Nonalcoholic fatty liver disease (NAFLD) encompasses: fatty liver (SS), steatohepatitis (NASH) with or without fi-brosis and cirrhosis. Patatine-like phosphatas in domain 3 (PNPLA3; adiponutrin; SNP rs738409 C/G, M148I) shows anabolic and catabolic activities on lipid metabolism and significant association to fatty liver content; however, I148M demographics and ethnics, as its role with NAFLD have not been fully elucidated.
Material and methodsPNPLA3 genotyping from peripheral blood DNA by polymerase chain reaction (PCR) and direct sequencing, 211 patients diagnosed with NAFLD including SS, NASH and fibrosis spectrum.
ResultsEighty nine per cent showed the G risk allele [CC: 23 (10.5%), GC: 73 (34.7%), GG 115 (54.7%)], the allele frequency was 77%, NASH (71%), SS (80%) and fibrosis (73%). GG genotype carriers showed 3.8 times (CI 95%: 3.03 - 4.79) of increased risk of steatohepatitis and 2.3 times more (CI 95%: 1.77 ~ 3.23) risk of having liver fibrosis (CC). PNPLA3 (GC, GG) conditioned higher probability of low levels of HDL cholesterol (p < 0.010), SS even in normal weight (p < 0.007), insulin resistance by HOMA (p < 0.029), NAFLD fibrosis score showed > 0.675 (p < 0.001) and altered serum alanine aminotransferase (p < 0.05).
ConclusionPNPLA3 expression in Hispanics could be decisive in NAFLD pathogenesis, it's highly prevalent and it's a key to condition and determine the spectrum associated, SS, NASH and fibrosis.
The NAFLD, Nonalcoholic fatty liver disease, is a clinical and morphological entity characterized by histo-logical findings similar to those seen in alcoholic liver, nevertheless affecting patients not consuming the amount of alcohol knowing to cause liver damage. Diagnosis is performed by ultrasonography (hepatic steatosis) and/or hepatic biopsy.1 It is the third worldwide leading cause of liver disease; however, in the west, conditioned by obesogenic environment, ranks the first place.2
The NAFLD spectrum includes: steatosis (SS), steato-hepatitis (NASH), fibrosis, cirrhosis and hepatocellular carcinoma; hepatic steatosis represents the first evidence of organ damage.3 Those who develop NASH; in high contrast to those with steatosis, are more likely to progress to more severe states; this differentiation is conditioned by different factors ongoing research.
In 2008, a Single Nucleotide Polymorphism (SNP) in the gene for phospholipase A3: PNPLA3 was identified and strongly associated with increased triglycerides in the hepatic content.1,4 The variant is due to the substitution of methionine by isoleucine at residue 148 of PNPLA3, which results in an increased lipogenesis (SNP rs738409 C/G, M148I). It is predominantly expressed in liver and adipose tissue, it has lipolytic and lipogenic activity in vitro and it is stimulated by glucose and insulin. Speliotes, et al. demonstrated that G allele of rs738409 PNPLA3 confers increased risk of NAFLD, histologically confirmed (odds ratio [OR] = 3.26, 95% [CI] = 2.11-7.21; P = 3.6 3 x 10-43).5 There was no observed association with IMC, triglycerides, HDL or diabetes, so it can be concluded that PNPLA3 genetic variations have no visible effects on metabolic syndrome components.2,6
It is, nowadays known, that PNPLA3 contributes to ethnic and differences regarding hepatic fat content and progression susceptibility in the spectrum, but not in metabolic syndrome components.7,8 This SNP is common in Hispanic population and it has been demonstrated to be associated to NAFLD in Mexican population with allele frequency of 59%.9,10 We aimed to analyze the association between SNP PNPLA3 and NAFLD in adult Mexican patients.
ObjectiveDetermine association; in Mexican adults, of SNP PNPLA3 with NAFLD and its spectrum related; fatty liver, steatohepatitis and fibrosis.
Material and MethodsEthnicity and ethicsParticipants were Mexicans, recruited in a consecutively fashion, half-caste, with more than 2 family generations living in Mexico City, between 18 and 70 years old and they all signed informed consent prior to participation. The study was approved by the Institutional Ethics Committee and was conducted according to WMA Declaration of Helsinki. The study was evaluated in conferences and research seminars by outside researchers.
DesignTransversal and observational case-control study was implemented. Comparison of NAFLD patients based on the presence (cases) or absence(controls) of risk allele (G) of PNPLA3. All patients belonging to Instituto Na-cional de Ciencias Médicas y Nutrición Salvador Zubirán from December 2013 to January 2015.
Study populationNAFLD patients according to 20121 Guides diagnose criteria. Patients with secondary causes of steatosis, such as those related to alcohol consumption were excluded. Fatty liver ultrasound diagnosis was performed by two standardized radiologists; equipment with 3.5-MHz transducer (Adama Siemens, Erlangen, Germany) was used. Liver biopsy was used in cases where both; the treating physician indicated it for diagnosis approach and, where the patient consented. The doctors who performed the imaging studies were blinded to patients’ diagnosis. It had previously been documented interobserv-er agreement kappa of 0.83 among radiologists who performed and interpreted studies. Two pathologists blinded to diagnosis and comorbidities performed biopsy interpretation according to Brunt and Kleiner classifi-cation.11,12
Clinical and demographic factorsAll patients received medical assessment; nutritional, anthropometrical, abdominal ultrasound and laboratory studies. The criteria for metabolic syndrome were consistent to the agreed definition published in Circulation 2009.13 The figures for abdominal circumference validated in Mexican population: > 90 cm in men and> 80 cm in women. Alcohol consumption – related liver disease was according to if in a 2 years period prior to the date of inclusion or liver biopsy; the patient had a higher intake of 21 cups for men and 17 cups for women, within a week.12 Five basal samples of peripheral venous blood were obtained at intervals of one minute. Total cholesterol, triglycerides, low-density lipoprotein cholesterol (HDL) and non-HDL; liver function tests, fasting glucose and insulin for calculating insulin resistance index (HOMA) was analyzed. Glucose alterations and diabetes were diagnosed according to the International Diabetes Federation.14
Ultrasound as diagnostic method for NAFLDUltrasound is the most common image method to assess the diagnosis for fatty liver due to its low cost, wide availability and noninvasive nature.15,16 The sonographic criteria for steatosis diagnosis include: liver hyperecho-genicity, far field attenuation and limited diaphragm visualization. They are rated from 1 to 3 (1 Mild, 2 Moderate and 3 Serious). Sensitivity goes from 85% to 95% and specificity from 90 to 100%.17-19
SNP selection and genotypingGenomic DNA was isolated from peripheral blood leukocytes by salt fractionation method (96 QIAmp DNA Blood Kit, Quiagen). Samples were genotyped for SNP rs738409 PNPLA3 using TaqMan probes for allelic discrimination (LightCycler 480 Real-Time PCR System, Roche). Genotyping quality was examined by a quality control procedure with a success rate of 95%, positive internal controls samples and Hardy-Weinberg balance test. Laboratory personnel was blinded to the source of samples and their diagnoses.
Statistics analysis- •
Calculation of sample size. Based on N. Méndez, et al.9 the allele frequency was 59% and the variant effect on the odds ratio for having fatty liver was in the range of 1.8 to 2.3 (OR 1.8 reported for NAFLD). The number of participants required for a case-control study by additive or dominant model is 53 for GG, 53 for GC, 53 for CC, with 80% of statistical power, using QUANTO® software.
Descriptive statistics was used; average ± standard deviation or median and interquartile ranges as appropriate to distribution type. We use T-Student for continuous variables and χ2 for categorical ones. For allele frequency associations, χ2 or Fisher exact test was used. Performance of correlation analysis between grade of steatosis determined by biopsy and that by ultrasound and also fibrosis determined by NAFLD fibrosis score and that by Spearman Rho biopsy. Clinical variables and genetic markers were analyzed with Pearson χ2 test and trend. Regression analyses were performed to control for the effect of age, AST, ALT, BMI, and GenotipoPNPLA3 additive and dominant. We consider statistically significant at p < 0.05. Statistical SPSS v.20 (Illinois USA) and SDS 2.2 softwares were used.
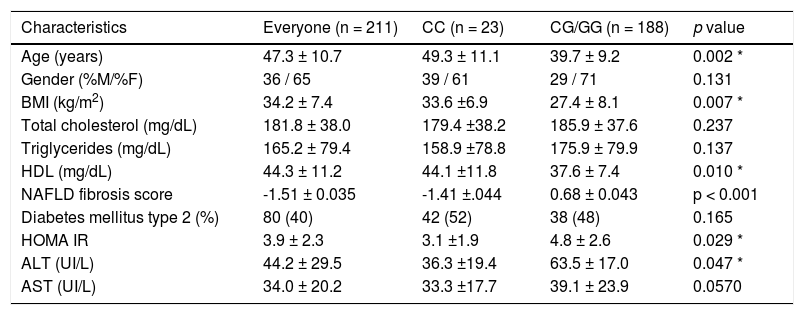
ResultsSamples from 211 patients, between 18 and 67 years old; 136 (64.5%) were women, 80 (40%) had diabetes mellitus type 2. One hundred percent had ultrasound: 78 patients (37%) had steatosis grade 1, 72 (34.1%) steatosis grade 2 and 61 patients (28.9%) steatosis grade 3. In 95 patients (45%) liver biopsy was done, from those 61/95 (64.2%) had NASH and 41/95 (43.1%) some grade of liver fibrosis. The correlation between the grade of steatosis determined by biopsy and that determined by ultrasound was r = 0.76 (p < 0.001). The correlation between fibrosis determined by NAFLD fibrosis score that by biopsy (presence or absence of fibrosis) was r = 0.83 (p < 0.001). Demographic characteristics and main variables are detailed in table 1.
Demographic characteristics and main variables.
| Characteristics | Everyone (n = 211) | CC (n = 23) | CG/GG (n = 188) | p value |
|---|---|---|---|---|
| Age (years) | 47.3 ± 10.7 | 49.3 ± 11.1 | 39.7 ± 9.2 | 0.002 * |
| Gender (%M/%F) | 36 / 65 | 39 / 61 | 29 / 71 | 0.131 |
| BMI (kg/m2) | 34.2 ± 7.4 | 33.6 ±6.9 | 27.4 ± 8.1 | 0.007 * |
| Total cholesterol (mg/dL) | 181.8 ± 38.0 | 179.4 ±38.2 | 185.9 ± 37.6 | 0.237 |
| Triglycerides (mg/dL) | 165.2 ± 79.4 | 158.9 ±78.8 | 175.9 ± 79.9 | 0.137 |
| HDL (mg/dL) | 44.3 ± 11.2 | 44.1 ±11.8 | 37.6 ± 7.4 | 0.010 * |
| NAFLD fibrosis score | -1.51 ± 0.035 | -1.41 ±.044 | 0.68 ± 0.043 | p < 0.001 |
| Diabetes mellitus type 2 (%) | 80 (40) | 42 (52) | 38 (48) | 0.165 |
| HOMA IR | 3.9 ± 2.3 | 3.1 ±1.9 | 4.8 ± 2.6 | 0.029 * |
| ALT (UI/L) | 44.2 ± 29.5 | 36.3 ±19.4 | 63.5 ± 17.0 | 0.047 * |
| AST (UI/L) | 34.0 ± 20.2 | 33.3 ±17.7 | 39.1 ± 23.9 | 0.0570 |
Risk allele G = C = wild-type allele. Data are expressed as means ± standard deviation/frequencies (percentages),* p < 0.05.
According to genotyping: 23 patients (11%) were CC, 73 (34.5%) were GC and 115 (54.5%) were GG. By dominant analysis, 188 patients (89.5%) carried the risk allele (G), (p 0.039, OR 4.34, CI 95% 1.43 - 5.2). The overall al-lele frequency was 77%. The allelic frequency as variant was: 80.47% for SS, 71.87% for NASH and 73.1% for fibro-sis. The observed frequencies were in balance to those expected.
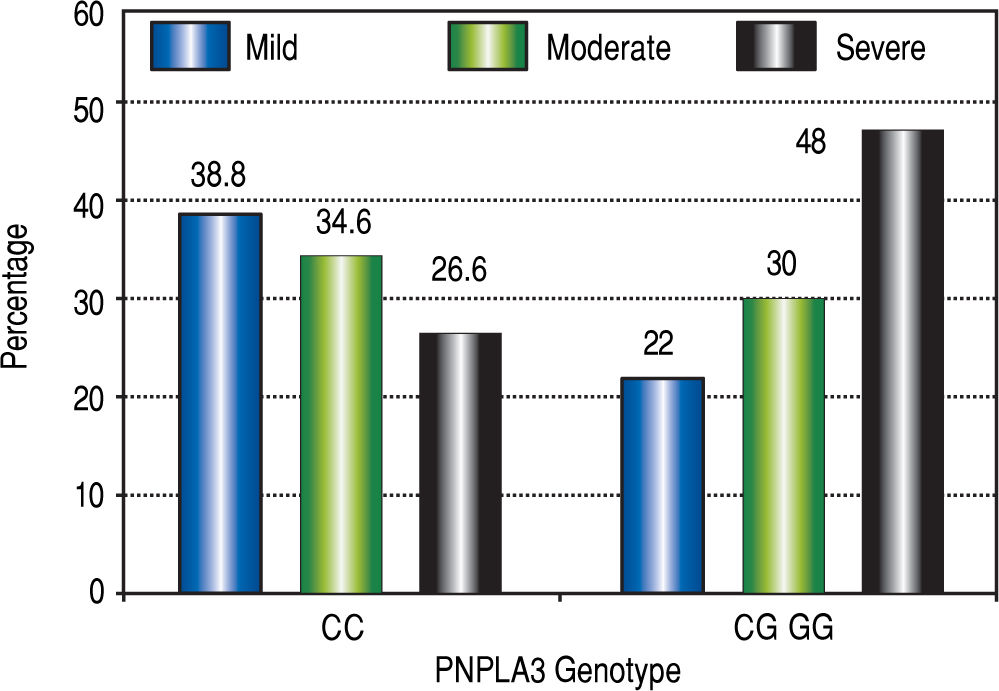
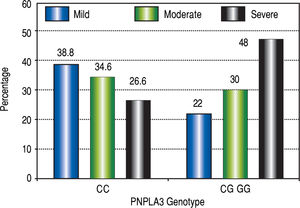
The effect of I148M PNLAP3 variant on the expression of seriousness of hepatic steatosis determined by ultra-sonography and liver biopsy was progressive depending on the number of copies present of the risk allele (Figure 1).
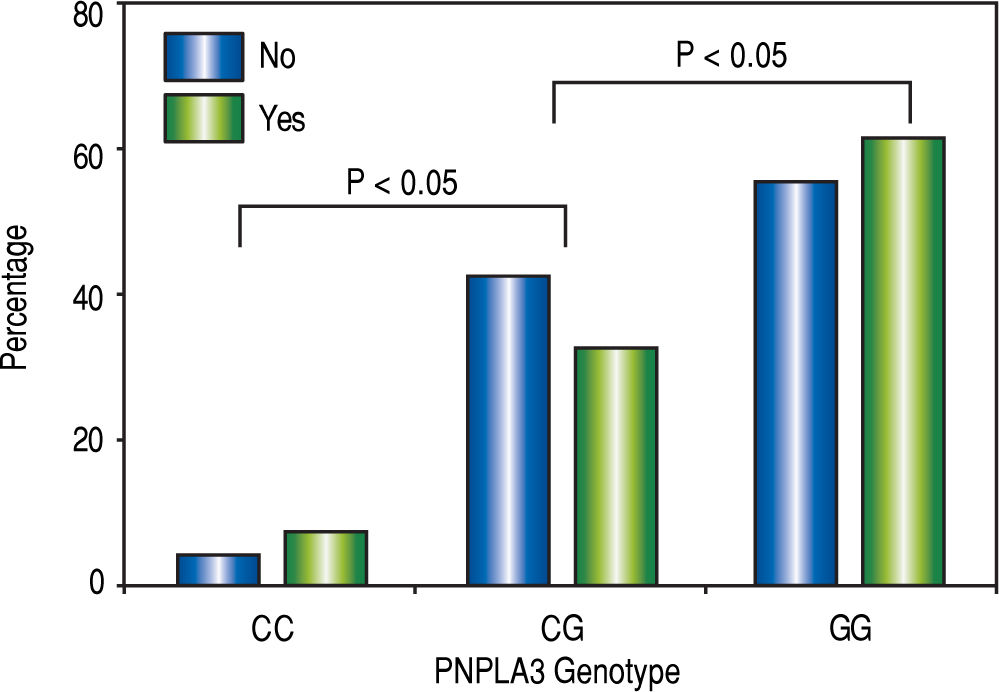
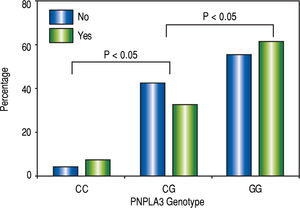
The homozygous risk allele G had 3.81 times more risk of having NASH (p < 0.05, IC 95%: 3.03-4.79) and 2.32 times more the risk of fibrosis (p < 0.05, 95% CI 1.77 ~ 3.23). The probability of having NASH was higher in homozygous and heterozygous carrying the risk allele (Figure 2).
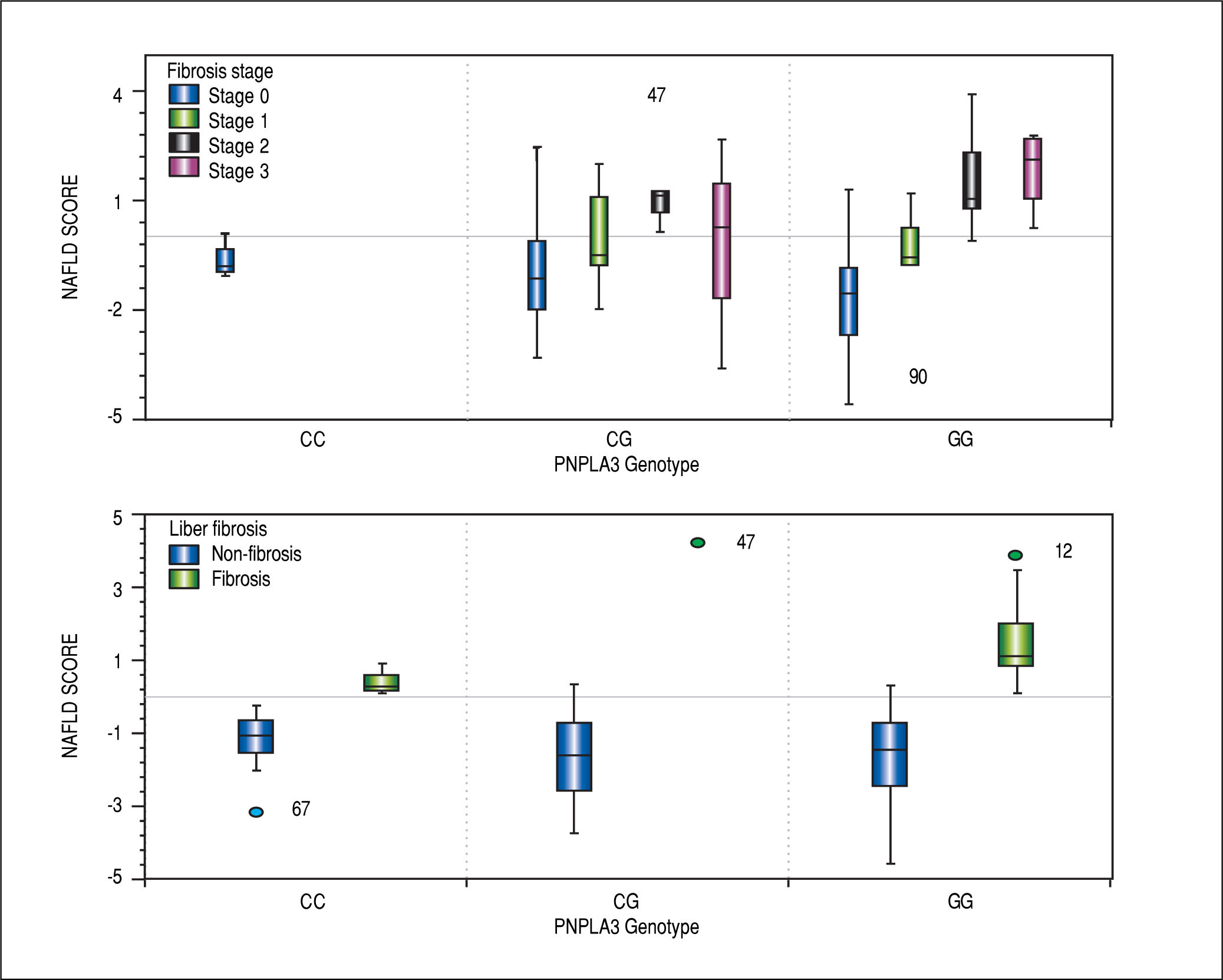
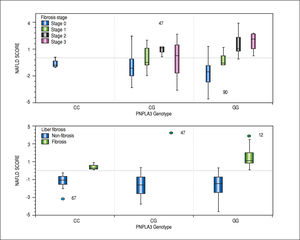
To greater number of copies of the risk allele, the more likelihood of having scores of NAFLD fibrosis score predictors of presence of significant fibrosis (> 0.675) and increased severity of liver fibrosis confirmed by biopsy (Figure 3).
Homozygous and heterozygous patients with the risk allele had higher percentages of severe steatosis vs. the ho-mozygous wild-type allele. The highest percentages of severe steatosis occurred in nondiabetic homo and heterozygous with the risk allele.
After controlling for ALT, AST, age, BMI, PNPLA3 genotype dominant in our multivariate models, fibrosis remains significantly associated with ALT (Odds Ratio [OR]: -0.2767, 95% Confidence Interval [CI]: -0.5518 to -0.0016, p = 0.049), AST (0.5542, 95% CI: 0.1257-9827, p = 0.012), IMC (-0.0124, 95% CI: -0.0048 to 0.0200, p = 0.001), and PNPLA3 genotype dominant (0.1918, 95% CI: 0.0117-0.3719, p = 0.037) but no longer for age. Steatosis remains significantly associated with PNPLA3 genotype dominant (-0.2255, 95% CI: -4194 to -0.0315, p = 0.023), but no longer for IMC, AST, ALT, or age.
DiscussionAlthough etiology and pathogenic mechanisms of NAFLD are not entirely known, all series are consistent in clinical situations called “risk factors”: women (65-83%), obesity (47-100%), diabetes mellitus type 2 (34-55%) and dyslipidemia (20-81%).19,20 The sum of risk factors seems to have an additive role in risk or severity of NAFLD. Obesity is the most associated condition with NAFLD (69-100%),22,23 followed by diabetes mellitus type 2 (34-75%). In some cases not only diagnosis is made at an earlier age, more often in males (53%)21 and most of them are found nondiabetics nor obese and but also in up to 46% cases, underlying factors are not identified then can be inferred that it's genetics what conditions NAFLD and its spectrum.
By ultrasonography was evidenced that severity of hepatic steatosis is greater given the presence of at least one copy of the risk allele for PNPLA3 even in patients without Diabetes Mellitus. The highest percentages of severe steatosis were more common at the sDM2 homo - or heterozygous to allele of risk group, while patients without the risk allele had the lowest percentages regardless co-morbidity DM2.
The allele frequency 77% evidenced in the present study, is higher than the previous 59% documented; finding consistent and alarming at a time, since adding lifestyle, diet and environmental stimuli prevalent in México, it is been enhanced the expression of PNPLA3 and the disease development. In other words, Mexicans have a high genetic predisposition to NAFLD and its complications, furthermore the own specific risk factors of metabolic syndrome that match with our current epidemiological situation; rich in carbohydrates and saturated fats diet, high Diabetes indexes and obesity, strengthens the disease onset since early ages and rapidly towards chronic phases. Not only, we do ignore prevention of a genetics inherent disease, but also we foster it in our habits, forging that way, troubling implications for individual and social health and for a Mexican Health System with no prevention and control schema.
Additive genetic model predominated in this investigation, variant conditioned the expression of fatty liver in heterozygous, but; the effect was even greater in ho-mozygous (Figure 1). PNPLA3 polymorphism PNPLA3 favored predisposition to atherogenesis and cardiovascular risks; primarily, low levels of HDL cholesterol and high levels of insulin resistance. Favored predisposition to increased severity of steatosis, more probability of altered liver function tests, increased risk of NASH and fibrosis, even at lower ages and body mass indexes than those ho-mozygous with wild-type allele. It is now known that NAFLD is an independent factor from cardiovascular risk and that it is associated with long-term morbidity and mortality of cardiovascular, liver and neoplastic complications; however, in non-diabetic population it had been little evidence to support such statements, etiologically related only to diabetes until now.
Being NASH the most likely stage to evolve toward fi-brosis and chronic inflammation its trigger; the risk allele I148M PNLAP3 carrying population would probably be more likely to evolve to chronic and irreversible phases; given that at a greater number of copies of the risk allele, major liver function test increase and higher scoring in NAFLD fibrosis score; which as it is known, these are indirect clinical surrogate markers for inflammation and possible evolution to fibrosis. Elevated levels of liver function test related to PNAPLA3 in Mexicans, had already been described, but only in pediatric24 population and regardless of weight. So it is plausible to imply that the beginning of the inflammation process at early ages and its perpetuation in adulthood may perform an important role in the evolution of the spectrum from a simple hepatic steatosis to steatohepatitis and fibrosis.
It is clear the usefulness and validity of NAFLD fibro-sis score as a tool to predict fibrosis, given that, the presence of scores at ranges of “indeterminate” or “presence” fibrosis, place certainty to diagnose liver fibrosis. Using this desktop tool in all NASH diagnosis, allows addressing a preventive perspective in chronic liver diseases such as cirrhosis.
Partial not total histological material collected from patients is the limitation in this study. Fact given by our adherence to research protocols bioethics committee recommendations; however, it is very evident the presence of this genetic polymorphism in our population.
ConclusionsThe documented polymorphism PNPLA3 I148M in the Mexican studied population, has a frequency higher than reported, which is 77% vs. 59%; this favors severe hepatic steatosis in patients of the risk allele, even in those with lower body mass indexes and at a younger ages. PNP-LA3 predisposes increased cardiovascular risk given lower levels of HDL cholesterol and increased probability of insulin resistance.
In the Mexican studied population, PNPLA3 conditioned more severe hepatic steatosis, also most likely to present steatohepatitis, liver fibrosis and abnormal liver function tests; so it can be assumed that PNPLA3 can play a decisive role in NAFLD pathogenesis. It is necessary that those environmental factors that promote its expression such as, obesity, diabetes, dyslipidemia, etc. must be countered by effective measures in national prevention programs.
Data Analysis and ResponsibilityAuthor's contributions: L.A.M., E.L., J.J.C, D.K. and A.T. contributed to the study design. L.A.M., A.T. and J.J.C. collected the data. E.L. performed the genetic data analysis L.A.M., J.J.C., A.T. performed the statistical data analysis and article preparation. D.K., A.T. and J.J.C. contributed to the editing and revision and also gave the final approval.
Potential Conflicts of InterestNone.
Financial SupportThe National Council of Science and Technology (CONACyT) provided a master degree scholarship for the principal investigator (L.A.M.). The CONACyT took no part in design, conduct of the study, collection, management, analysis, interpretation of the data, preparation, review, and approval of the manuscript.