Various cryopreservation techniques have been investigated to extend the storage of isolated hepatocytes; however, most have a reduced viability after rewarming due to ice crystal formation. Subzero nonfreezing conditions could theoretically reduce organ metabolism without damage due to ice crystal formation. In the present work we evaluated the viability and metabolic parameters of isolated rat hepatocytes preserved in subzero nonfreezing condition. Cell suspensions were maintained in modified University of Wisconsin (mUW) solution using 8% -1,4-butanediol as cryoprotectant, up to 120 h at -4°C. The time course evolution of hepatocytes viability were measured by LDH release and propidium iodide assay. The cellular concentrations of glutathione, ATP, glycogen and the lactate production during cold storage were also determined. Finally, results were compared with conventional hypothermic storage at 0 °C in mUW solution without cryoprotectant. After 5 days of subzero storage, we found an improvement in the ability of rat hepatocytes to maintain the metabolic resources in comparison with the cold preserved group.
This work was funded by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), PICT-03-14492, BID 1728 OC/AR (Argentina) and Progetti Alta Rilevanza, Ministero degli Esteri, Italy, Prot. 269/P/0093044 and prot. 269/P-0114337.
Presented in part at the 2nd Workshop of Cryobiology in Medical Sciences, held May 15-17, 2007 in Rosario, Argentina.
IntroductionCold storage of mammalian cells in preservation solutions is a well-known methodology to protect these cells against injurious or degradative processes and is widely used in experimental and clinical applications.
Considering that chemical reactions of metabolism are controlled by temperature, it is logically assumed that organs and cells should be stored at temperatures as low as possible without damage by freezing to maintain their vitality for extended periods. The widespread and well-accepted preservation method of maintaining isolated hepatocytes is the hypothermic storage in the UW solution,1,2 in which the biological material is maintained between 0 and 4 °C. This temperature range was chosen based on the premise that the water content of the cell would freeze below 0 °C and also that the cellular structures would be destroyed.3 Regarding energy metabolism, cooling of cellular systems delays all non enzymatic and enzymatic processes usually by a factor of 1.5 to 3 per 10 °C of temperature decrease.4 Further decrease of the storage temperature to subzero temperature as -4 °C, would theoretically decrease the metabolic rate of a cell/organ 17-fold, but the decreased rate of metabolism would be associated with the deleterious effects caused by the supercooling and freezing. In this way, these mechanisms of damage have been extensively studied and are naturally overcome by several species of amphibians, fish and insects,5 as for example, several species of frogs survive low temperatures by a rapid synthesis and accumulation of low-molecular cryoprotectans such as glucose and glycerol.6
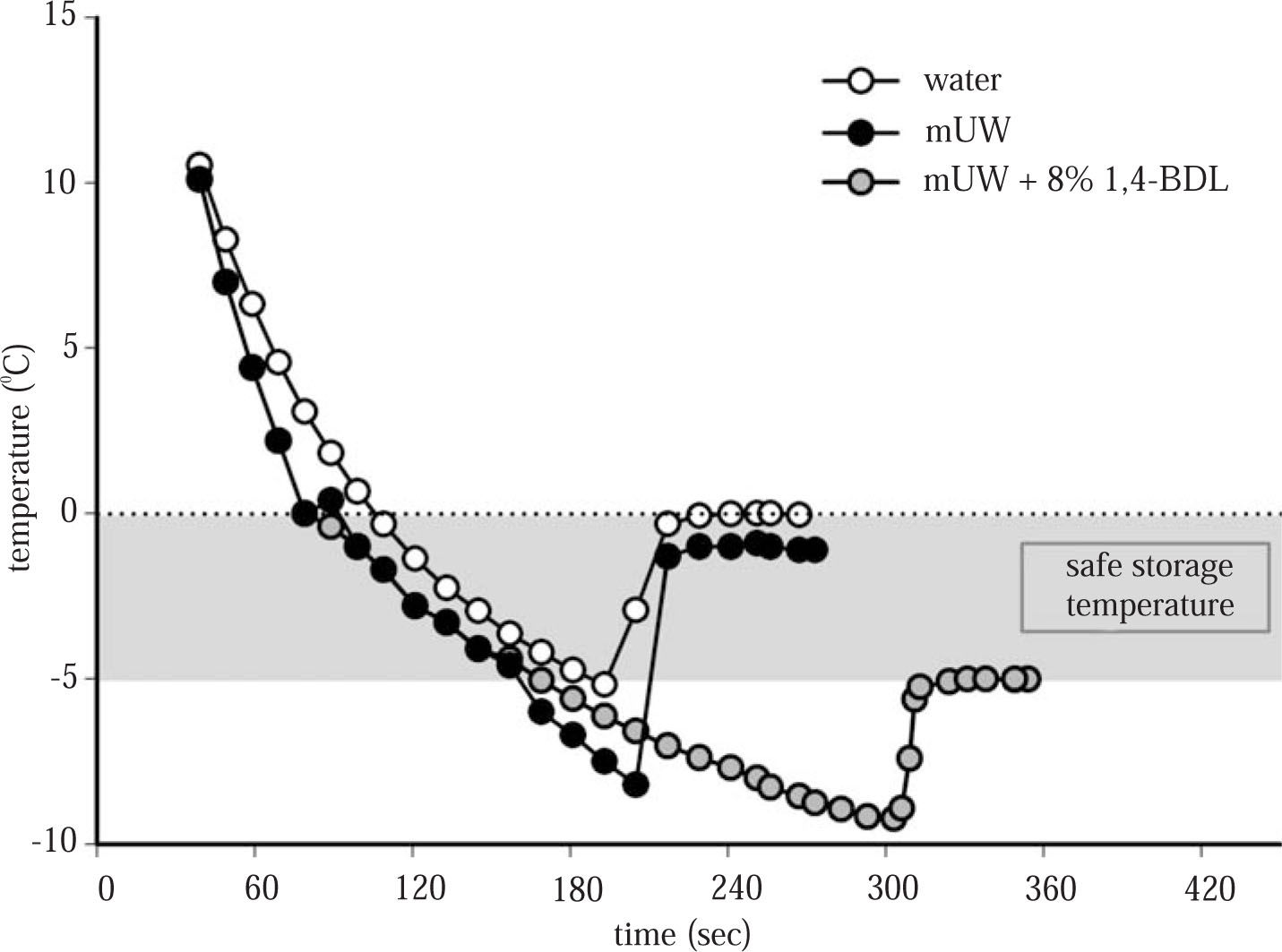
However it cannot always be assumed that subzero temperatures will freeze the cell. Recently, some subzero nonfreezing preservation protocols have been described,7,8 where livers, and isolated hepatocytes were stored at a temperature between 0 °C and the freezing point of the sample (-T °C). But, there are some limitations in the application of this method in the clinical setting. Subzero temperatures without cryoprotective agents (CPA) (supercooling state in which the preservation solution is below the freezing point but not yet frozen) poses the hazard of initiation of ice nucleation and catastrophic freezing of the sample due to a slight impact or change in temperature. The addition of CPA to the preservation solution prevents ice formation, decreases the freezing point of the solution and in this temperature range (0 °C - (-T) °C), the solution is not susceptible to freezing and provided a safety subzero nonfreezing temperature for cell/organ preservation (seeFigure 1). Regarding their results, this method seems to make longer the period when organs and cells can be stored, without a drastic loss of viability.
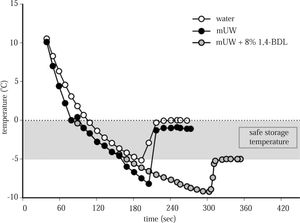
Cooling curves and freezing point of distilled water, mUW solution and mUW + 8% 1,4-BDL. The freezing point of distilled water was 0.00 ± 0.14 °C, mUW = -1.50 ± 0.10 °C, (401 ± 10 mOsm/kg H2O) and mUW + 8% 1,4-BDL = -5.08 ± 0.41 °C, (1.653 ± 99 mOsm/kg H2O). Data are expressed as mean ± SD for 5 experiments.
Several chemicals as ethylene glycol, dimethylsulfoxide, 1,3-propanediol, glycerol and 2,3-butanediol (2,3-BDL) have been employed as CPA in experimental models.9 However the toxicity and osmotic stress of these cryoprotective agents has been implicated with the impaired function of tissues in prolonged subzero preservation,10 like this 2,3-BDL has been found to be toxic at high concentrations (> 10%).11
We have developed a protocol to preserve isolated rat hepatocytes under subzero temperature without freezing.12–14 We have used 1,4-butanediol (BDL) as a cryoprotectant agent, which permitted to reduce the freezing point of the modified UW solution about 4 times. The diol 1,4-BDL was selected because is a molecule suitable to be used as CPA and a component of the mammalian internal and external milieu, this is a compound of biochemical as well as toxicological interest.15 In this protocol, the optimal concentration of 1,4-BDL was 8% (W/V) adequate to do not produce toxic and osmotic effects in the cells and to give a subzero non freezing storing temperature of -4 °C.12
The aim of the present study was to evaluate the viability and metabolic functions of isolated rat hepatocytes during the cold subzero storage period (-4 °C), and also compares the results obtained with conventional hypothermic storage (0 °C). The isolated hepatocytes were cold stored up to 120 hours.
Material and methodsAnimals. Male Wistar rats weighing 250-300 g were used in all experiments. Rats were allowed free access to standard laboratory diet and water ad libitum prior to the experiment and received care in compliance with international regulations. The National Council Committee of Argentine approved animal protocols.
Hepatocyte isolation. Rat hepatocytes were isolated by collagenase perfusion as it was described previously.16
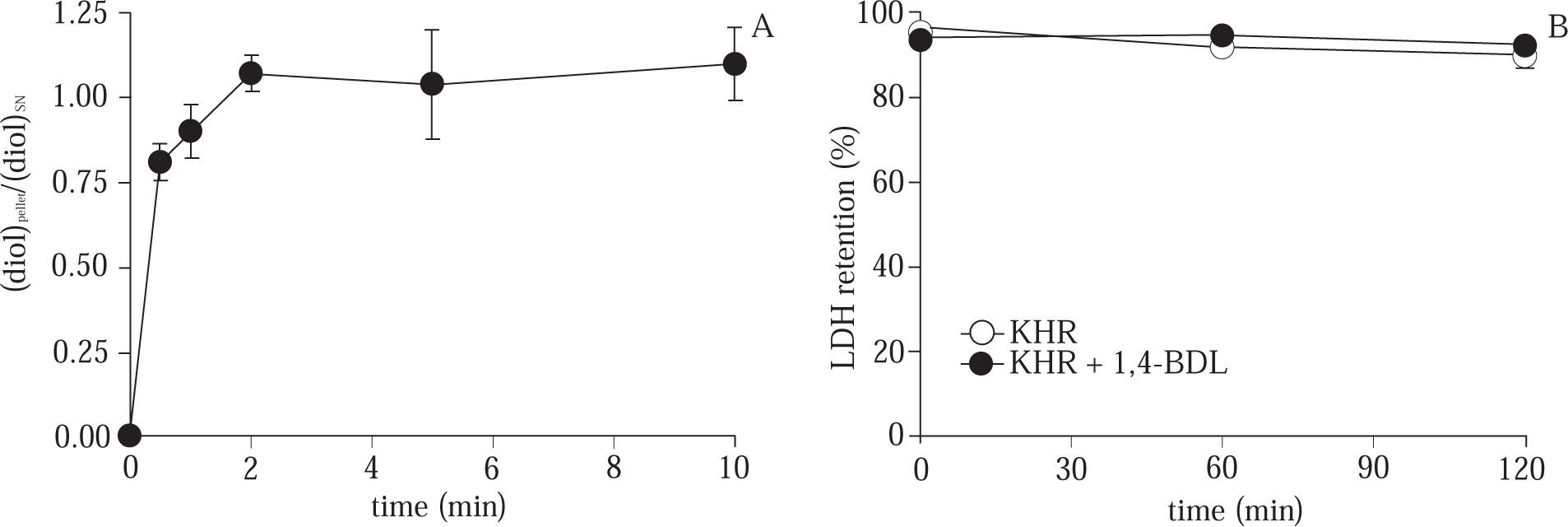
Effect of acute cryoprotector exposure at 37 °C. To explore the possibility of CPA toxicity upon hepatocytes, we studied the acute effect of 8% 1,4-BDL on cells incubated in Krebs – Henseleit solution (KHR) (17) at 37 °C during 10 min in a Dubnoff metabolic bath with constant shaking (50 rpm) equilibrated with O2+CO2 (95/5%). During the incubation, samples of cells and supernatant were taken and CPA concentration was measured13 to determine the rate of CPA penetration into the cells. After that, the CPA was removed according the procedure described in12 and the cells were resuspended in KHR (2-3. 106 cells/mL) incubated 120 min in a Dubnoff metabolic bath with constant shaking and O2+CO2 (95/5%) atmosphera. To evaluate the cell viability, samples of the cell suspension were taken at 0, 60 and 120 min and the LDH retention test measured.
Hepatocyte cold storage. Isolated hepatocytes were rinsed twice and resuspended in freshly prepared cold (0 °C) modified UW solution (mUW). The composition of the mUW solution was previously described,16 this solution does not contain hydroxyethyl starch, insulin and dexametasone. In the mUW solution, polyethylene glycol was included due to its effective antioxidant activity which prevents lipid peroxidation and hypothermic cell swelling in cold stored hepatocytes.18 15 mM glycine was also included since it has been shown19 to be effective in preventing cold ischemic damage. Hepatocytes (120.106 cells in 40 mL UW solution) were allowed to settle to the bottom of a 50 mL screw cup polycarbonate tubes, and left undisturbed at 0 °C up to 120 h.
Hepatocyte subzero nonfreezing storage. Isolated hepatocytes were rinsed twice and resuspended in freshly prepared cold (0 °C) modified UW solution.14 Hepatocytes (110.106 cells in 36 mL UW solution) were put in a thermostatized suspension culture flask maintained at 0 °C under N2 atmosphere and constant stirring (30 rpm). Subsequently, 4 g of 1,4-BDL were added at a rate of 0.17 g/min with an infusion pump (Sage Instruments, syringe pump mod. 355, Cambridge, Mass, USA) to get a final concentration of 8% (v/v). Hepatocytes were allowed to settle to the bottom of 50 mL screw cap polycarbonate tubes and were put in a refrigerated bath (Polyscience Mod. 9501) that initially was set on at 0 °C and then, the temperature was decreased at a rate of -0.07 °C/min until reach the storage temperature (-4.0 °C). In this condition, hepatocytes were cold stored for up to 120 h.12
Experimental protocol. Hepatocytes from Wistar rats isolated by collagenase digestion were resuspended in UW solution and divided into the following groups: 1-subzero nonfreezing stored group (Subzero Storage), and 2-cold stored group (Cold Storage). They were stored for up to 120 hr at the temperatures indicated. It is important to point out that based on previous works performed in our laboratory,2,17,20 72 hours of cold storage has been demonstrated the time limit for a good cell preservation in mUW solution. In this paper we are comparing a cold storage time of 120 hours for both groups.
To evaluate the viability and metabolic function, at different times 0, 48, 72 and 120 h, one of the stored tubes with the cells suspensions was removed daily, hepatocytes were resuspended in the storage solution and aliquots were obtained.
Cell viability measurements.LDH retention. The capacity of the cells to retain macromolecules as Lactate Dehydrogenase (LDH) was determined by measuring the LDH activity in the cell suspension (total activity) and in the supernatant (extracellular activity).14 Results were expressed as the percentage of LDH retention by the cells.
Cell viability % = 100 - [100 x (extracellular LDH total activity)]
PI assay. The capacity of the cells to exclude the fluorescent marker propidium iodide (PI) was established as follows: the cells were incubated with the fluorescent marker and the fluorescence intensity is measured. As previously described,14 cell viability is expressed as the ratio between the fluorescence originated from non-viable cells that have membrane damage and the fluorescence originated from all cells in the sample.
Cooling curves and freezing point determination. The cooling curves and freezing point determination of distilled water, mUW and mUW + 8% 1,4-BDL were determined as it was described previously.12 The osmolality of the preservation solutions was measured using a freezing point osmometer Osmomat 030 (Gonotec, GMBH, Germany).
Evolution of cellular metabolites during cold and subzero storage period.Glutathione assay. GSH is an important cellular anti-oxidant and is a major factor in the protection of cells from reactive oxygen intermediates. Total glutathione-GSH plus GSSG-concentration (GSHt) was determined by the enzyme coupled spectrophotometric assay of Tietze,21 as previously described.16 Results were expressed as nmol of GSH/106 cells.
ATP assay. Cellular ATP concentration was measured as an index of energy status and was determined by a HPLC technique, as previously described.22 Results were expressed as nmol of ATP/106 cells.
Total lactic acid production. Lactic acid concentration was determined as an index of hypothermic metabolic suppression and was determined using the method described by Gutmann y Wahlefeld.23 Results were expressed as total lactic acid (intracellular plus extracellular) production, estimated from the measured values using the formulae 100 * ([lact]t - [lact]0)/ [lact]f, where [lact]t, [lact]0 and [lact]f are the total lactic acid produced after t hours, t = 0 h and t = 120 h of preservation, respectively.
Glycogen assay. Intracellular glycogen concentration was assessed by the amyloglucosidase enzyme assay, followed by an enzymatic determination of released glucose, technique described by Carr and Neff.24 Results were expressed as % of initial glycogen, measured as follow: % initial glycogen = (100 * ([GLN]t/[GLN]0)), where [GLN]t is the glycogen concentration after t h of preservation, and [GLN]0 is the glycogen concentration at t = 0 h.
Statistical analysis. Statistical significance of the differences between values was assessed by analysis of variance (ANOVA) followed by Scheffe’s multiple range tests. A p value less than 0.05 were considered statistically significant.
ResultsFigure 1 shows the cooling curves of distilled water, mUW solution and mUW + 8% 1,4-BDL, the specific freezing point of mUW + CPA was -5.08 ± 0.41 °C, then we chose a temperature of - 4 °C to store the cells safely.
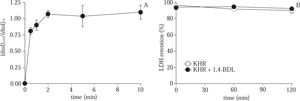
As shown in Figure 2A, the ratio of CPA concentration in cell/supernatant was equilibrated after 2 min of 8% CPA exposure to hepatocytes at normothermic temperature. After 10 min of CPA exposure, the cells were washed and incubated in Krebs-Henseleit solution during 120 min. The results shown in Figure 2B, stablished that there is not effect on cell viability measured by the LDH retention test.
(A) Time course evolution of the CPA concentration ratio in cell pellet / supernatant during the acute exposure of hepatocytes to CPA at 37 °C. (B) Evolution of % LDH retention during 120 min of normothermic incubation of control cells and hepatocytes exposed to 8% CPA. Data are expressed as mean ± SD for 3 experiments.
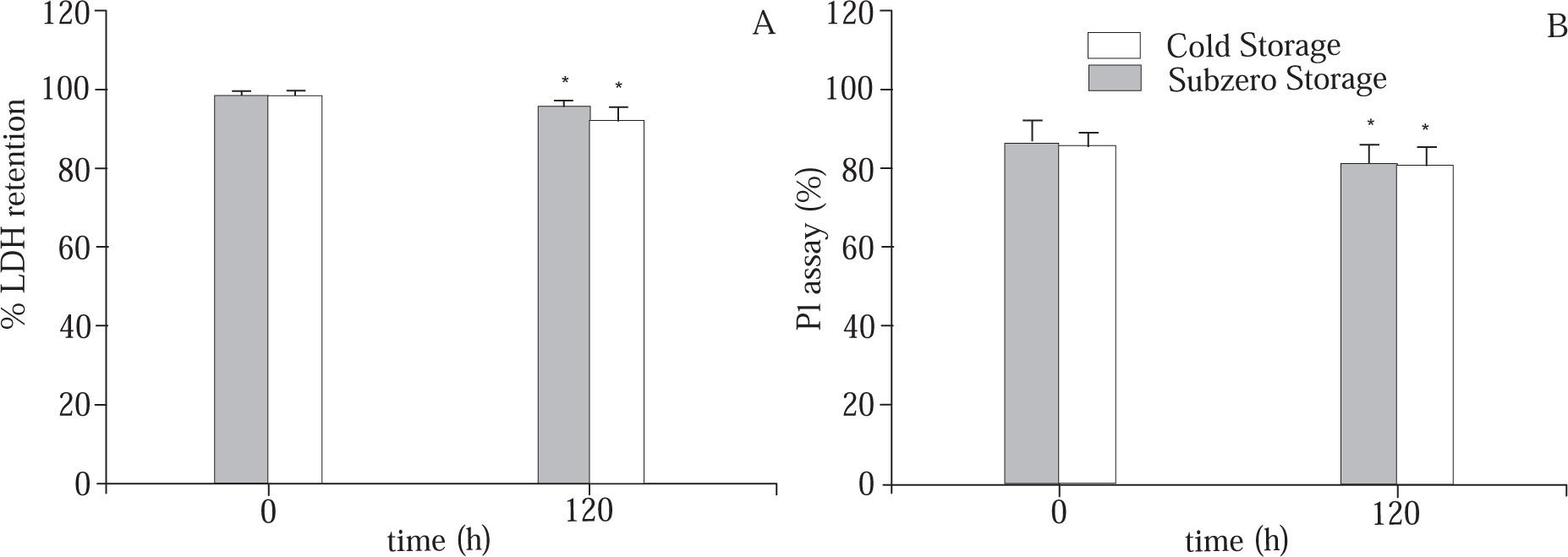
In relation to the preservation period, Figure 3A shows that during the cold or subzero storage there is a significant diminution of the ability of hepatocytes to retain the intracellular levels of LDH, and they are more permeable to the fluorescent marker PI as well (Figure 3B). These results suggest alterations in the membrane integrity during the cold storage. However, there is no statistical difference between both storage protocols.
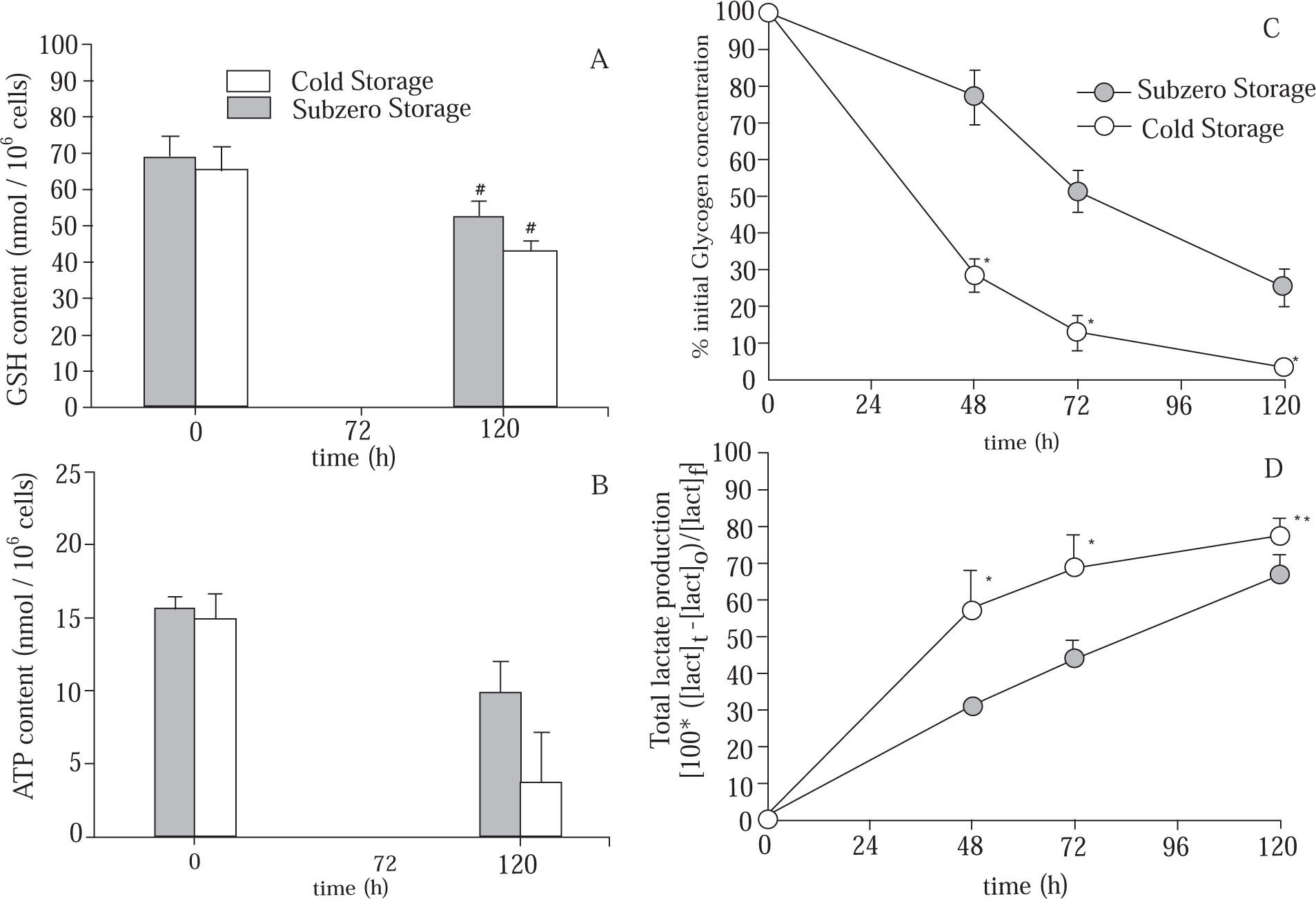
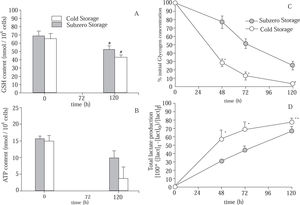
Figure 4A shows the cellular GSH content of cold and subzero stored hepatocytes. Statistical differences were found after 120 h of preservation for both groups. However, at the end of the preservation period, hepatocytes suspensions that were subjected to subzero temperature were able to maintain more efficiently the initial cellular GSH content (Subzero Storage: 75.89 ± 5.74% of GSHt=0h; Cold Stored: 65.68 ± 3.13% of GSHt=0h).
Evolution of (A) intracellular GSH content; (B) intracellular ATP content; (C) glycogen content (% of initial content), and (D) total lactate produced, in suspensions of hepatocytes stored in mUW solution at 0 °C (Cold Stored) and in mUW solution + BDL at -4 °C (Subzero Stored), for up to 120 h. Data are expressed as mean ± SD for 4 hepatocyte preparations. # P < 0.05 versus t = 0 h. * P < 0.05 versus Subzero Storage. ** P < 0.10 versus Subzero Storage.
We here further characterize the time course evolution of the energy resources during the storage period, by studying the ATP and GLN contents (Figure 4B) and (Figure 4C), respectively. Results have shown a time-dependent depletion of ATP in both groups. However, after 120 h of storage, the diminution of cellular ATP content in the Subzero Stored group was approximately 38%, while in the other group the ATP content was decreased approximately by 76%. On the other hand, after 48 h of storage, Cold Stored group has shown a sudden decrease in the GLN content. Hepatocytes suspensions stored at subzero conditions were able to attenuate this decrease. Besides, at the end of the storage period, Subzero Stored hepatocytes retained approximately 26% of the initial GLN content, whereas Cold Stored cells maintained only 3%.
As shown in Figure 4D, during the storage period there were an increment in the amount of total lactate produced by both groups, however, Subzero Stored group shows the lowest production of lactate.
DiscussionThe purpose of organ or cell preservation is to determine a condition of storage for as long as possible without a reduction of viability. According to the temperature setting, preservation techniques are currently divided into two categories, one is the hypothermic immersion method at 0 - 4 °C or «cold storage», the other, is the cryopreservation at -150 or -196 °C. Nonfreezing preservation at subzero temperature is an alternative method that avoid ice formation and may prolong the storage time with respect to the cold storage method. To date, few studies of preservation methods utilizing subzero storage without freezing have been reported.7,8,11,12,25–27 That is why, we have chosen 1,4-BDL as a novel CPA; the addition of this diol to mUW solution prevents ice formation besides the low concentration used might diminished the risk of CPA toxicity on cells.
Because CPA toxicity is known to be a function of CPA concentration, exposure time and temperature,27,28 we also investigated the effect of the acute exposure of CPA at normothermic temperature on viability of hepatocytes. It has been shown that 1,4-BDL will completely equilibrate all cellular water in hepatocytes with 2 min of incubation at 37 °C. After 10 min exposition, the CPA was removed and the cells were washed, then incubated up to 120 min in a physiological environment. Our test of acute exposition to CPA at normothermic temperatures showed that 1,4-BDL has not a deleterious effect on LDH retention test as compared to a hepatocyte control suspension.
An interesting observation was that after 120 h of cold storage, the cell membrane integrity estimated by a LDH retention test and propidium iodide test was affected, the use of propidium iodide only assesses the integrity of the plasma membrane. While a loss of membrane integrity has been correlated with a loss in post-preservation cell function, a loss of membrane integrity is not necessarily a determinant of gross cell function. There have been numerous reports in the literature where cells have been shown to repair membrane injury and survive low temperature exposure. This point will be studied in the following paper.29
As far as the energy status of the cells stored at -4 °C is concerned, a significant increase of ATP content, a considerable GLN retention and suppression of lactic acid production, reflected the decrease of the intracellular process of anaerobic catabolism. Lowering the storage temperature from 0 °C to -4 °C, retarded the ATP depletion almost twofold, also the metabolic conversion of large molecules as glycogen was considerably reduced.
The observation that subzero stored cells retain more efficiently their GSH content after 120 h of storage, might have led to a significant improvement of cell function. It is known that the cell capacity to maintain an effective level of glutathione will be an important determinant of cell survival during the rewarming step.
The results presented in the current study suggest that during the storage period, hepatocytes suspensions stored at -4 °C in mUW solution plus 1,4-BDL as CPA, were able to maintain a reduced basal metabolism in comparison with cold stored hepatocytes suspensions. Subzero temperature storage is supposed to protect stored hepatocytes against hypoxic injury by greather suppression of cellular degradative metabolism in comparison with the conventional cold preservation. Therefore, this new strategy could increase the storage period without a considerable loss of viability and provide a regular source of viable and metabolically competent hepatocytes for bioartificial liver devices.