Bhattacharya et al.[1] have called our attention to the well-known risk that patients with cirrhosis face for developing associated and frequently deleterious infections. By definition, liver cirrhosis concurs with an immune-depressive status; it is therefore not surprising that the natural history of cirrhosis is linked to an acute or chronic infection or, as is frequently the case, to recurrent infections. Most works on the subject describe the medical measures recommended for preventing infections. However, the rules for controlling the sale of antibiotics worldwide, but particularly in developing countries, are weak, thus making it necessary to establish more rigorous preventive actions to control the side effects of infections that are resistant to antibiotics (usually unnecessarily prescribed) or even multi-drug-resistance (MDR).
Consistent with the findings of other studies, Bhattacharya et al. have confirmed the importance of infections –specifically spontaneous bacterial peritonitis (SBP)- among individuals with liver cirrhosis. These patients also suffer from other bacterial infections such as those affecting the lower respiratory tract (15-21%), spontaneous bacteremia (12%), bacterial enterocolitis (13.9%), skin infections (11%) and urinary tract infections (20-25%). Moreover, they are highly vulnerable to medical iatrogenic factors [2–4]. Infections in patients with cirrhosis contribute to 30-50% of deaths, with a higher prevalence of nosocomial infections [3]. Patients with cirrhosis are exposed to antibiotics for prophylaxis and treatment. In very few cases have controlled studies demonstrated that prescribing antibiotics is beneficial from a cost-benefit perspective.
We believe that is time to introduce global policies and establish government guidelines based on evidence from controlled studies in order to effectively regulate activities such as the over-the-counter sale of drugs. Such studies are urgently needed for guiding the proper use of antibiotics in treating cirrhosis if efforts to prevent MDR infections are to succeed in the case of this and other chronic diseases. Unfortunately, Auta et al [5]. recently demonstrated that acquiring antibiotics without restrictions is possible in many countries through prescriptions at community pharmacies. In their 2000-2017 meta-analysis on the effects of the unregulated sale of antibiotics, these authors presented data suggesting that the overall pooled proportion of antibiotics sold without a prescription had reached 72%. Proportions are high even in partially developed countries such as Mexico, where a ban on the sale of antibiotics without a prescription was instituted only nine years ago. With regard to Latin America specifically, aside from Mexico, only Chile, Costa Rica, Peru and Venezuela have enacted such restrictions.
In their article, “Infection in Cirrhosis: A Prospective Study,” Bhattacharya et al. demonstrate the importance of infections in patients with cirrhosis as a factor associated with increased mortality and morbidity. Infections in these patients represent a public-health issue of critical importance, reflected in a greater prevalence of drug resistance, a diminished quality of life and the need for increasingly larger health budgets. According to a recent retrospective study, the mortality rates in patients with cirrhosis and a number of other infections have risen by 18% [6]. In decompensated patients, survival depends on controlling the factors leading to decompensation, including infections [2]. Failure to establish clear differences –as defined by disease scores or biochemical markers- has rendered identification of the at-risk population more difficult. Throughout the world, clinicians face a new epidemiological challenge: antibiotic and drug resistance. Cirrhosis-related infections are very much a part of this growing public-health problem.
MDR, defined as antimicrobial resistance to at least one agent across three or more antimicrobial categories, results from one of two mechanisms: [1] multiple genes accumulated by bacteria, each coding for resistance to a single drug within a single cell, and [2] increased expression of genes that code for multidrug efflux points [7,8].
The prevalence of MDR has been reported as ranging from 4% to 20%, with higher prevalence in nosocomial infections [2,7]. MDR affects both Gram-positive and Gram-negative bacteria, but therapeutic options are more limited [7] for the latter. MDR is a major global public-health concern, magnified by antibiotic overuse and unwarranted prescribing antibiotics [7]. The use of inappropriate empiric antimicrobials increases the risk of MDR and mortality.
MDR has been identified as occurring after the prescription of third-generation cephalosporins, an antimicrobial agent frequently used as a standard treatment for SPB [7,9]. On the other hand, in the curative treatment of cirrhosis, infections by MDR organisms affects patients both prior to and following liver transplants, representing a major cause of morbidity and mortality. It has recently been observed that MDR increases the risk of hepatic encephalopathy, hepatorenal syndrome and hydropic decompensation among patients on the waiting list for liver transplants, with MDR representing an independent risk for poor survival among this subpopulation [10]. In addition to its clinical impact, MDR has important socioeconomic consequences and has been estimated to incur expenses greater than US$ 4.5 billion. These high costs could have a negative impact on health budgets around the world [11].
Patients with bacterial infections related to MDR require repeated hospitalization and invasive procedures involving a high risk of exposure to other infections. Repeated and prolonged hospitalization represents increased individual, institutional and governmental costs. These factors also increase the level of MDR prevalence in health-care environments, especially those without effective policies in place for epidemiological surveillance.
Various alternatives have been developed for non-antibiotic prophylaxis such as the fecal microbiota transplant, pre- and pro-biotics and bile acids; however, studies on the cost-effectiveness of these options are needed in order to evaluate the advisability of their inclusion as part of public-health policies. The empiric administration of certain broad-spectrum antimicrobial agents seems to be effective on an individual level but does not represent a beneficial option from a public-health perspective [7].
1Potential measures for reducing MDRIn clinical practice, hepatologists must consider the presence or absence of MDR risk factors when formulating antibiotic strategies for treating patients with cirrhosis. Prophylaxis for the most common infection in patients with cirrhosis (SBP) could be useful in the case of other bacterial infections; however, it is necessary to establish how prophylaxis affects the frequency of MDR. It is also essential to improve and monitor medical education in order to avoid the overuse of antibiotics, ensure the appropriate prescribing of antibiotics and implement early de-escalations strategies – these representing the previous rules for preventing infections and MDR in patients with cirrhosis. Several studies have demonstrated the risk of infections in hospitalized patients. However it is imperative to focus, as well, on MDR prevention among the non-hospitalized population, often exposed to the indiscriminate use of antibiotics in primary-care centers.
Is important to note that the risk of infection is higher in the presence of other comorbidities that compromise immunologic function, such as type 2 diabetes mellitus. This acquires additional importance given that non-alcoholic fatty liver disease, such as hepatic manifestations of metabolic syndrome, will be the major cause of chronic hepatic disease within a few years; a higher prevalence of cirrhosis will also increase the risk of infections and MDR.
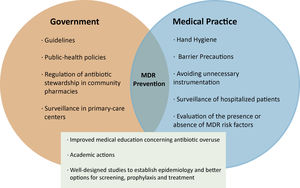
Prevention of infections and MDR begins with simple steps, some related to medical practice and others to government regulations. These include measures to improve hand hygiene and barrier precautions, and avoid unnecessary instrumentation in order to reduce the risk of cross-transmission. Additionally, surveillance strategies must be implemented not only in hospital units, but also in primary-care centers, ideally with specific teams dedicated to monitoring the presence or absence of risk factors for the development of MDR prior to the prescribing of antibiotics [12].(Figure 1). Multicenter studies focused on the epidemiological characteristics of MDR in patients with cirrhosis are also needed in order to establish better options for screening, prophylaxis and the treatment of infections in this clinical scenario.
Beyond this, health ministries and the World Health Organization must help to establish policies and guidelines to regulate over-the-counter sale of antibiotics. We understand that pharmaceutical corporations are large and influential actors, not only in Mexico. Prior to the implementation of the medical prescription requirement, the market of antibiotics sold without prescription accounted for 6,500 billion Mexican pesos annually. Unfortunately, to date, no studies have been conducted comparing the effects of such bans on the MDR ratio before and after the free versus controlled sale of antibiotics in various countries.