Budd-Chiari syndrome (BCS) is a heterogeneous group of disorders characterized by hepatic venous outflow obstruction. Abernethy malformation is a congenital vascular malformation defined by diversion of portal blood away from the liver. Both conditions are rare vascular diseases. We report here the first case of a patient with combined type II Abernethy malformation and BCS from China. The inferior vena cava obstruction was treated with percutaneous balloon angioplasty; close follow-up was elected for the Abernethy malformation.
BCS is a heterogeneous group of disorders characterized by obstruction of hepatic venous outflow at various levels from the small hepatic veins (HVs) to the junction of the inferior vena cava (IVC) and the right atrium.1 It is a rare disease, with a reported prevalence in China of 0.0065%.2 BCS can be classified into primary and secondary types according to its cause: The primary type refers to congenital obstruction of the hepatic veins or the hepatic portion of the IVC. The secondary type refers to obstruction of the same anatomic structures by a tumor or, more commonly, thrombus or thrombi in patients with systemic disease, usually myeloproliferative disorders.3 The primary type of BCS is most common in Oriental countries and Africa, whereas the secondary type is most common in Western countries.
Abernethy malformation is a rare congenital abnormal portosystemic shunt, first described by John Abernethy in 1793. It constitutes a diversion of portal blood away from the liver by either end-to-side or side-to-side shunting.4 Morgan and Superina classified congenital extrahepatic portosystemic shunt into two types:5 Type I, in which portal blood is diverted completely into the IVC with absence of the portal vein (PV) and is associated with hepatic failure and hepatic tumors; and Type II, in which the PV is intact and thus has better prognosis. Until 2016, only 101 cases of Abernethy malformation had been reported,6 and none were combined with BCS. Herein, we present a case of combined BCS and type II Abernethy malformation diagnosed using duplex ultrasonography and computed tomography (CT) angiography.
Case ReportThis study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. A 60-year-old woman was admitted to the Department of Intervention with a 10-year history of prominent veins of the abdominal wall, abdominal distention, and mild swelling of both legs. Physical examination revealed many prominent dilated subcutaneous veins with upward flow in the abdominal wall, palpable liver and spleen, and mild edema of the legs without varices, pigmentation, or ulcers. Hepatic encephalopathy was not evident. Blood ammonia values and indices of liver and renal function were in the normal range, with the exception of platelet count (33 x 109/L) consistent with mild hypersplenism.
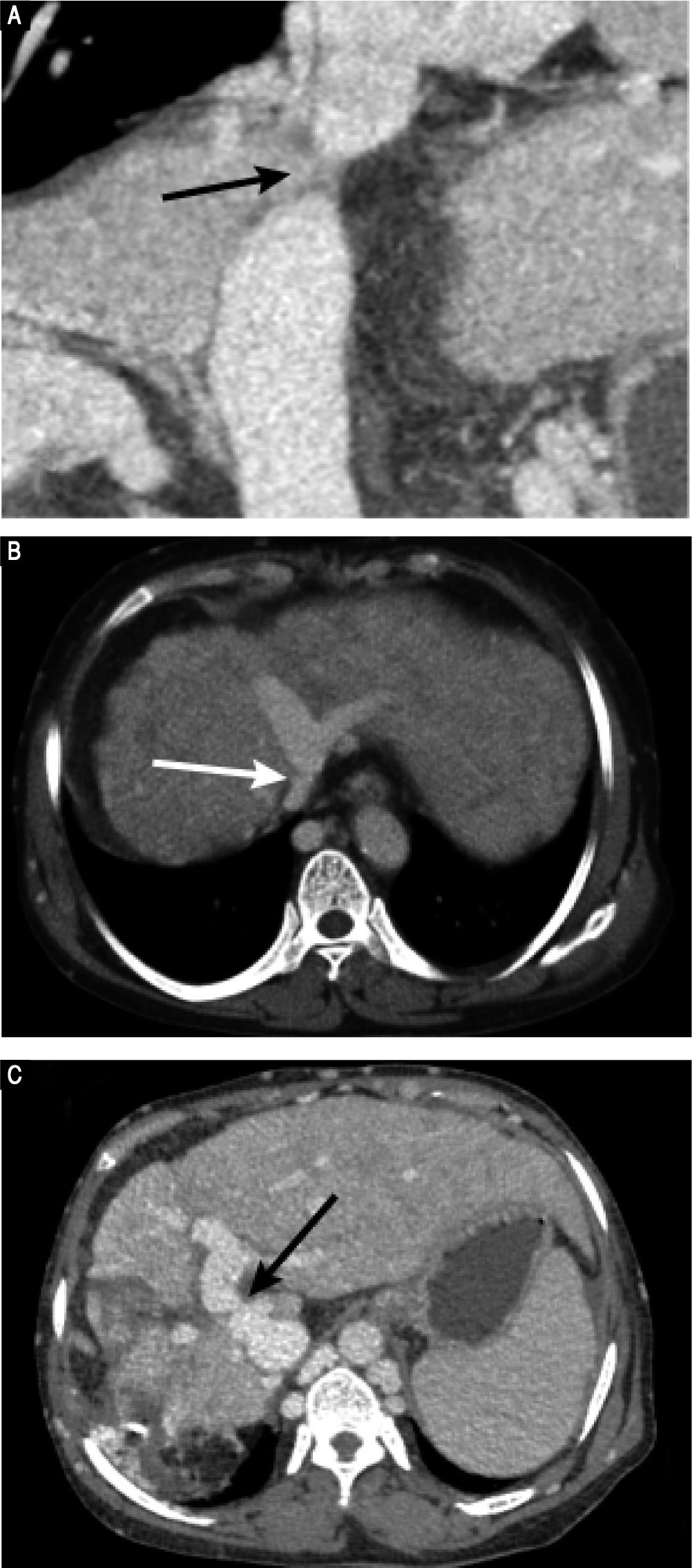
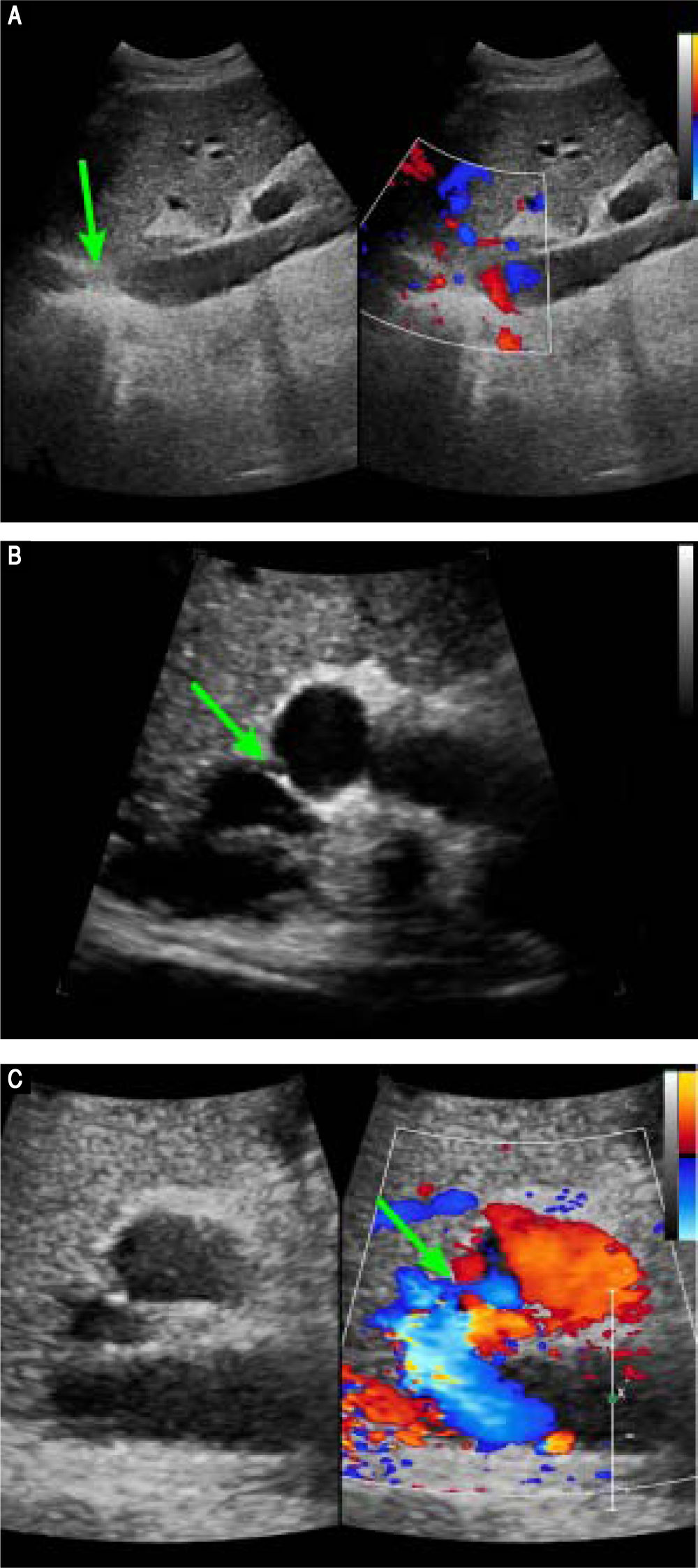
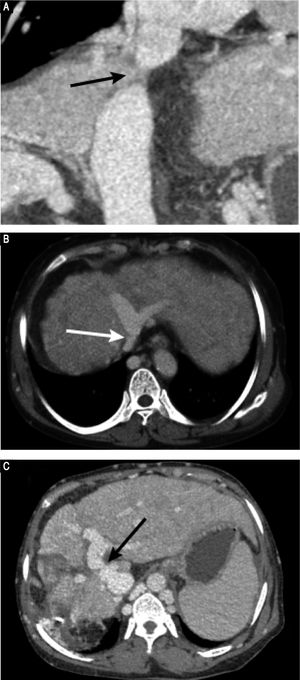
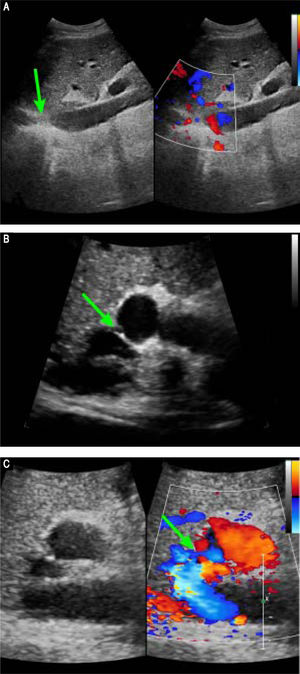
Preoperative contrast-enhanced CT revealed marked atrophy of right lobe of the liver and compensatory enlargement of left lobe, no liver mass, and splenomegaly. Vascular findings were a membranous obstruction of the IVC with dilatation of the upper lumbar tributaries and hemiazygous/azygous veins (Figure 1A); a whole-course obstruction of the right HV; a membranous obstruction of the middle HV and left HV at the orifice of the HV (Figure 1B); and a dilated phrenic vein and pericardial vein, draining blood from the middle HV and the left HV to the superior vena cava. Portal venous-phase images revealed a side-to-side anastomosis of 2 mm diameter between the PV and IVC (Figure 1C). The splenic and superior mesenteric veins were normally oriented and joined to form the main PV. These results were consistent with those of abdominal Doppler ultrasound (Figure 2). The diagnosis of type II Abernethy malformation and BCS was made.
Preoperative CT images. A. CT-angiography reveals a membranous obstruction of the intrahepatic IVC (arrow). B. Axial porta venous phase CT scan reveals that the right HV cannot be visualized; the co-orifice of the middle HV and left HV have a membranous obstruction; and the azygos and hemiazygos veins are markedly dilated (arrow). C. Axial porta venous phase CT scan reveals a side-to-side anastomosis (arrow) between the portal vein and IVC, a finding compatible with type II Abernethy malformation.
Preoperative Doppler ultrasound images. A. Abdominal two-dimensional ultrasound and three-dimensional Doppler ultrasound images reveal a membranous obstruction of the intrahepatic IVC without blood flow (arrow). B. Abdominal two-dimensional ultrasound and Doppler ultrasound images reveal that the co-orifice of the middle HV and the left HV have a membranous obstruction and no blood flow, C. Abdominal two-dimensiona ultrasound and Doppler ultrasound images reveal an abnormal connection (arrow) between the porta vein and the IVC.
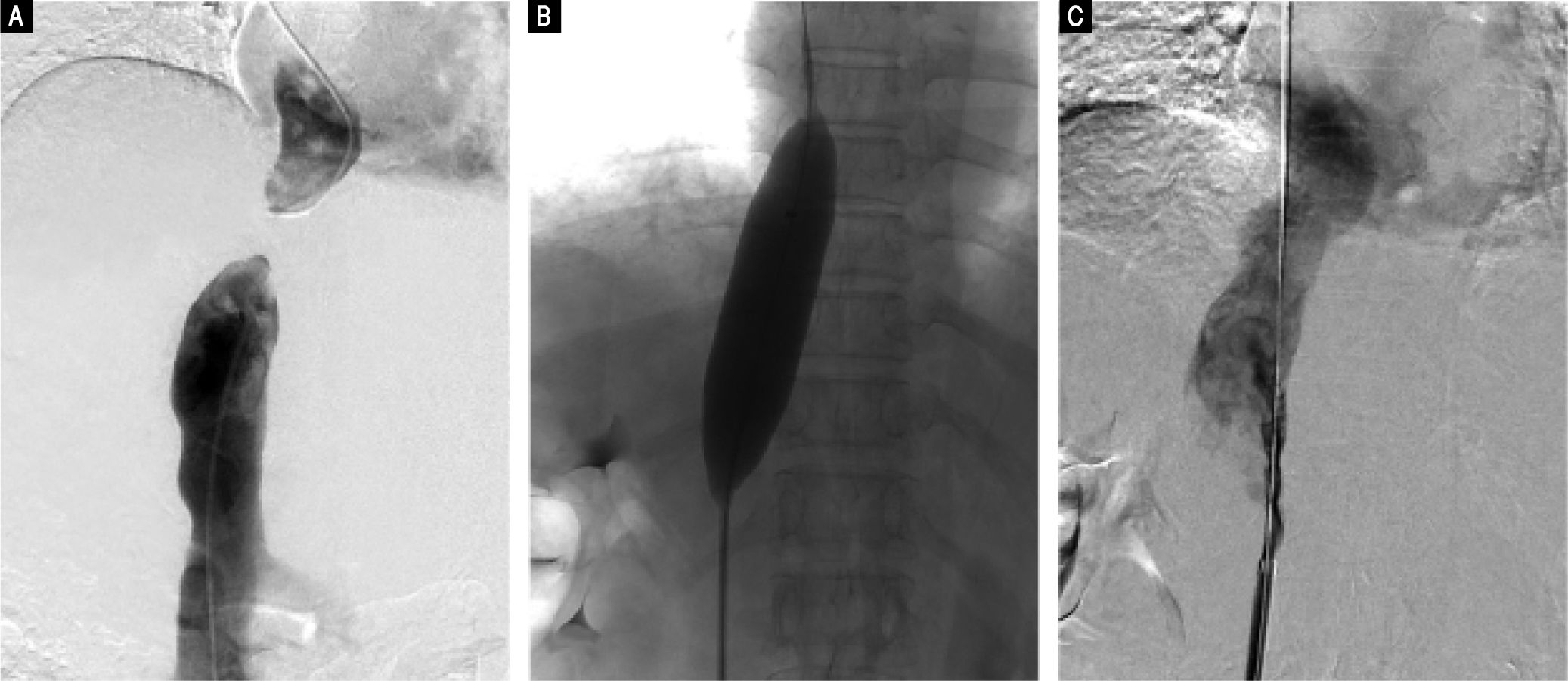
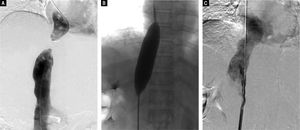
Based on these findings, we performed percutaneous balloon angioplasty (PTBA) for the obstructed IVC. For the Abernethy malformation, we elected conservative treatment because the patient was asymptomatic and the diameter of the shunt was small. For the PTBA procedure, we first performed inferior vena cavagraphy through the right femoral vein to identify the anatomy and location of the occluded section of the IVC (Figure 3A). Next, a selfmade, blunt, steel wire needle was introduced transfemorally into the distal portion of the obstruction to rupture the obstructive membrane of the IVC. A super stiff exchange wire was then inserted and positioned in the superior vena cava through the obstructed IVC. PTBA of the IVC membranous obstruction was performed with a 25 mm diameter balloon catheter (Cook Medical, Bloomington, IN, US) (Figure 3B). After the PTBA, the IVC pressure had decreased from 19 to 10 cm H2O, and inferior vena cavagraphy revealed adequate IVC blood flow and no stenosis (Figure 3C).
Digital subtraction angiography images. A. Inferior vena cavogram via both the jugular approach and the femoral approach reveal complete obstruction of the intrahepatic IVC, which confirmed the results of Doppler ultrasound and CT-angiography. B. Dilation of the obstructed IVC with a 25 mm diameter balloon after successful rupture of the membrane, C. Inferior vena cavogram immediately after balloon angioplasty reveals patency of the IVC without residua stenosis.
Low-molecular-weight heparin (4,100 U/12 h, subcutaneously) was given immediately after the procedure and continued for 5 days. Warfarin (5 mg/day, orally) was administered from the second day for one year after the procedure at an international normalized ratio of 2-3. Subcutaneous varicose veins of the abdominal wall had decreased remarkably by the second day after the procedure. Hepatic encephalopathy has not occurred at any time since the procedure was done, and, at 6 months follow-up, Doppler ultrasound revealed that the IVC was patent.
DiscussionIn our patient, both the HV and IVC had membranous obstruction. The HV obstruction was compensated by dilated phrenic and pericardial veins, so the patient had no signs or symptoms of portal hypertension, such as ascites and esophageal and gastric variceal bleeding. Because IVC hypertension was the patient’s main clinical manifestation, only obstructed IVC was treated, and neither HV recanalization nor transjugular intrahepatic portosystemic shunt was used.
Since our patient’s obstructed IVC was of the membranous type, PTBA was the most appropriate treatment. We performed it, and it was successful. PTBA is the most common treatment of BCS in Asian and African countries and has been proven effective in most patients, with relief of symptomatic venous obstruction and reestablishment of normal venous flow and prevention of progressive liver disease.7 When there is residual stenosis or re-occlusion of vessels shortly after PTBA, stent placement may be successful treatment.8
In general, treatment of congenital malformations of the portal system depends on the type of shunt, the presenting symptoms, coexisting congenital anomalies, hepatic insult, complications and comorbidity. The most important determinant appears to be the type of the lesion.5 For children with a type I shunt, surgical closure should be considered, but if that is not possible, close follow-up is indicated. With clinical experience and technological advances, more patients are becoming surgical candidates. However, no surgical strategy has been described for adult patients with a type II shunt. When these patients have serious symptoms, such as hepatic encephalopathy or collateral bleeding, the shunt should be closed soon, which may help avoid the development of mesenteric venous congestion.4,9,10
Reported treatments of Abernathy malformations include operations, such as closure of shunt, liver nodule resection, liver transplantation, and interventional treatment, such as percutaneous balloon-occluded obliteration, percutaneous trans-catheter embolization with metallic coils or plug, stent placement, and stent-graft placement.11,12 In recent years, there has been a trend towards the use of interventional treatment. For the reasons stated above, we elected to not treat our patient’s Abernathy malformation, but to closely monitor the patient’s clinical, biochemical, and imaging findings.
In summary, we have presented the first case we are aware of with combined BCS and type II Abernathy malformation in China. The membranous occlusion of the IVC was treated with PTBA; the Abernathy malformation was not treated because of its small diameter and the patient’s asymptomatic status.
Abbreviations- •
BCS: Budd-Chiari syndrome.
- •
CT: computed tomography.
- •
HV: hepatic vein.
- •
IVC: inferior vena cava.
- •
PTBA: percutaneous balloon angioplasty.
- •
PV: portal vein
The authors declares that there is no conflict of interest regarding the publication of this case report.
AcknowledgementsWe are grateful to Dr. Yan Zhang from the Department of Ultrasound, and the First Affiliated Hospital of Zhengzhou University for assistance with patient followup and preparation of ultrasound images.