Background. Bacterial infections are frequent complications in patients with cirrhosis. Since they are associated with poor outcomes, antibiotics are frequently over-prescribed. Surrogate markers of bacterial infections, like procalcitonin, are needed to better discriminate between infected and not infected patients.
Aims. To evaluated the diagnostic accuracy of an ultra-sensitive procalcitonin assay for the diagnosis of bacterial infections in patients with cirrhosis.
Material and methods. In a single-center prospective study, we determined the basal levels of procalcitonin in 106 episodes of admissions to the emergency department in 84 cirrhotic patients. Patients were classified as infected or not infected by two independent hepatologists blinded to the procalcitonin result.
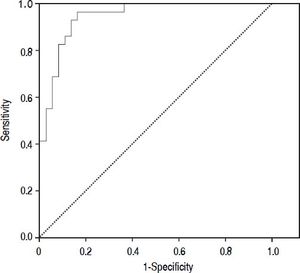
Results. The prevalence of bacterial infection was 28% (29 episodes). The median procalcitonin was significantly higher in the infected group than in the not infected group (0.45 vs. 0.061 ng/mL, p < 0.001). The diagnostic accuracy of procalcitonin for bacterial infection estimated by the ROC curve was 0.95 (CI: 95%, 0.91-0.99). When selecting a cutoff value of 0.098 ng/mL a sensitivity of 97% and a negative predictive value 98% were found.
Conclusions. The use of an ultra-sensitive procalcitonin assay identifies patients with cirrhosis at very low risk of bacterial infections.
Bacterial infections are frequent complications in patients with cirrhosis.1–4 This has been reported in different scenarios, such as in hospitalized cirrhotic patients, in whom bacterial infections occur in up to 34% of the cases.3,5 The risk of bacterial infection is even higher in patients with cirrhosis and gastrointestinal bleeding, namely as high as 45%.6–8
Bacterial infections in patients with cirrhosis are associated with poor outcomes.9–12 The incidence of renal failure and sepsis-related mortality is higher in the cirrhotic population than in non-cirrhotic patients. Infections increase mortality 4-fold, with 30% of patients dying within 1 month after infection and another 30% by 1 year.13,14
From the above mentioned data, early diagnosis and treatment of bacterial infection is mandatory in the management of patients with cirrhosis.
However, some unique characteristics of cirrhotic patients make the diagnosis of bacterial infections challenging. For example, Systemic Inflammatory Response Syndrome (SIRS) has been described to be present in up to 30% of the not infected cirrhotic patients and in only 57-70% of the infected ones. Leukocytosis may be masked by hyperesplenism, and spontaneous bacterial peritonitis might be oligosymptomatic, among other possible confounders.2,15 On the other hand, encephalopathy or degradation of liver function may be the only expression of a masked infection.
The initial work-up to identify bacterial infections includes blood and urinary cultures, ascitic fluid analyses and a chest X-ray. With this approach, spontaneous bacterial peritonitis can be rapidly diagnosed. However, the diagnosis of other bacterial infections is not always straightforward, especially in patients with encephalopathy. For example, since dyspnea and atelectasis are frequent in patients with ascites and malnutrition, difficulties exists to diagnose pneumonia; the presence of leukocyturia does not always correlate with urinary tract infection and the diagnosis of spontaneous bacteremia can only be established once the results of blood cultures are received.
Thus, when treating decompensated cirrhotic patients in whom bacterial infections are suspected but cannot be ruled out, antibiotics are usually prescribed, leading to possible over-prescription.
In the constant search for surrogate markers of bacterial infections, procalcitonin (PCT) has arisen as an attractive candidate. Procalcitonin, a precursor of calcitonin, produced ubiquitously by thyroidal and extra-thyroidal tissues, is a marker of bacterial infections that has been evaluated in different settings.16–18 In the presence of a bacterial infection, PCT’s gene expression is induced, and PCT is released from all tissues.16
Conflicting results exist regarding threshold values and diagnostic accuracy of the PCT for the diagnosis of bacterial infections in patients with cirrhosis, since few studies have been conducted and different assays with variable measuring ranges have been tested.19–23
In this study, we prospectively evaluated the diagnostic accuracy of an ultra-sensitive PCT assay for the early diagnosis of bacterial infections in patients with cirrhosis.
Material and MethodsStudy populationThis prospective study was performed in the Hospital Italiano from Buenos Aires, Argentina. All cirrhotic patients aged ≥ 18 years consecutively evaluated in the Emergency Department were considered for eligibility. In order to be enrolled in the study, patients had to sign the informed consent approved by the Institutional Review Board. Patient had to fulfill at least one of the following criteria, considered to be frequently present in cirrhotic patients with bacterial infections: axillary temperature ≥ 37.5 oC, chills, SIRS,24 new-onset encephalopathy or worsening of preexisting encephalopathy by at least one grade according to West Heaven Criteria,25 new onset ascites or hydrothorax or worsening of preexisting ascites or hydrothorax, abdominal pain, respiratory tract symptoms (cough, dyspnea or tachypnea), urinary tract symptoms (back pain and dysuria, polaquiuria or tenesmus), clinical evidence of pyodermitis (cellulitis or erysipelas), upper gastrointestinal bleeding (esophageal or gastric varices or portal hypertensive gastropathy), white blood cell (WBC) count ≥ 10,000/mm3, increase in creatinine by 50% from baseline or increase in bilirubin by 3 mg/dL from baseline. Patients receiving therapeutic antibiotics at screening or over the previous 7 days and/or receiving immunosuppressors were excluded. Patients receiving prophylaxis for spontaneous bacterial peritonitis could be included. In patients with upper gastrointestinal bleeding, the clinical assessment and the complimentary studies had to be performed before the administration of prophylactic antibiotics in order to be included. Patients could be re-enrolled in the study in different episodes of admission to the Emergency Department.
The study was conducted according to the principles of the Declaration of Helsinki and approved by the local ethics committee. The Standards for Reporting of Diagnostic Accuracy Studies criteria (STARD) were used.26
Clinical assessmentsAfter enrollment, data was collected for the medical history and a physical examination was performed. Blood samples were collected in all patients for chemical and hematological determination and PCT. Blood cultures, urinary cultures and a chest X-ray were performed in all patients. In patients with ascites or hydrothorax, samples were obtained for chemical analysis and cultures. Additional studies were performed in case the treating physician considered necessary.
Final diagnosisTwo independent hepatologists classified the patients as infected or not according to pre-defined criteria (see below). Investigators were blinded to PCT result. In cases in which the hepatologists did not agree with the diagnosis, a third hepatologist defined. Infection was defined as any of the following:
- •
Spontaneous bacterial peritonitis. Absolute polimorfonuclear count ≥ 250 cells/mm3 in ascitic fluid without an evident intra-abdominal, surgically treatable source of infection.
- •
Spontaneous bacterial empyema. Absolute polimorfonuclear count ≥ 250 cells/mm3 in thoracic fluid without an evident intra-thoracic source of infection.
- •
Spontaneous bacteremia. At least 1 positive blood culture (excluding those considered contaminated) without an evident source of infection.
- •
Urinary tract infection, pneumonia, skin and soft tissue infection and other less frequent bacterial infections, were defined based on standard criteria.27
A blood sample was collected and stored at -20 °C at screening before antibiotics were eventually prescribed. Procalcitonin was measured by electrochemiluminescence immunoassay (Elecsys Brahms PCT-Roche Diagnostics). The assay has a measuring range between 0.02 and 100 ng/mL.
Statistical analysisA sample of 94 episodes was estimated. This was calculated accepting an expected test sensitivity and specificity of 92% and 78%, respectively, considering an estimated proportion of bacterial infection in cirrhotic patients assisted at the emergency department of 25%19,22 and a 95 confidence interval range of 0.11.28
In the descriptive analysis, continuous variables are expressed as mean and standard deviation or median and interquartile range (IQR), depending on the observed distribution. Continuous variables are compared using t-Test or Mann-Whitney test. Categorical variables are compared using χ2 test. Procalcitonin diagnostic performance was evaluated using receiver-operating characteristic (ROC) curves. After selecting a clinical relevant cut-off point, the sensitivity, specificity, negative predictive value and positive predictive value and their respective 95% confidence interval, were estimated.
A multiple logistic-regression model was used to evaluate possible confounders.
A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 19).
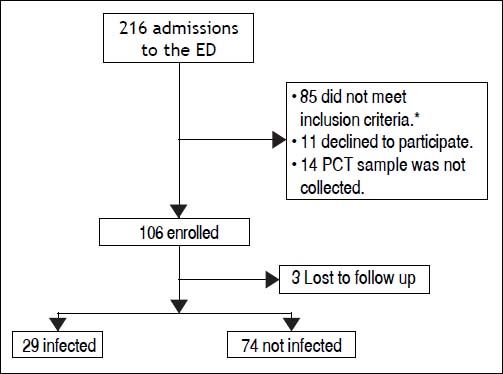
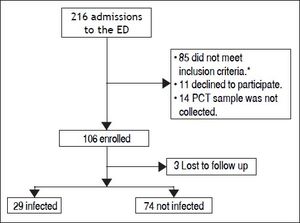
ResultsBetween the November 1, 2010 and June 1, 2012, 216 consecutive admissions to the Emergency Department of the Hospital Italiano from Buenos Aires were registered in 156 cirrhotic patients. Eligibility criteria were fulfilled in 128 episodes. Finally, 106 episodes in 84 patients were included. Three patients were lost to follow up (Figure 1).
The prevalence of bacterial infection was 28% (29 episodes).
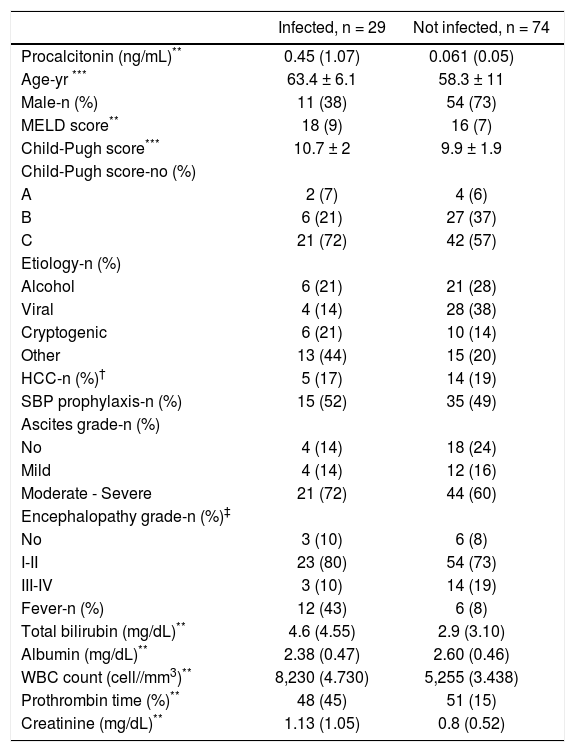
The baseline characteristics of the study population are shown in table 1. Patients in the infected group were older, more likely to have fever and to be female, and had higher creatinine, bilirubin and WBC, than patients in the not infected group.
Baseline characteristics of the study population according to the final diagnosis.*
| Infected, n = 29 | Not infected, n = 74 | |
|---|---|---|
| Procalcitonin (ng/mL)** | 0.45 (1.07) | 0.061 (0.05) |
| Age-yr *** | 63.4 ± 6.1 | 58.3 ± 11 |
| Male-n (%) | 11 (38) | 54 (73) |
| MELD score** | 18 (9) | 16 (7) |
| Child-Pugh score*** | 10.7 ± 2 | 9.9 ± 1.9 |
| Child-Pugh score-no (%) | ||
| A | 2 (7) | 4 (6) |
| B | 6 (21) | 27 (37) |
| C | 21 (72) | 42 (57) |
| Etiology-n (%) | ||
| Alcohol | 6 (21) | 21 (28) |
| Viral | 4 (14) | 28 (38) |
| Cryptogenic | 6 (21) | 10 (14) |
| Other | 13 (44) | 15 (20) |
| HCC-n (%)† | 5 (17) | 14 (19) |
| SBP prophylaxis-n (%) | 15 (52) | 35 (49) |
| Ascites grade-n (%) | ||
| No | 4 (14) | 18 (24) |
| Mild | 4 (14) | 12 (16) |
| Moderate - Severe | 21 (72) | 44 (60) |
| Encephalopathy grade-n (%)‡ | ||
| No | 3 (10) | 6 (8) |
| I-II | 23 (80) | 54 (73) |
| III-IV | 3 (10) | 14 (19) |
| Fever-n (%) | 12 (43) | 6 (8) |
| Total bilirubin (mg/dL)** | 4.6 (4.55) | 2.9 (3.10) |
| Albumin (mg/dL)** | 2.38 (0.47) | 2.60 (0.46) |
| WBC count (cell//mm3)** | 8,230 (4.730) | 5,255 (3.438) |
| Prothrombin time (%)** | 48 (45) | 51 (15) |
| Creatinine (mg/dL)** | 1.13 (1.05) | 0.8 (0.52) |
Procalcitonin (p < 0.001), age (p 0.004), sex (p 0.001), fever (p < 0.001), creatinine (p 0.02), WBC count (p < 0.001) and bilirubin (p 0.013), differed significantly between groups. No other significant differences were found.
According to West Heaven Criteria, with higher scores indicating more severe impairment. The Model for End-Stage Liver Disease (MELD) score can range from 6 to 40, with higher scores indicating more severe disease. The Child-Pugh score (range, 5 to 15, where 5 indicates good liver function and 15 indicates poor liver function) was calculated on the basis of the presence and degree of hepatic encephalopathy, the presence and degree of ascites, the serum bilirubin level, the serum albumin level, and the prothrombin time.
Liver function assessed by Child-Pugh and MELD scores was similar in the two groups.
The most common etiologies of cirrhosis were viral hepatitis, alcohol related and cryptogenic.
Infected patientsAmong the 29 infections, the following diagnoses were made: 13 (45%) spontaneous bacterial peritonitis, 7 (24%) spontaneous bacteremias, 4 (14%) urinary tract infections, 2 (7%) pneumonias, 2 (7%) cellulitis and 1 (3%) spontaneous bacterial empyema. Fifty two percent of the infections were community-acquired, 22% health-related and 26% nosocomial. At least one culture was positive in 14 (48%) episodes. The most frequent isolated bacteria were E. coli, K. pneumonia, S. viridians and Staphylococcus spp. The cultures of the remaining cases were negative.
Not infected patientsThe diagnosis of the 74 episodes considered not infected were worsening or new-onset ascites, hydrothorax or encephalopathy due to disease progression or dietary or treatment non compliance (37), acute renal failure (11), increase in bilirubin due to disease progression (8), diarrhea and abdominal pain(5) and upper gastrointestinal bleeding (3), among others (10).
At least one dose of antibiotic was empirically prescribed by the treating physician in 28% of the episodes of the not infected patients. Cefriaxone and piperacillin-tazobactam were the most frequently prescribed antibiotics.
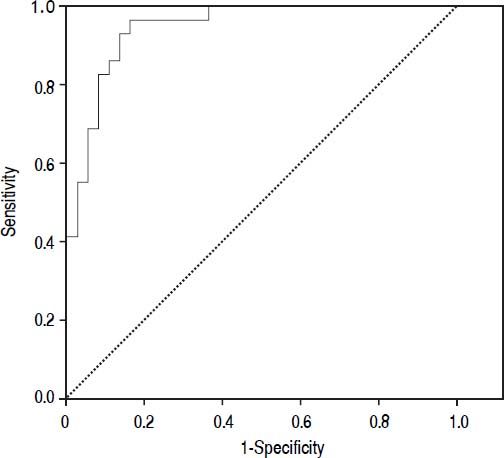
Diagnostic accuracy of procalcitoninThe median PCT was significantly higher in the infected group than in the not infected group (0.45 ng/mL vs. 0.061 ng/mL, p < 0.001). The diagnostic accuracy of PCT for bacterial infection estimated by the ROC curve was 0.95 (CI 95%: 0.91-0.99).
The statistical significance was still maintained after performing a multivariate analyses adjusting for age, prothrombin time, Child-Pugh, creatinine, WBC count and fever (p < 0.001).
Figure 2 shows the diagnostic accuracy of PCT. When selecting a cutoff value of 0.098 ng/mL, the following results were obtained for the diagnosis of bacterial infection: sensitivity 97% (95% CI: 86-100%), specificity 82% (95% CI: 72-94%), negative predictive value 98% (95% CI: 94-100%) and positive predictive value 68% (95% CI: 51-88%).
Six patients were included in the study in more than one episode of admission to the emergency department. This gave us the opportunity to observe the intra-individual variation of PCT in different clinical situations. In cases in which both episodes of infection and not infection were observed in the same patient, PCT varied significantly. In patients in whom all episodes were unrelated to bacterial infections, PCT was persistently below 0.098 ng/mL. Finally, in patients in whom all episodes were related to bacterial infections, PCT was persistently above 0.098 ng/mL.
DiscussionIn this study we found a great accuracy of PCT to identify patients with cirrhosis at very low risk of bacterial infections.
Procalcitonin plasmatic concentration has been reported to rise in bacterial infections in different clinical settings in the non-cirrhotic population.16,18
Studies in patients with cirrhosis were performed with different PCT assays and vary greatly in design. However, median PCT levels were consistently higher in infected patients in comparison with not infected patients, regardless of the PCT assay that was used.20–23,29 Moreover, PCT levels in patients with stable cirrhosis are in the physiological range20 and rise in cases of bacterial infections, even in patients with advanced liver disease.29 These characteristics make PCT an attractive surrogate marker of bacterial infections in patients with cirrhosis.
Most of the studies that evaluated PCT in patients with cirrhosis proposed different cut-off points in order to support the diagnosis of bacterial infections. Consequently, relatively high PCT cut-off points were selected, and, as a result greater specificity than sensitivity results were obtained. The initial studies used assays with sub-optimal lower limits of detection, ranging from 0.1 ng/mL to 0.5 ng/mL.20,21,23 These studies reported sensitivities between 60 and 72%.20,23
A highly sensitive PCT assay (lower limit of detection 0.02 ng/mL) such as the one we used, was explored more recently in a study performed by Lazzarotto, et al.30 In this study, using a cut-off value of 0.1 ng/mL, a sensitivity of 96% and a negative predictive value of 96% was found. We also selected a low PCT cut-off point, in order to obtain a high sensitivity and negative predictive value. In our study with a cut-off value of 0.098 ng/mL we found a sensitivity of 97% and a negative predictive value of 98% (AUROC of 0.95). These findings suggest a potential role of PCT to early identify patients who are at very low risk of bacterial infections.
Comparing results among different studies assessing PCT in patients with cirrhosis should carefully be performed because inclusion and exclusion criteria might vary. In our study, we decided to include patients expressing a wide range of clinical and/or biochemical signs of deterioration frequently observed in patients with cirrhosis, and that in practice usually lead to perform a work-up to rule out bacterial infections. We did not include all patients with cirrhosis admitted to the emergency department, because that would have resulted in the enrolment of a great number of patients without a significant risk of bacterial infection.
Moreover, we found that PCT accuracy to diagnose bacterial infections was observed in different episodes of admission to the Emergency Department in the same patient.
Recurrent admissions in patients with endstage liver disease, particularly due to ascites and encephalopathy are frequent.31–33 Procalcitonin might be a useful tool to apply in these cases, especially because this population usually requires broad spectrum antibiotics for epidemiological reasons.
In our study, the treating physician prescribed at least one dose of antibiotics in 28% of the admissions of the not infected patients. This reflects the difficulties to diagnose bacterial infections in the emergency department in patients with cirrhosis.
The PCT assay has a total duration of 18 minutes, resulting in valuable information immediately available to the treating physician. Furthermore, in our region, this PCT assay is inexpensive. Its cost is comparable to two blood cultures.
Our study has some limitations. First, PCT was not compared with other inflammatory markers, such as C-reactive protein (CRP). Prior studies compared the performance of CRP and PCT in patients with cirrhosis.23,29 These studies found a better performance of CRP over PCT. However, the PCT assay that was used was sub-optimal, with lower limits of detections not better than 0.1 ng/mL. It would be of interest in future trials, to compare the performance of PCR with this ultra-sensitive PCT assay. Second, although the number of patients enrolled might seem small, it adequately represents the sample size estimated to provide the specific power. Further validation is desired in order to confirm our findings. Finally, serial determinations of PCT were not performed, which could have added valuable information to guide antibiotic therapy and predict outcomes.
In conclusion, we found a great accuracy of PCT to identify patients with cirrhosis at very low risk of bacterial infections. We believe this might be of great help, especially in patients in whom the diagnosis of bacterial infection cannot be confirmed or ruled-out with the initial work-up. Further studies evaluating PCT-guided antibiotic therapy should specifically address its role in avoiding antibiotic over-prescription.
Abbreviations- •
AUROC: area under the ROC curve.
- •
HCC: hepatocellular carcinoma.
- •
IQR: interquartile range.
- •
PCT: procalcitonin.
- •
ROC: receiver-operating characteristic.
- •
SB: spontaneous bacteremia.
- •
SBP: spontaneous bacterial peritonitis.
- •
SIRS: systemic inflammatory response s yndrome.
- •
UTI: urinary tract infection.
- •
WBC: white blood cell.
No conflict of interest or financial support to declare.