Aim. The aim of this study was to assess the effects of the molecular absorbent recirculating system (MARS) on patients with acute liver failure (ALF) and liver failure with cirrhosis (AoCLF) as well as in cholestatic patients with intractable pruritus in a Mexican population.
Material and methods. From August 2003 to December 2011, MARS was used in 38 patients with ALF, 15 patients with AoCLF, and 17 cholestatic patients with intractable pruritus. The patients were examined using a standard liver function test and for vital signs, presence of ascites and encephalopathy before and after each treatment. The therapeutic response, patient status, follow-up status, and need for liver transplantation were determined.
Results. Seventy-nine MARS procedures were performed. MARS was used for ALF in 54.3% of patients, AoCLF in 24.2%, and cholestatic disease in 21.5%. There were significant improvements in serum bilirubin (p = 0.000), aspartate aminotransferase (p = 0.000), alanine aminotransferase (p = 0.030), gamma-glytamyl transpeptidase (p = 0.044), alkaline phosphatase (p = 0.006), and encephalopathy grade (p = 0.000). Thirty-eight ALF patients were listed for emergency liver transplantation and treated with MARS; 20 of these patients died on a waiting list, 18 survived. only four underwent liver transplantation and 14 (37%) recovered without transplantation after the MARS procedure.
Conclusion. MARS is a safe and effective procedure, especially for ALF patients. Our results suggest that MARS therapy can contribute to native liver recovery in ALF patients.
The molecular absorbent recirculating system (MARS) is an extracorporeal liver support system based on albumin dialysis because albumin is one of the most important molecules involved in detoxification and liver regulation processes. It is an important antioxidant, drug carrier, and transporter for endogenous and physiological anions, including bilirubin, bile salts, long-chain fatty acids, aromatic amino acids, nitric oxide, and cytokines. MARS was introduced in 1993 by two German nephrologists, Mitzner and Stange, from Rostock University, Germany. The major goal of liver support is to increase the patient survival time.1,2 The MARS technique adds the albumin removal (both the bound and water-soluble forms) to acute liver failure (ALF)3–5 and acute on chronic liver failure (AoCLF) management;6,7 It has also been used to treat intractable pruritus in cholestatic diseases.8,9
The principal aim of MARS was to stabilize the patient and allow the liver to recover; further, it is occasionally used as a “bridging” procedure in patients with severe liver disease awaiting recovery or transplantation.
The first MARS treatment in Mexico was performed on August 18, 2003 at Hospital San José Tec de Monterrey on a patient with primary biliary cirrhosis and intractable pruritus. After two sessions, the patient’s pruritus disappeared for a year, but its recurrence prompted another MARS session. After the patient’s symptoms recurred a second time, liver transplantation was performed on January 23, 2005. Through 2011, 79 procedures were performed in various Mexican hospitals.
The aim of this study was to assess the effects of MARS in patients with ALF and AoCLF as well as cholestatic patients with intractable pruritus in a Mexican population.
Material and MethodsBefore the procedure was performed, each patient provided written informed consent. The study protocol conformed to the ethical guidelines in the Declaration of Helsinki 1975 and was approved by the Hospital San Jose’s Ethics Committee Ignacio Morones Prieto # 3000 Col. Los Doctores, Monterrey. N.L., Mexico. It is a transverse retrospective, observation-based, descriptive analysis of patients who underwent MARS treatment in seven different tertiary Mexican centers from August 2003 to December 2011. We extracted the following information: the date; hospital location; demographic data; type of liver failure (acute or acute on chronic) or cholestatic liver disease; liver disease etiology; mean age; sex; proportion of females; MARS session number and duration; maximum, minimum, and medium blood pressures; heart rate; temperature; presence of ascites; hepatic encephalopathy grade; Child-Turcotte-Pugh index baseline; and laboratory test results (hemoglobin; hematocrit; white blood cells; platelets; glucose; creatinine; cholesterol; alanine amino-transferase (ALT); aspartate aminotransferase (AST); bilirubin; alkaline phosphatase (ALP); gamma-glytamyl transpeptidase (GGT); total protein; albumin; globulin; and prothrombin time). The international normalized ratio (INR) for each parameter was measured before and after each treatment. We also recorded the therapeutic response, follow-up duration, patient status, and whether liver transplantation was required.
The inclusion criteria for MARS treatment in patients with ALF (all ALF etiologies, including ALF after liver surgery and after liver transplant dysfunction) were the criteria used to identify patients considered for liver transplantation: King’s College prognostic criteria,10,11 such as level 2 or greater hepatic encephalopathy, elevated intracranial pressure, renal dysfunction, progressive cholestasis with serum bilirubin > 10 mg/dL, INR > 1.5 or prothrombin activity < 40%, and AST or ALT > 1,500 U/L, and the Clichy prognostic criteria with Factor V less than 20% of normal in patients less than 30 years old and less than 30% of normal in patients greater than 30 years old.
In acute on chronic liver failure we included patients with progressive hepatic cholestasis who did not respond to three days of standard therapy; alcoholic liver disease patients with Maddrey’s scores > 32;12 patients with hepatic encephalopathy (HE) grades 3 or 4 on the West Haven criteria,13–15 which involves clinically assessing the HE grade and a validated neuropsychological test (number connection test [NTC-A]); and patients with progressive renal failure or type 1 or 2 hepatorenal syndrome (HRS).
For patients with cholestatic diseases, we included patients with intractable pruritus or an indication for liver transplantation to facilitate long-lasting or temporary relief from pruritus or or avoid or delay liver transplantation. These patients were treated with two 7 h MARS sessions, one day apart.8 The pruritus level was recorded before and after each session, first week post-procedure, monthly for the first three months and every three months during a follow-up using a visual analogue scale (VAS).16 A standard liver test was performed, and serum bile acid levels were measured.
The exclusion criteria were hemodynamic instability; median arterial pressure < 55 mmHg with inotropic drugs; active bleeding; and severe coagulopathy or thrombocytopenia < 50,000.
Severe coagulopathy often correlates with ALF; very few doses of anticoagulant were provided to maintain systemic recirculation while avoiding systemic coagulation only in such cases.
During treatment, we administered saline solution or heparin (100 UI) at alternate hours.
MARS was performed using an extracorporeal device that dialyzes the blood against albumin. Albumin-bound molecules are transferred to the dialysate albumin, which is continuously regenerated by passage through charcoal and anion-exchange columns as well as conventional dialyzer. The system includes three different fluid compartments: a blood circuit, a circuit with 600 mL of 20% human serum albumin as well as charcoal and anion-exchange resin columns, and a dialysate circuit. MARS requires a standard dialysis machine to control the blood and dialysate circuits. The extracorporeal blood circuit was controlled by dialysis equipment (Fresenius 2008 H; Fresenius Medical Care, Bad Homburg, Germany). An albumin-impregnated highly permeable dialyzer (Mars Flux; Gambro Lundia AB, Lund Sweden) was used as the membrane, which facilitated removal of protein-bound toxins during the blood treatment. A closed loop with 20% commercial albumin and the dialysate solution was used to remove the dialysate toxins. The album dialysate recirculated at a 250 mL/min mean flow rate throughout the dialysate compartment and regenerated online through perfusion over an anion-exchange column and uncoated charcoal column as well as a low-flux dialyzer for dialysis. The treatment was performed every day for ALF and AoCLF over 8 hours.
Patients with cholestatic disease were treated with two 7 h MARS sessions, one day apart.8
Throughout the study, patients scored the pruritus severity using a visual analog scale (VAS).16,17 The scales were designed according to standard principles16 and comprised 100-mm horizontal lines without marks, on which the patient was asked to indicate the itching severity. The left side (0 mm) was labeled “no-itching”, and the right side (100 mm),”unbearable itching”. The pruritus level was recorded before and after each session, throughout the first week post-procedure, monthly for the first three months and every three months during follow-up. Serum bile acid levels were measured.
Statistical analysisThe data are expressed as means ± standard errors of the means (SEM). An unpaired Student’s t test or Wilcoxon test was used when appropriate to analyze the differences before and after the procedure. A 5% probability was regarded as statistically significant.
ResultsFrom 2003 to 2011, 79 MARS procedures were performed in Mexico. We recovered records for 88.6% (70) procedures.
The procedures were performed in seven different Mexican hospitals. Three hospitals were in north Mexico: Hospital San José Tec de Monterrey Instituto Tecnológico y de Estudios Superiores de Monterrey, 34 patients (48.9%); Unidad de Alta Especialidad #25 Instituto Mexicano del Seguro Social Monterrey, 14 patients (14%); and Hospital Universitario “José Eleuterio González”, Secretaría de Salubridad y Asistencia, 13 patients (18.3%). One hospital was in west Mexico: Hospital Civil de Guadalajara, Secretaría de Salubridad y Asistencia, four patients (5.6%). Three hospitals were in the center of Mexico: Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, four patients (5.6%); Hospital de la Beneficiencia Española, in Puebla, one patient (1.4%); and Instituto de Investigación Biomédica, Fundación Clínica Médica Sur.
The baseline demographic data are in table 1.
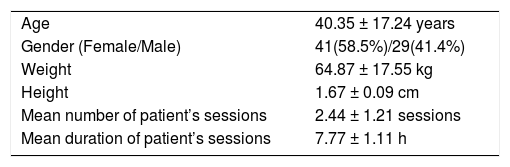
Approximately two (2.44 ± 1.21) treatment sessions were provided to each patient with 7.77 ± 1.11 h per procedure mean duration; 41 patients were female (58.6%) and 29 (41.4%) male. The mean age was 40.35 ± 17.24 years, the mean body weight 64.87 ± 17.55 kg, and the mean height 1.67 ± 0.09 cm. The patients were divided into three groups: ALF, AoCLF, and chronic cholestatic disease. ALF was the principal indication for MARS treatment in 38 patients (54.3%), but MARS was also required in 15 patients (21.5%) with AoCLF and 17 patients (24.2%) with cholestasis and intractable pruritus.
The ALF etiology was principally drug-related with 20 patients (29.2%), severe acute alcoholic hepatitis in six patients (8.5%), hepatitis A virus in four patients (5.6%), subacute hepatitis in four patients (5.6%), autoimmune disease in two patients (2.8%), and idiopathic in two patients (5.6%). In Ao-CLF, hepatic encephalopathy was the principal cause of chronic liver disease with ALF in six patients (8.5%), HRS in five patients (7%), acute alcoholic hepatitis in three patients (4.2%), and icterus in one patient (1.4%). The principal etiology for patients with resistant and intractable pruritus was primary biliary cirrhosis in nine patients (12.7%), post-liver transplantation cholestasis in three patients (4.2%), cholestatic hepatitis in three patients (4.2%), cholestasis secondary to hepatitis C virus in one patient (1.4%), and secondary to sepsis in one patient (1.4%).
Albumin dialysis was associated with a statistically significant improvement in the HE level (p = 0.000). For NCT, the time (in minutes) decreased from 4.41 ± 0.98 to 1.08 ± 0.08 (p = 0.050) with significant reductions in ALT, AST, ALP, and bilirubin (p = 0.000)
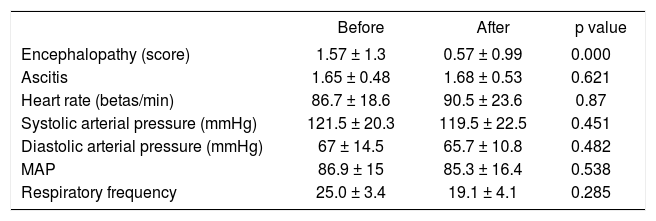
We did not find significant differences in hemodynamics, heart rate, systolic arterial pressure, diastolic arterial pressure, or medial arterial pressure for the patient group treated (Table 2).
Clinical data before and after MARS treatment.
| Before | After | p value | |
|---|---|---|---|
| Encephalopathy (score) | 1.57 ± 1.3 | 0.57 ± 0.99 | 0.000 |
| Ascitis | 1.65 ± 0.48 | 1.68 ± 0.53 | 0.621 |
| Heart rate (betas/min) | 86.7 ± 18.6 | 90.5 ± 23.6 | 0.87 |
| Systolic arterial pressure (mmHg) | 121.5 ± 20.3 | 119.5 ± 22.5 | 0.451 |
| Diastolic arterial pressure (mmHg) | 67 ± 14.5 | 65.7 ± 10.8 | 0.482 |
| MAP | 86.9 ± 15 | 85.3 ± 16.4 | 0.538 |
| Respiratory frequency | 25.0 ± 3.4 | 19.1 ± 4.1 | 0.285 |
Data are expressed as mean ± SDM. MAP: median arterial presure.
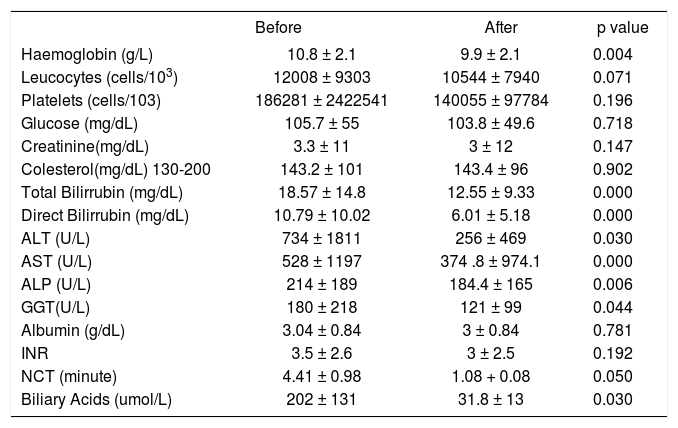
In the biochemical tests, the baseline was significantly reduced for the bilirubin level (p = 0.000), ALT (p = 0.030), AST (p = 0.000), ALP (p = 0.006), and GGT (p = 0.044) after MARS treatment. MARS treatment did not cause significant changes in the albumin, cholesterol, or INR levels. The hemoglobin level was significantly reduced after treatment, but the platelet count was not reduced significantly (Table 3).
Clinical and biochemical data before and after MARS treatment.
| Before | After | p value | |
|---|---|---|---|
| Haemoglobin (g/L) | 10.8 ± 2.1 | 9.9 ± 2.1 | 0.004 |
| Leucocytes (cells/103) | 12008 ± 9303 | 10544 ± 7940 | 0.071 |
| Platelets (cells/103) | 186281 ± 2422541 | 140055 ± 97784 | 0.196 |
| Glucose (mg/dL) | 105.7 ± 55 | 103.8 ± 49.6 | 0.718 |
| Creatinine(mg/dL) | 3.3 ± 11 | 3 ± 12 | 0.147 |
| Colesterol(mg/dL) 130-200 | 143.2 ± 101 | 143.4 ± 96 | 0.902 |
| Total Bilirrubin (mg/dL) | 18.57 ± 14.8 | 12.55 ± 9.33 | 0.000 |
| Direct Bilirrubin (mg/dL) | 10.79 ± 10.02 | 6.01 ± 5.18 | 0.000 |
| ALT (U/L) | 734 ± 1811 | 256 ± 469 | 0.030 |
| AST (U/L) | 528 ± 1197 | 374 .8 ± 974.1 | 0.000 |
| ALP (U/L) | 214 ± 189 | 184.4 ± 165 | 0.006 |
| GGT(U/L) | 180 ± 218 | 121 ± 99 | 0.044 |
| Albumin (g/dL) | 3.04 ± 0.84 | 3 ± 0.84 | 0.781 |
| INR | 3.5 ± 2.6 | 3 ± 2.5 | 0.192 |
| NCT (minute) | 4.41 ± 0.98 | 1.08 + 0.08 | 0.050 |
| Biliary Acids (umol/L) | 202 ± 131 | 31.8 ± 13 | 0.030 |
ALT: alanine aminotrasferase. AST: aspartate aminotrasferase. ALP: alkaline phosphatase. GGT: gamma-glytamyl transpeptidase. INR: International Normalized Ratio. NCT: Number Connection Test.
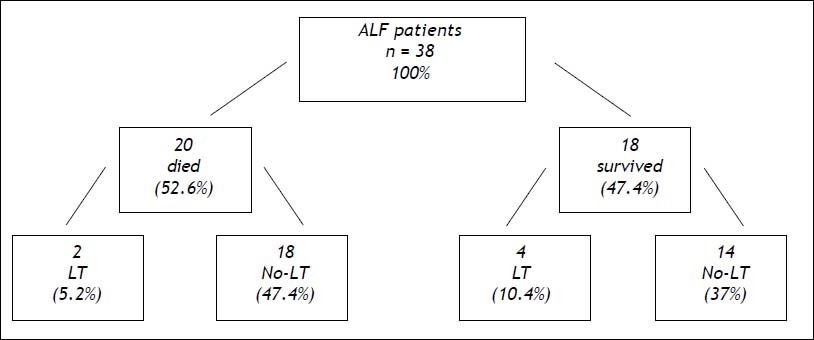
Thirty-eight patients with ALF and listed for emergency liver transplantation were treated with extracorporeal albumin dialysis sessions using MARS; 20 patients died (52.6%), two patients after liver transplantation (5.2%) and 18 patients awaiting liver transplantation (47.4%). Of the 18 (47.4%) surviving patients, four underwent liver transplantation (10.4%), and 14 (37%; 95% confidence interval [CI] 0.5%-93%) avoided transplantation because their liver function improved, and they fully recovered thereafter (Figure 1).
Schematic evolution of 38 ALF patients listed for emergency liver transplantation treated with MARS LT could be avoided in 14 patients (37%, 95% confidence interval [CI]0.5-93%). ALF: acute liver failure. LT: liver transplant. No-LT: no liver transplant. MARS: molecular adsorbent recirculating system.
We noted that the best survival rate in the ALF patients for our population was for patients with a hepatitis A virus etiology (100%) (Table 4).
In the AoCLF group, albumin dialysis was associated with a statistically significant improvement in the hepatic encephalopathy score (p = 0.004) and significant reductions in ALT, AST, ALP, GGT and bilirubin (p = 0.000) (Table 3).
Six patients with hepatic encephalopathy had a significantly improved hepatic encephalopathy grade from stage 3 to stage 1 (p = 0.000).
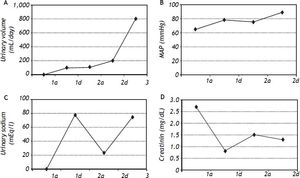
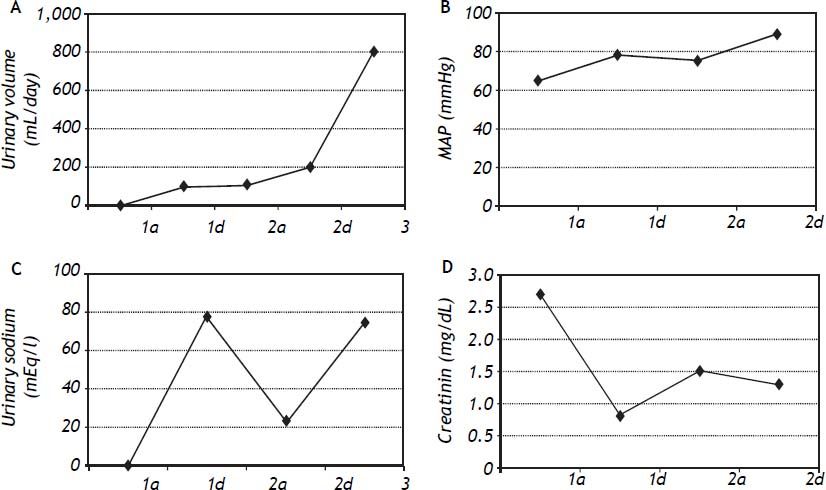
We used MARS in five patients with type 1 HRS (two female and three male patients) and three survived (60%); one was transplanted (20%), and two patients died (40%). The hemodynamic parameters (paired-sample correlations) were significantly different for this patient subgroup from their baseline after MARS treatment with increased mean blood pressure from 117 mmHg to 129 mmHg (p = 0.006) and mean arterial pressure from 78 to 90.75 mmHg (p = 0.030) (Figure 2).
We used MARS in 17 patients with cholestatic diseases, predominantly at Hospital San José Tec de Monterrey (13 patients, 76.5%). Of these 17 patients, 11 (64.7%) were female, 14 (82.4%) survived, and three (17.6%) died; six patients were transplanted (35.3%), and 11 patients were not (64.7%). Only two 8-h MARS sessions were provided, one day apart. We observed a clear association between the MARS treatment and ameliorated itching with VAS scores at 96.3 ± 3.2 before treatment and 3.3 ± 2.8 after treatment. The biliary acid significant declined after treatment (202 ± 131 umol/L to 31.8 ± 13 umol/L (p=.030)). Although the bile acids gradually increased during evolution of the disease, pruritus decreased one month after treatment, and the most of the patients did not develop pruritus thereafter.
DiscussionThere are two different types of liver-assistance devices. Devices with a synthesis function include liver cells or tissue in synthetic housing; although promising, such devices have not been successful in clinical trials. The factors that limit studies on such devices include small numbers of patients, lack of controls and randomization, and heterogeneous etiologies.1 The second type of device includes detoxification processes, such as the Molecular Absorbent Recirculating System (MARS)2 dicussed above, and Prometheus, which involves fractionated plasma separation and adsorption (FPSA). Prometheus was introduced by Falkenhagen et al in 1999.18,19 In this system, a special albumin-permeable filter with a membrane cut-off close to 300,000 Daltons is used. Albumin and the protein-bound toxins pass through the membrane and are directly removed from the blood by a special adsorber in the secondary circuit. This technique combines the FPSA method with high-flux hemodialysis in an extracorporeal detoxification system. Blood from the patient is pumped through a dialysis catheter and passes through an albumin-permeable polysulfone filter (Albu-Flow; Fresenius Medical Care AG, Bad Homburg, Germany). Molecules up to the size of albumin pass easily from the blood into the secondary circuit. There, the filtered albumin-rich plasma flows through one or two adsorbers (Prometheus 01* and Prometheus 02*), where the toxins are directly adsorbed. The purified plasma is then returned to the blood side.This device’s filtration and adsorption capacities are enhanced by using a plasma pump that recirculates the albumin-rich plasma. To eliminate water-soluble toxins, the blood then undergoes hemodialysis through a conventional high-flux dialyzer (FX50). Both the MARS and Prometheus systems have been successful but have not been shown clinically effective at enhancing patient survival.20
ALF remains at an unacceptably high mortality rate absent transplantation. Great effort has been exerted to develop liver support systems as temporary measures for ALF patients to bridge the period before transplantation or ensure patient survival until his/her native liver function recovers.
Unlike other countries, where an organ can be obtained for urgent liver transplantation within 24 h, Mexico has a shortage of donor organs, and the time required to obtain a liver is approximately one week. We use the MARS liver-assisting device to treat liver failure and allow time for native liver recovery or as a bridging step to liver transplantation. Although ALF mortality is very high, we have demonstrated that using MARS for waiting-list patients contributes to native liver recovery in 37% of ALF patients. This result suggests that MARS therapy is an optimal treatment for ALF, can contribute to native liver recovery, and is safe in patients on transplantation waiting lists. The timing of intervention using the MARS device is critical to the ALF outcome, and we recommend that treatment begins immediately after the criteria for liver transplantation are met. Novelli21 and Kantola22 showed the importance of MARS for native liver recovery or serve as bridging therapy to liver transplantation in ALF. Recently, a French group treated 18 ALF patients listed for emergency liver transplantation with MARS. Due to the improved liver function, transplantation was avoided in 9 patients, who fully recovered thereafter (50%, 95% confidence interval [29-71%]).23
In the subgroup of AoCLF patients with HRS, we observed improved hemodynamic parameters, which is consistent with Schmidt et al., who demonstrated beneficial effects in ALF and AoCLF patient hemodynamics.5,7 The improvements included increased median arterial pressure, heart rate, cardiac output, and systemic vascular resistance, which was attributed to reduced vasoactive agents, such as renin, angiotensin, aldosterone, and nitric oxide.24–26
The MARS device, but not the Prometheus device, significantly attenuated hyperdynamic circulation in AoCLF patients, presumably through a different removal rate for certain vasoactive substances, which suggests conceptual differences between the devices.27,28
The current therapy for liver failure with cirrhosis is limited to treating the precipitating event and managing the existing and potential liver failure complications. However, the MARS objective was to eliminate the precipitating event, while facilitating liver recovery to its previous compensated state. In our study, MARS improved severe hepatic encephalopathy (p = 0.000) in ALF and AoCLF patients, consistent with a randomized controlled trial by Hassanein, et al. in 2007.29 The 70 patients were used in the trial (56% with grade 3 hepatic encephalopathy and 44% with grade 4). The patients were randomized for MARS or a standard medical therapy (SMT). The proportion of patients that improved was greater in the MARS group than the SMT group (34 vs. 18.9%, respectively; p = 0.044), and improvement was faster and more frequent for the MARS group compared with the SMT group (p = 0.045).
Parés, et al. also showed improved hepatic encephalopathy and reduced total amino acid levels (causing an increase in Fisher’s ratio), which was attributed to significantly reduced circulating phenolic aromatic amino acids in patients with severe liver failure treated with MARS.30
A review of MARS for hepatic encepalopathy in liver failure’s treatment was made and a case report of three cases treated with MARS one with ALF associated with EBV infection, one with AoCLF chonic hepatitis B infection, and one with cryptogenic hepatitis MARS was an effective strategy in this three cases, greatly improved the biochemical variables with no impact on mortality rate.31,32
For treating resistant pruritus in patients with chronic cholestasis, we demonstrated a clear association between the MARS treatment and itching relief. The scratched skin lesions improved or disappeared in parallel with the alleviated itching. The decrease in itching was significant, as measured by VAS and circulating bile acids.
Parés, et al. demonstrated that MARS is an effective alternative for treating patients with cholestatic pruritus, who have not responded to other therapies.8 Acevedo, et al. also concluded that MARS is an effective treatment for intractable pruritus in cholestasic liver disease.9
ConclusionOur results for this Mexican population suggest that patients with ALF or cholestatic diseases should benefit from MARS treatment. This treatment also improves the clinical condition of patients with cirrhosis and superimposed acute injury. The procedure appears to be safe and well-tolerated. This study has especially demonstrated that ALF can be reversed using MARS, which may also act as a bridging step to liver transplantation. Furthermore, in 37% of patients, transplantation was entirely avoided because liver function improved, and they fully recovered. Therefore, MARS is another therapeutic option for transplantation centers. In Mexico with a shortage of donor organs, we use MARS as a bridge to transplantation. Our results suggest that MARS therapy is the optimal treatment for ALF, can contribute to native liver recovery, and is safe for patients on liver transplantation waiting list.
Abbreviations- •
ALF: acute liver failure.
- •
AoCLF: acute on chronic liver failure.
- •
FPSA: fractionated plasma, separation adsorption and dialysis.
- •
HE: hepatic encephalopathy.
- •
HRS: hepatorenal syndrome.
- •
MARS: molecular adsorbent recirculating system.
- •
NCT: number connection test.
- •
SMT: standard medical therapy.
- •
VAS: visual analogue scale.
This trial was neither based on grants nor financial support.





![Schematic evolution of 38 ALF patients listed for emergency liver transplantation treated with MARS LT could be avoided in 14 patients (37%, 95% confidence interval [CI]0.5-93%). ALF: acute liver failure. LT: liver transplant. No-LT: no liver transplant. MARS: molecular adsorbent recirculating system. Schematic evolution of 38 ALF patients listed for emergency liver transplantation treated with MARS LT could be avoided in 14 patients (37%, 95% confidence interval [CI]0.5-93%). ALF: acute liver failure. LT: liver transplant. No-LT: no liver transplant. MARS: molecular adsorbent recirculating system.](https://static.elsevier.es/multimedia/16652681/0000001300000002/v1_201906020852/S1665268119308877/v1_201906020852/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)