Background and aims. The Barcelona Clinic Liver Cancer (BCLC) staging system is the algorithm most widely used to manage patients with hepatocellular carcinoma (HCC). We aimed to investigate the extent to which the BCLC recommendations effectively guide clinical practice and assess the reasons for any deviation from the recommendations.
Material and methods. The first-line treatments assigned to patients included in the prospective Bern HCC cohort were analyzed.
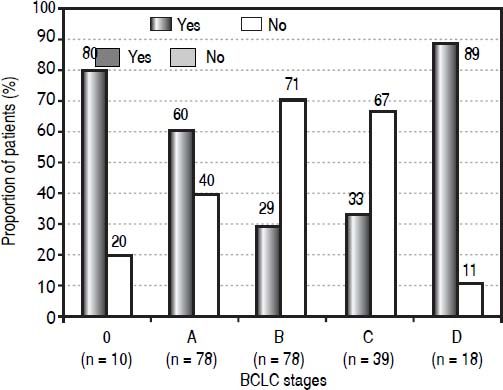
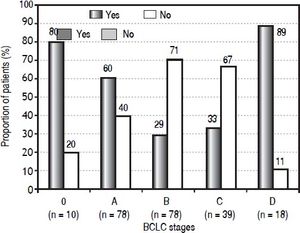
Results. Among 223 patients included in the cohort, 116 were not treated according to the BCLC algorithm. Eighty percent of the patients in BCLC stage 0 (very early HCC) and 60% of the patients in BCLC stage A (early HCC) received recommended curative treatment. Only 29% of the BCLC stage B patients (intermediate HCC) and 33% of the BCLC stage C patients (advanced HCC) were treated according to the algorithm. Eighty-nine percent of the BCLC stage D patients (terminal HCC) were treated with best supportive care, as recommended. In 98 patients (44%) the performance status was disregarded in the stage assignment.
Conclusion. The management of HCC in clinical practice frequently deviates from the BCLC recommendations. Most of the curative therapy options, which have well-defined selection criteria, were allocated according to the recommendations, while the majority of the palliative therapy options were assigned to patients with tumor stages not aligned with the recommendations. The only parameter which is subjective in the algorithm, the performance status, is also the least respected.
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer-related death worldwide and, consequently, represents a major global health problem.1 The prognosis assessment is a decisive step in the management of HCC patients. The most widely used algorithm that classifies patients according to both prognosis and treatment allocation is the Barcelona Clinic Liver Cancer (BCLC) staging system.2 The BCLC algorithm is endorsed by the European and American clinical practice guidelines,3,4 whereas in Japan, for example, other guidelines are followed.5
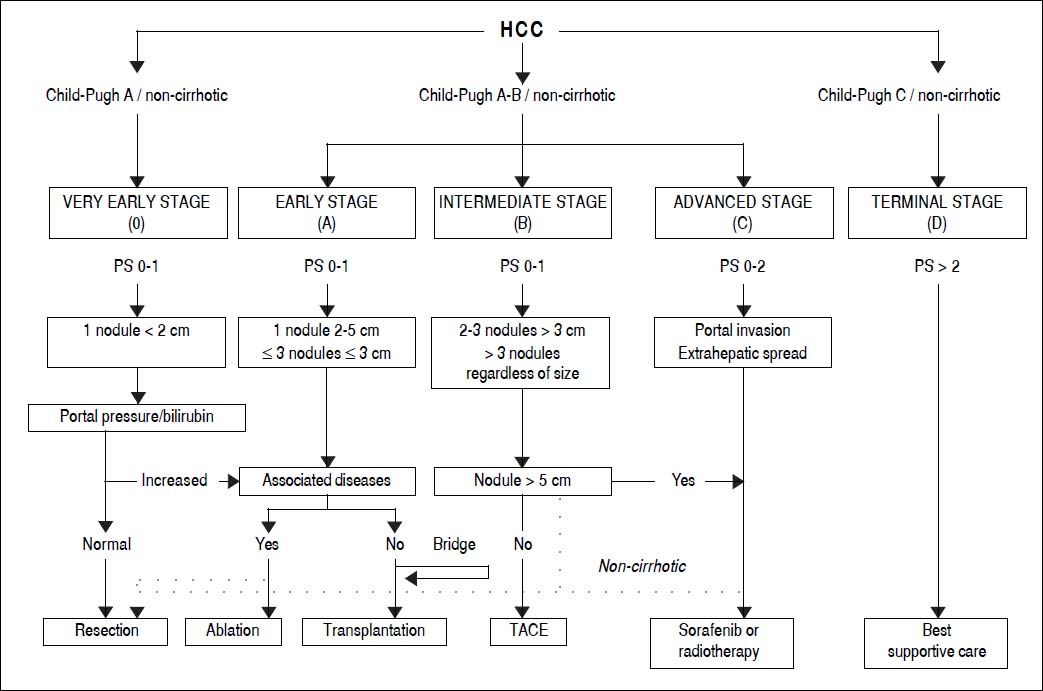
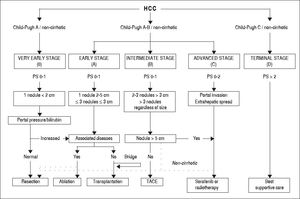
The BCLC algorithm classifies patients into five HCC stages (0, A, B, C, and D) and allocates either curative or palliative therapy recommendations according to three main prognostic variables: tumor status (number, size, vascular invasion, extrahepatic localization), liver function (Child-Pugh score) and performance status (PS, defined by the Eastern Cooperative Oncology Group scale6). Of the three sets of variables, PS is the most subjective. The very early stage (BCLC 0) is defined as the presence of a single nodule < 2 cm in diameter and without vascular invasion or metastases in patients with good performance status (PS 0) and well-preserved liver function (Child-Pugh A). The early stage (BCLC A) corresponds to patients with one nodule < 5 cm or up to three nodules each < 3 cm. Patients with BCLC stages 0 and A are candidates for potentially curative treatment options, i.e. surgical resection, liver transplantation, or local ablation. The intermediate stage (BCLC B) includes asymptomatic patients with large or multifocal tumors limited to the liver parenchyma. The advanced stage (BCLC C) characterizes patients with cancer-related symptoms, macrovascular invasion, or extrahepatic spread. Patients with BCLC B and C are treated with palliative approaches, such as transarterial chemoembolization (TACE) or systemic therapy with sorafenib. Patients in the terminal stage (BCLC D) present with a poor PS or liver function (Child-Pugh C), reflecting a severe tumor or cirrhosis-related disability; these patients receive best supportive care.
The BCLC algorithm does not include all therapeutic options for HCC, such as transarterial radioembolization (TARE) or external radiotherapy, even though both treatments have shown promising antitumoral activity.7-11 In addition, the intermediate stage includes a heterogeneous group of patients, although the BCLC system does not provide any subclassification. A panel of experts has made a proposal for a subclassification, involving the up-to-seven criterion,12 to facilitate therapeutic management of patients with BCLC B.13
The extent to which the BCLC recommendations are followed in clinical practice is currently unknown. The main objective of this study was to investigate whether first-line treatments for HCC are assigned in line with the BCLC algorithm to patients included in a prospective cohort from a single center in Switzerland (the Bern HCC Cohort).
Material and MethodsStudy designThe characteristics of patients who entered the Bern HCC cohort were evaluated over a period of 48 months between August 2010 and August 2014. All adult patients with an HCC that had been diagnosed in the 18 months prior to entering the cohort were invited to participate. Standardized prospective information was collected through questionnaires, clinical examination, and laboratory investigation. The therapeutic decision was taken at a weekly tumor board involving specialists in oncology, radiotherapy, nuclear medicine, interventional radiology, visceral surgery, and hepatology. The protocol was approved by the local ethics committee and all enrolled patients signed an informed consent.
Data collection and processingBaseline data were collected on entry into the Bern HCC cohort, which have been previously described in an analysis of factors that affect screening for HCC.14 Follow-up investigations were carried out every 3 months since inclusion and progression of the following variables were documented: tumor status (stationary/regressive/ progressive), BCLC classification, Milan Criteria, Child-Pugh and its variables (albumin, bilirubin, prothrombin time, ascites, encephalopathy), Model of End-stage Liver Disease (MELD) score, PS and therapy. Standardized information regarding quality of life was also collected on every follow-up visit. A “drop-out” form was completed when the patient was either transplanted or lost to follow-up, or had died. All data were gathered in the REDCap database.15
Subclassification of intermediate HCC13A proposal for subclassification of the intermediate HCC has been made by a panel of experts. This proposal involves the up-to-seven criterion12 and classifies the B patients into four substages (B1-B4). The up-to-seven criterion means that the sum of the number of nodules and the size of the largest tumor (in centimeters) is no more than seven. The B1 group comprises patients with PS 0, Child-Pugh A or B (up to a score of 7 and without clinical ascites nor jaundice) and within the up-to-seven criterion. The B2 group includes patients with PS 0, Child-Pugh A (without clinical ascites or jaundice) and beyond the up-to-seven criterion. The B3 group comprises patients with PS 0, Child-Pugh B (score of 7) and beyond the up-toseven criterion. The B4 group includes patients with PS 1, decompensated Child-Pugh B (score of 8 or 9) and with any up-to-seven criterion.
Statistical methodsFor comparison of patients treated according to BCLC system and patients who were not, Fisher’s exact test (for categorical outcomes) and the Mann-Whitney-Wilcoxon test (for continuous outcomes) were used. To compare the estimated overall survival of patients with PS 0 and 1, the log-rank test (Mantel Cox) was conducted using R version 3.1.1. A p-value of < 0.05 was considered statically significant.
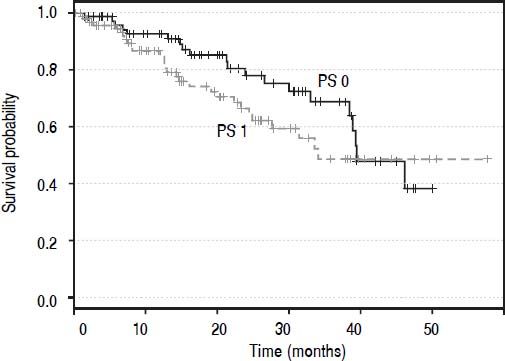
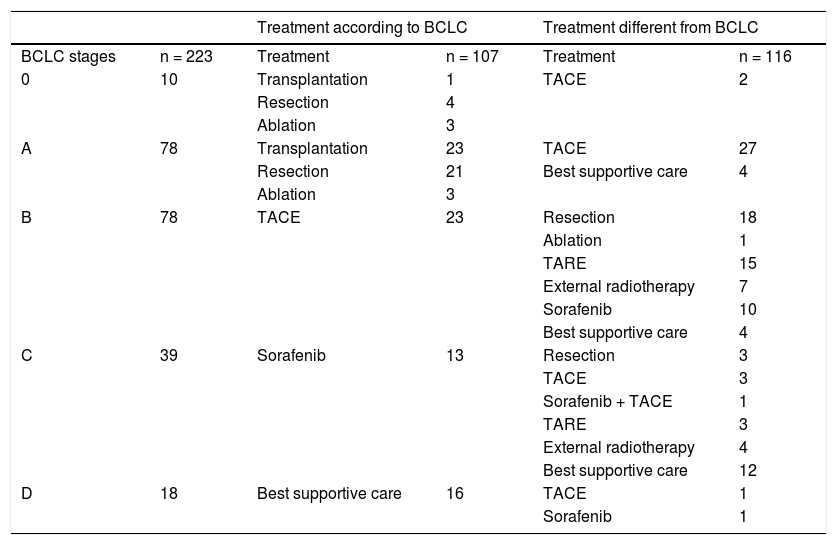
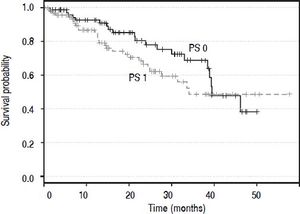
ResultsTwo hundred and twenty-eight patients were enrolled. One patient was excluded because the first-line treatment was assessed externally. Four patients were diagnosed with an HCC recurrence after being transplanted; as these patients had already received the first-line treatment (liver transplantation), they were excluded. Seventy-three patients died (32.7%) during the period of observation and 150 are alive or censored (67.3%). Thus, the BCLC staging system was applied to 223 patients as shown in table 1. Ten patients (5%) were assigned in the very early stage (0), 78 patients (35%) in the early stage (A), 78 (35%) in the intermediate stage (B), 39 (17%) in the advanced (C) stagem and 18 (8%) in the terminal (D) stage. Survival curves (Kaplan-Meier estimator) (Figure 1) were compared for PS 0 and 1 and the log-rank test demonstrated that these two levels were not statistically different (p = 0.203). Thus, patients with PS 1 were also accepted in the BCLC 0 (five patients), A (41 patients) and B (46 patients) stages, and six patients with PS 0 were furthermore classified in the BCLC C stage. One-quarter of patients (10/39, 26%) in the advanced stage category had a PS score of 2, and 44% (8/18) of patients in the terminal stage category had PS scores of 3 or 4. The treatment allocation among all patients is described in table 1. One hundred and seven patients (48%) were treated according to the BCLC recommendations and 116 patients (52%) were not. Of the 116 patients treated differently from the algorithm, the majority (74%) received a treatment recommended for other tumor stages, while the remaining 26% of patients (30/116) received a treatment not mentioned in the BCLC algorithm, such as TARE, external radiotherapy, or a combination of sorafenib and TACE.
Treatment allocation among all patients (n = 223) included in the Bern HCC cohort.
| Treatment according to BCLC | Treatment different from BCLC | ||||
|---|---|---|---|---|---|
| BCLC stages | n = 223 | Treatment | n = 107 | Treatment | n = 116 |
| 0 | 10 | Transplantation | 1 | TACE | 2 |
| Resection | 4 | ||||
| Ablation | 3 | ||||
| A | 78 | Transplantation | 23 | TACE | 27 |
| Resection | 21 | Best supportive care | 4 | ||
| Ablation | 3 | ||||
| B | 78 | TACE | 23 | Resection | 18 |
| Ablation | 1 | ||||
| TARE | 15 | ||||
| External radiotherapy | 7 | ||||
| Sorafenib | 10 | ||||
| Best supportive care | 4 | ||||
| C | 39 | Sorafenib | 13 | Resection | 3 |
| TACE | 3 | ||||
| Sorafenib + TACE | 1 | ||||
| TARE | 3 | ||||
| External radiotherapy | 4 | ||||
| Best supportive care | 12 | ||||
| D | 18 | Best supportive care | 16 | TACE | 1 |
| Sorafenib | 1 | ||||
BCLC: Barcelona Clinic Liver Cancer. N: number of patients. TACE: transarterlal chemoembollzatlon. TARE: transarterlal radloembollzatlon.
The proportion of patients in each BCLC stage receiving a treatment that was or was not recommended for that particular stage is shown in figure 2. Of 10 patients with 0 stage HCC, 80% were treated according to the algorithm, i.e. with transplantation (one patient), resection (four patients) or ablation (three patients), with the remaining 20% receiving TACE. Of 78 patients in stage A, 60% were treated with transplantation (23 patients), resection (21 patients) or ablation (three patients), while the remaining patients received either TACE (27 patients) or best supportive care (four patients). The therapy received by patients with B and C stages differs mostly from the BCLC algorithm. Of patients with intermediate stage, 23 (29%) were treated with TACE, while the remaining 71% (55/8) received heterogeneous therapies: 18 patients were resected, one underwent ablation, 15 were treated with TARE (all of them had a heavy tumor burden), seven with external radiotherapy (also with a heavy tumor burden), 10 with sorafenib and four received best palliative support. One-third (13/39, 33%) of patients with an advanced-stage tumor were treated with sorafenib, while the others were treated with resection (three patients), TACE (three patients), a combination of sorafenib and TACE (one patient), TARE (three patient), external radiotherapy (four patients), or best supportive care (12 patients). In the terminal stage, the majority received best supportive care (16/18 patients, 89%). Of the remaining two terminalstage patients, one patient was treated with sorafenib and one with TACE.
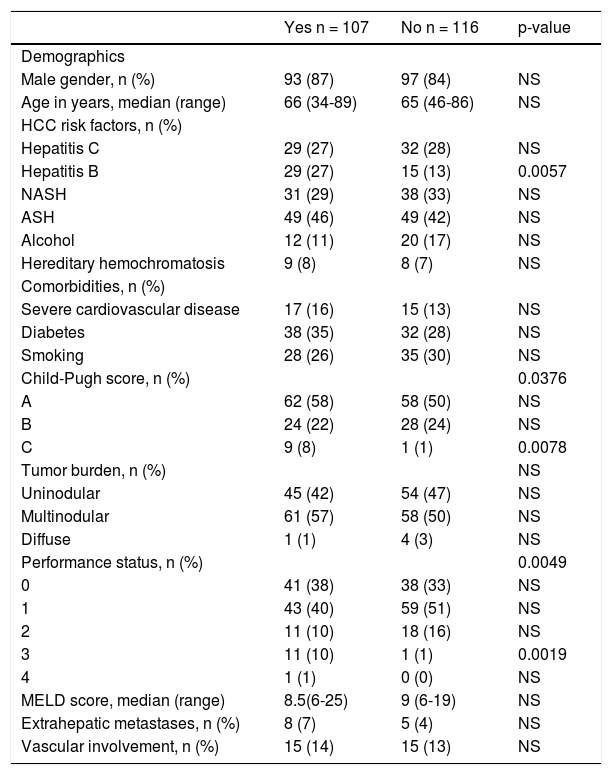
The characteristics of all patients treated either according to or different from their BCLC stage are listed in table 2. There was no difference in gender, age or comorbidities (severe cardiovascular disease, diabetes, smoking habit) between the patients who received the BCLC recommended therapy and those who did not. Regarding parameters involved in the BCLC staging system, the two groups showed no differences in tumor burden, or the presence of extrahepatic metastases and vascular involvement. In contrast, a significantly (p = 0.0078) greater proportion of patients with a Child-Pugh C score were allocated to a BCLC recommended therapy. Patients with a PS of 3 were significantly (p = 0.0019) more likely to receive a therapy aligned with their BCLC stage. Patients with a hepatitis B had a significant (p = 0.0057) tendency to be treated according to the BCLC and were also younger (57.8 ± 7.8 years, p < 0.0001) than patients without hepatitis B (66.8 ± 9.1 years) and more often transplanted (27.3 vs. 13.6%, p = 0.039). No difference was found between the two groups for other HCC risk factors (hepatitis C, alcoholic/nonalcoholic steatohepatitis, consumption of > 30 g of alcohol/day, hereditary hemochromatosis) and the MELD score.
Characteristics of patients included in the Bern HCC Cohort and treated according to (Yes) or different from (No) the BCLC algorithm.
| Yes n = 107 | No n = 116 | p-value | |
|---|---|---|---|
| Demographics | |||
| Male gender, n (%) | 93 (87) | 97 (84) | NS |
| Age in years, median (range) | 66 (34-89) | 65 (46-86) | NS |
| HCC risk factors, n (%) | |||
| Hepatitis C | 29 (27) | 32 (28) | NS |
| Hepatitis B | 29 (27) | 15 (13) | 0.0057 |
| NASH | 31 (29) | 38 (33) | NS |
| ASH | 49 (46) | 49 (42) | NS |
| Alcohol | 12 (11) | 20 (17) | NS |
| Hereditary hemochromatosis | 9 (8) | 8 (7) | NS |
| Comorbidities, n (%) | |||
| Severe cardiovascular disease | 17 (16) | 15 (13) | NS |
| Diabetes | 38 (35) | 32 (28) | NS |
| Smoking | 28 (26) | 35 (30) | NS |
| Child-Pugh score, n (%) | 0.0376 | ||
| A | 62 (58) | 58 (50) | NS |
| B | 24 (22) | 28 (24) | NS |
| C | 9 (8) | 1 (1) | 0.0078 |
| Tumor burden, n (%) | NS | ||
| Uninodular | 45 (42) | 54 (47) | NS |
| Multinodular | 61 (57) | 58 (50) | NS |
| Diffuse | 1 (1) | 4 (3) | NS |
| Performance status, n (%) | 0.0049 | ||
| 0 | 41 (38) | 38 (33) | NS |
| 1 | 43 (40) | 59 (51) | NS |
| 2 | 11 (10) | 18 (16) | NS |
| 3 | 11 (10) | 1 (1) | 0.0019 |
| 4 | 1 (1) | 0 (0) | NS |
| MELD score, median (range) | 8.5(6-25) | 9 (6-19) | NS |
| Extrahepatic metastases, n (%) | 8 (7) | 5 (4) | NS |
| Vascular involvement, n (%) | 15 (14) | 15 (13) | NS |
Statistical analyses were based on Fisher’s exact test for categorical outcomes and Mann-Whitney-Wilcoxon’s test for continuous outcomes. ASH: alcoholic steatohepatltls. HCC: hepatocellular carcinoma. MELD: Model for End-Stage Liver Disease. N: number of patients. NASH: nonalcoholic steatohepatltls. NS: not significant.
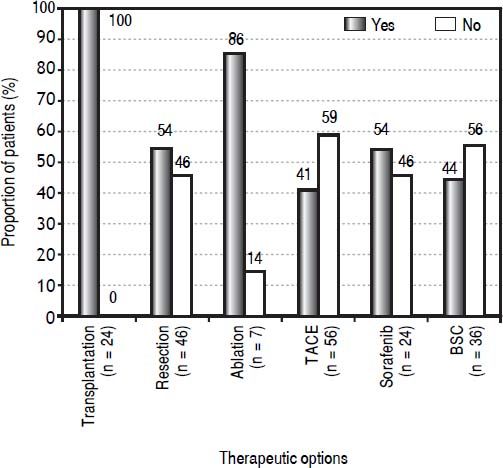
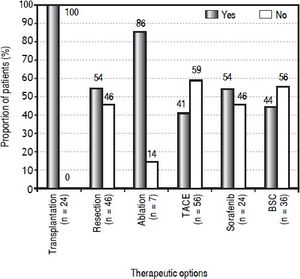
The proportion of patients by therapeutic option treated according to or different from their BCLC stage is shown in figure 3. The curative therapy options followed BCLC recommendations with most accuracy. All 24 transplanted patients had a BCLC 0 or A tumor stage. Resection was offered to 46 patients, but 21 (46%) did not have very early or early HCC, for which it is recommended. Of those, 18 patients had intermediate HCC and three had advanced HCC; none had portal hypertension, 13 were non-cirrhotic (62%; cirrhotic patients all had a Child-Pugh A score), 19 had normal bilirubin (90%) and 14 had a single tumor (67%). Ablation was offered to seven patients, six of whom had a very early or early HCC and one of whom had an intermediate HCC. The majority of the palliative therapy options were allocated to patients with tumor stages not corresponding to the recommendations. Of 56 patients treated with TACE, 33 (59%) did not have an intermediate HCC; instead, 29 patients were stage 0-A either on the transplantation waiting list or not eligible for transplantation or refused it (8/29 had a Child-Pugh B score), three were in the advanced stage but without vascular invasion or extrahepatic spread (all had a Child-Pugh B score), and one was in the terminal stage, classified because of a PS of 3 (Child-Pugh B). For patients treated with sorafenib, 46% (11/24) did not have advanced HCC, but 10 patients had intermediate HCC (9/10 were not eligible for TACE and 1/10 refused it) and one had terminal HCC. For patients who received best supportive care, 56% were not in a terminal stage (20/36), but rather four patients had early HCC (patient’s choice), four had intermediate HCC (3/4 refused any treatment, 1/4 was not eligible for any other treatment), and 12 had advanced HCC (9/12 were not eligible for sorafenib, 2/12 refused any other treatment, 1/12 died before any treatment could be started).
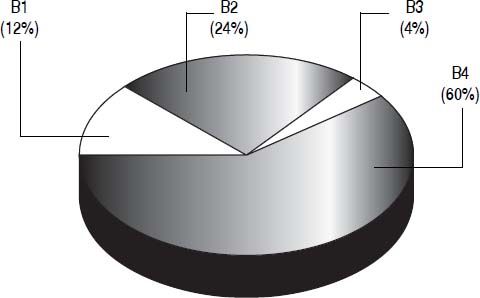
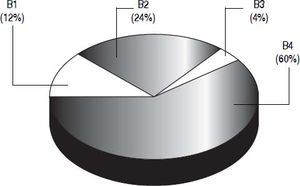
Patients with intermediate HCC were classified into one of four substages (B1-B4) based on the proposal from Bolondi, et al. (Figure 4).13 Based on a PS of1, 60% (47/78) of stage B patients were categorized as B4 substage. According to their Child-Pugh score and their up-to-seven criterion, nine patients (12%) were classified as B1, 19 (24%) as B2, and three (4%) as B3. As for the therapies allocated among the four sub-stages, they were very heterogeneous and no one therapy was more prevalent in any of the substages.
Distribution of the 78 BCLC B patients into the four substages (B1-B4) according to the Bolondi, et al. proposal for subclassification.13
This study assesses prospectively the treatment allocated to patients with HCC. Of 52% of patients whose firstline treatments were not aligned with the BCLC algorithm, most were patients with intermediate or advanced HCC, with two-thirds not receiving a recommended therapy.
The majority of patients in the 0 and A stages received recommended treatment. This can be explained by the international acceptance of well-defined criteria for transplantation (known as the Milan Criteria), which select eligible patients according to their tumor burden; no restriction is mentioned regarding Child-Pugh and PS score.16,17 Liver transplantation was indeed exclusively offered to patients with BCLC stages 0 and A. Resection, which was mostly allocated to patients with stages 0-A, also has precise criteria: absence of cirrhosis (18,19) and, in case of cirrhosis, normal portal pressure and bilirubin.20 In 89% of BCLC D patients, the recommended best supportive care was received. The prognosis of these patients is dictated by the advanced liver disease rather than by the HCC, with oncology treatments not altering the prognosis. The situation is different for patients in BCLC B and C stages. Only one-third of these patients were treated according to the algorithm. In 32 cases, tumor burden of BCLC B patients was too extensive to be treated with TACE and these patients received various treatments including TARE, external radiotherapy, sorafenib, or best supportive care. In nine cases, BCLC C patients were not eligible for sorafenib and instead received best supportive care. The clinical profile of the B and C stages is heterogeneous and is based on Child-Pugh class A or B, tumor burden, presence or absence of vascular invasion and/or metastatic spread, and PS 0-2. The BCLC B stage particularly encompasses a highly heterogeneous population, as shown by Bolondi, et al. in their proposal for subclassification of this stage.13 The 78 patients with intermediate HCC were classified according to this proposal and in 60% of the cases, their PS determined the substage. As a result of the emphasis placed on PS in the Bolondi, et al. proposal, the distribution in each substage was completely different from that demonstrated in an Italian study that aimed to investigate the prognostic capacity of this subclassification.21
Resection was allocated to 18 patients with intermediate HCC and to three patients with an advanced HCC, given that tumor size is not a clear-cut limiting factor and multifocality is not a contraindication for resection.2 Resection may be performed safely and with substantial survival benefit in patients with intermediate stage tumors with Child-Pugh A and in properly selected patients with vascular invasion.22 The best candidates for TACE are patients with preserved liver function and intermediate HCC < 5 cm not amenable to curative treatments, whereas vascular invasion and extrahepatic spread are major contraindications.23-25 TACE was allocated to 33 patients who were not in an intermediate HCC stage. Most were BCLC 0-A, although three were BCLC C and one was BCLC D without vascular invasion or extrahepatic spread. Patients with BCLC 0-A either received TACE as a bridge therapy or refused/were not eligible for transplantation.
Our results identify differences between HCC patients allocated to the recommended treatments and patients who were not. Hepatitis B was present in younger patients and was thus associated with a treatment allocation corresponding to the guidelines and also with more frequent transplantation. A significantly greater proportion of patients with a PS of 3 and with Child-Pugh class C (corresponding to patients with a terminal tumor stage) were treated with best supportive care as recommended. Surprisingly, neither the presence of comorbidities nor advanced age in the overall population were significant factors influencing a deviation from the algorithm.
The BCLC algorithm is not only referring to treatments with randomized controlled trials (RCTs) showing a benefit, i.e. TACE, sorafenib, but also to treatments perceived as well established despite the lack of RCTs, i.e. liver transplantation.3 The present version of the algorithm does not incorporate important treatments that are nevertheless widely used: TACE as a bridge therapy to the transplantation, TARE, and external radiotherapy. Also, BCLC B stage does not have any limitation regarding tumor burden, though it was shown that a tumor > 5 cm negatively affects response to TACE.25 Also, the algorithm does not allow intermediate and well-selected advanced patients to be resected, although it is a practice that may have benefits.22
Performance status is a decisional parameter in the BCLC algorithm which is subjective, whereas the two other criteria used to classify HCC in the BCLC algorithm are objective, i.e. tumor burden and liver function. Although we considered cancer-related symptoms, PS can be differently appreciated and it is difficult to differentiate the symptoms due to cancer from symptoms due to comorbidities. Moreover, the difference between the Kaplan-Meier estimated overall survival curves of patients with PS 0 and PS 1 was not significant. Therefore, a PS of 0 or 1 was not taken into account when assigning patients to a BCLC stage. Besides, a recent study has also shown that the difference in prognosis between PS 0 and 1 was not as substantial as that between PS 1 and 2-4.22 In the advanced and terminal stages, PS plays an important role as it is often the decisive criterion used to assign patients to these stages.
These considerations have led to the development of a proposed alternative staging system, which is a revised version of the BCLC-the Bern Clinic Liver Cancer (Be-CLC) algorithm (Figure 5). This new version lends less weight to PS, as patients with a PS of 1 are also accepted in the very early, early and intermediate stages, and patients with a PS of 0 in the advanced stage. The BeCLC algorithm also incorporates treatments missing from the BCLC staging system. Furthermore, it allows patients with an intermediate or advanced HCC to undergo surgical resection, especially if they are noncirrhotic. The original description of the BCLC staging classification was designed for cirrhotic patients,26 whereas subsequent versions are used in clinical practice to manage both cirrhotic and noncirrhotic patients. Finally, the BeCLC algorithm includes a limitation of the tumor burden (< 5 cm) for the allocation of TACE among stage B patients, assigning these patients to sorafenib, TARE or external radiotherapy.
The proposed Bern Clinic Liver Cancer (BeCLC) algorithm for the management of hepatocellular carcinoma. The dotted line allows patients with an intermediate or an advanced HCC to undergo surgical resection, especially if they are nonclrrhotlc. HCC: hepatocellular carcinoma. TACE: transarterlal chemoembollzation.
The main limitation of this study is the relatively small number of patients. However, these data were acquired prospectively, in contrast to many publications in the field, and represent the management of HCC in a tertiary university center in Switzerland. It is unclear to what extent this population and these data are representative of those from other centers. The goal of our study is not to provide an outcome analysis. Therefore, there is no evidence that patients who were treated outside the BCLC guidelines did better than if they had been treated within.
In conclusion, this analysis indicates that the majority of curative therapeutic options, which follow specific selection criteria, were allocated in accordance with the algorithm, whereas most of the palliative therapies, whose allocation criteria are less precise, were offered to patients with different tumor stages outside the recommendations. In particular, the PS, which is the sole subjective parameter in the algorithm, appears to be the main reason for deviating from the algorithm.
Abbreviations- •
BCLC: Barcelona Clinic Liver Cancer.
- •
BeCLC: Bern Clinic Liver Cancer.
- •
BSC: best supportive care.
- •
HCC: hepatocellular carcinoma.
- •
MELD: Model for End-Stage Liver Disease.
- •
PS: performance status.
- •
TACE: transarterial chemoembolization.
- •
TARE: transarterial radioembolization.
None.
Financial SupportSwiss Foundation against Liver Cancer, Philanthropic Committee of the Firmenich Family, Bern League against Cancer.
AcknowledgmentsWe would like to thank Malcolm Sturdy of the Clinical Trial Unit and Peter Jüni of the Institute of Social and Preventive Medicine, University of Bern, for their support.