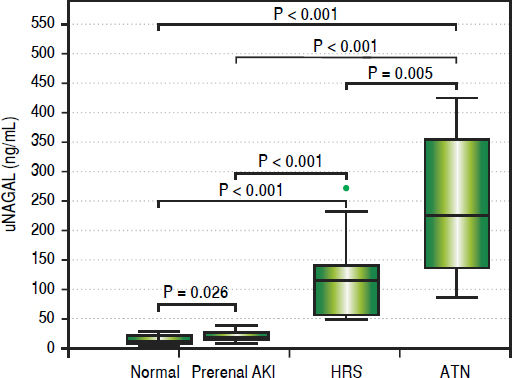
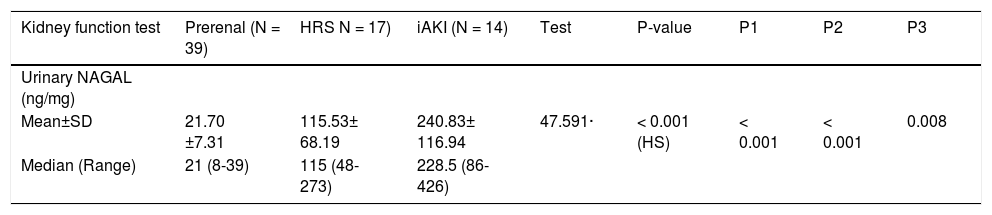
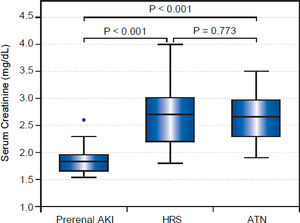
On the other hand, uNGAL showed evident variation of values among different groups with mean value of urinary NGAL in prerenal, HRS and iAKI was 21.70 ± 7.31, 115.53 ± 68.19 and 240.83 ± 116.94 ng/mg creatinine respectively as showed in table 4 and figure 2.
Urinary NAGAL (ng/mg) in studied groups.
| Kidney function test | Prerenal (N = 39) | HRS N = 17) | iAKI (N = 14) | Test | P-value | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|---|
| Urinary NAGAL (ng/mg) | ||||||||
| Mean±SD | 21.70 ±7.31 | 115.53± 68.19 | 240.83± 116.94 | 47.591· | < 0.001 (HS) | < 0.001 | < 0.001 | 0.008 |
| Median (Range) | 21 (8-39) | 115 (48-273) | 228.5 (86-426) |
HRS: hepatorenal syndrome. iAKI: acute tubular necrosis. uNGAL: urinary neutrophil gelatinase associated lipocalin. PI: prerenal against HRS. P2: prerenal against iAKI. P3: HRS against iAKI.
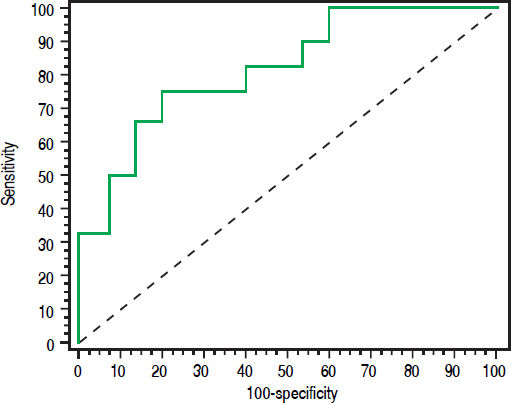
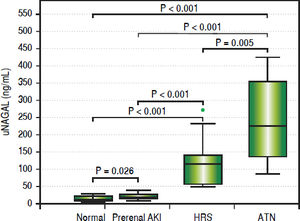
Urinary NAGAL > 143 (ng/mL) has the ability to differentiate iAKI from HRS in cirrhotic patients with area under ROC curve is 0.822 (sensitivity 75% and specificity 80%) with a positive predictive value (PPV) of 84.3% and negative predictive value (NPV) of 69.1%. ROC curve shown in figure 3.
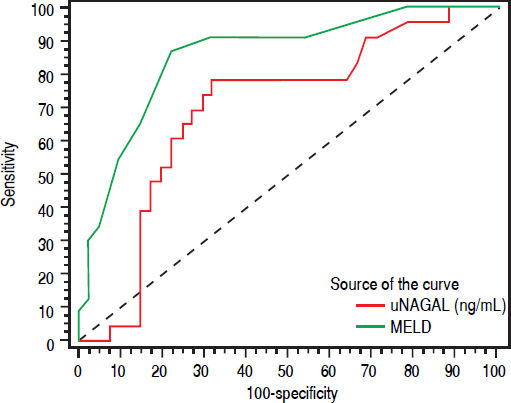
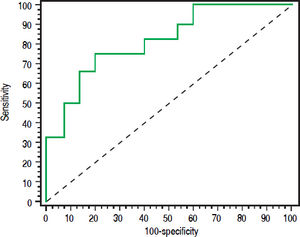
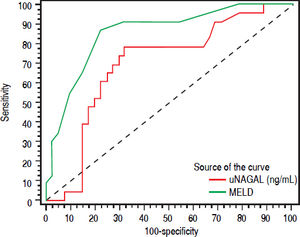
uNGAL and prognosis26 patients (37.1%) died during the same admission. Patients who died had higher baseline NGAL and MELD scores than patients who survived. Median MELD and uNGAL in patients who died was 23 (16-34) and 88 (14337) ng/mL respectively while they were 18 (12-30) and 24 (8-426) ng/mL in patients who improved and discharged. ROC curve analysis showed that Both MELD score more than 20 and urinary NGAL more than 32 has the ability to predict short-term mortality (during the same hospitalization). Figure 4 shows Receiver operating characteristic (ROC) curve of Urinary NAGAL (ng/mg) and MELD as predictors for mortality in cirrhotic patients with AKI.
DiscussionUrinary NGAL is a novel and promising biomarker that shows good sensitivity and specificity for detection and differentiating different causes of AKI in cirrhotic patients.7
After renal injury uNGAL is elevated in both urine and plasma, however, its urinary concentration is at least five times more than its plasma level making its detection in urine easier.7
Although serum creatinine levels are elevated in patients with AKI, it poorly differentiates AKI types, serum creatinine levels are higher in patients with HRS compared to prerenal azotemia but similar to patients with intrinsic acute kidney injury (iAKI), a finding that was reported in the previous manuscripts.7 That’s why the diagnosis of the cause of AKI may be delayed and this explains the need for another biomarker that can easily and early diagnose type of AKI in cirrhotic patients.
In the current study, uNGAL levels were significantly different in each category of AKI: highest in iAKI, intermediate in HRS and low in prerenal disease.
It is well known that in patients with prerenal elevation in kidney functions there is no intrinsic tubular damage; on contrary patients with ATN have severe tubular damage.
On the other hand and although hemodynamic changes in HRS with renal vascular constriction and diminished GFR can be considered prerenal state, however, minor kidney tubular and glomerular damage in HRS kidneys was reported in pathological studies, mostly due to chronic activation of angiotensin-aldosterone signaling. That is why uNGAL levels in patients with HRS are midway between those with prerenal and ATN.
The mean values of urinary NGAL in prerenal azotemia, HRS and ATN patients in our study were 21.70 ± 7.31 ng/mg creatinine, 115.53 ± 68.19 ng/mg creatinine, and 240.83 ± 116.94 ng/mg creatinine respectively. These results were consistent with previous reports with similar inclusions criteria.7,10 On the other hand higher values of urinary NGAL were noticed by research groups11,12 who didn’t exclude patients with urinary tract infections which itself induces uNGAL production.
Urinary NAGAL (ng/mg) and MELD score values in patients who survived and in those who died.
| Variable | Alive (n = 44) | Died (n = 26) | Test | p-value (Sig.) |
|---|---|---|---|---|
| Urinary NAGAL (ng/mg) | ||||
| Mean ± SD | 73.46 ±111.87 | 104.96 ± 84.17 | -2.610· | 0.009 (S) |
| Median (Range) | 24 (8-426) | 88 (14-337) | ||
| MELD | ||||
| Mean ± SD | 18.12 ± 3.65 | 23.78 ± 4.26 | -4.750- | < 0.001 (HS) |
| Median (Range) | 18 (12-30) | 23 (16-34) |
uNGAL: urinary neutrophil gelatinase associated lipocalin. MELD: model for end stage liver disease.
In the current study, urinary NGAL at 143 ng/mg creatinine showed the ability to differentiate between patients with iAKI and other causes of AKI.
Our results also emphasized that serum creatinine alone can’t differentiate ATN from HRS. Also, urinary creatinine and FeNa showed overlap in value ranges between patients with prerenal injury and HRS and mean values in both groups were less than 1%.
Higher MELD score was associated with mortality risk and levels more than 20 can predict inpatient mortality with area under the curve 0.867, sensitivity 87.5% and specificity 80.43%. Urinary NGAL level more than 32 also has the ability to predict mortality with area under the curve 0.698, sensitivity 79.17% and specificity 65.22%. However, ROC curve for both showed that MELD score (more than 20) had better predictive ability than uNGAL level more than 32. Combining both uNGAL and MELD didn’t add additional predictive benefit.
Verma and colleagues found that uNGAL more than 110 is a predictor of mortality in cirrhotic patients with AKI, however, in our study, the highest levels of uNGAL were noticed in patients with iAKI which may be reversible. On the other hand, patients with HRS showed moderate elevation in uNGAL however they had the worst prognosis.
ConclusionsA single baseline uNGAL measurement obtained at hospitalization has the ability to assist in determining type of kidney injury, and this may help decision taking in those critical patients and improving outcome. Urinary NGAL and MELD score are predictors of mortality in cirrhotic patients with AKI. However, MELD score above 20 showed a better predictive ability.
Abbreviations- •
AKI: acute kidney injury.
- •
ANOVA: analysis of variance.
- •
ATN: acute tubular necrosis.
- •
eGFR: estimated glomerular filtration rate.
- •
FENa: fractional excretion of sodium.
- •
HRS: hepatorenal syndrome.
- •
iAKI: intrinsic acute kidney injury.
- •
MDRD: modification of Diet in Renal Disease.
- •
MELD: model for end-stage liver disease.
- •
ROC: receiver operating characteristic.
- •
TBRI: theodor Bilharz Research Institute.
- •
uNGAL: urinary Neutrophil Gelatinase-Associated Lipocalin.
The authors report that there are no disclosures relevant to this publication.