This article discusses the infeasibility of adhering to the Brazil-Milan Criteria for determining whether patients with hepatocellular carcinoma (HCC) should be offered liver transplantation. The Criteria are currently used widely in Brazil. However, since they expand the net of the transplant-eligible population, and that transplantation is shown to improve clinical outcomes in HCC patients relative to other treatments, they are inherently attractive and may be adopted by other countries in determining whether HCC patients should receive liver transplantation. I argue that the Criteria unjustifiably disregard the number of tumour nodules found in the patient. This number may be indicative of the recurrence potential of the disease, since these nodules can originate from different clonal populations. The greater the number, the higher the risk these nodules are associated with clones which contain genetic mutations conferring even greater potential for proliferation and dissemination. Clusters of tumour cells may be micro-disseminated to vascular structures surrounding the liver, as well as extrahepatic systems. This increases recurrence potential and reduces the benefit of liver transplantation in these patients. Thus, HCC patients harbouring fewer but larger tumour nodules may present with a more favourable genomic and epigenomic profile than those with more, albeit smaller, tumour nodules. Patients with more tumour nodules may reap greater clinical benefit if initiated on systemic therapy early, rather than wait for a suitable donor liver. Although the Criteria are reached by consensus, with reference to findings from recent studies, as well as scientific theory, it is ripe for review.
Hepatocellular carcinoma (HCC) remains an issue of immense public health importance. Liver transplantation is indicated for treatment of HCC patients who satisfy the Milan Criteria [1]. The Brazil-Milan Criteria are less stringent. The transplant-eligible population is enlarged by excluding from consideration tumour nodules measured at < 2 centimetres in diameter [2]. This strategy is inherently attractive since liver transplantation improves tumour-free, and overall survival in HCC patients [3]. Providing more HCC patients with this therapeutic option carries positive public health benefits. Although not widely adopted outside Brazil at the moment [2], the Brazil-Milan criteria have the potential to replace the Milan Criteria, especially when current opinion indicates that the Milan Criteria may be too restrictive: some HCC patients not satisfying the criteria who would reap roughly the same benefits as those satisfying them might be unjustifiably excluded [4]. Nevertheless, completely ignoring the number of tumour nodules in clinical decision-making is undesirable and should not be adopted. While the Brazil-Milan Criteria has been agreed by virtue of consensus, its applicability to modern medicine is ripe for review.
The number of tumour nodules can be used as a surrogate marker for the recurrence potential of HCC. According to Ding and colleagues [5], initial ‘gatekeeper’ (epigenomic) changes in DNA methylation may predispose certain hepatocytes to greater potential of growth. Unlike other cancers, HCC usually arises as a sequela of background cirrhosis and fibrosis. Such changes occur in the backdrop of substantial selection pressure among hepatocytes. Subsequently, mutations in driver genes, such as TP53, GOF, CTNNB1, and the TERT promoter, arise and potentially promote malignant transformation [5]. These mutations are inherently variable and occur independently in different tumoural clusters. If an HCC patient has a single tumour nodule for a long period of time, it may suggest that the bulk of the malignant potential of the affected liver tissue, derived from mutations mentioned afore, is the highest in the said tumour nodule. The genomic profile of the surrounding tissue is less aggressive, in which the mutation rate is presumed to be lower. This contrasts the case of the HCC patient who has multiple tumour nodules where the number of which increases rapidly in a short period of time. Although the tumour nodules that have newly arisen might be smaller than the older ones (< 2 cm in diameter), the sheer fact that they evolve rapidly from surrounding hepatocellular tissue to malignant tissue suggests they have a more aggressive pathological profile as they acquire mutations more quickly. With greater aggressiveness comes greater potential for micro-dissemination of clusters of tumour cells, arising from the tumour nodules, to neighbouring vascular structures and outside the liver. The extent of micro-dissemination is expected to be positively correlated with the number of tumour nodules detectable on imaging. With a greater degree of micro-dissemination, even if the patient receives liver transplantation, due to the presence of extrahepatic HCC tumour clusters, it is doubtful whether the patient can reap the greatest benefit from the transplantation since the recurrence potential is substantially higher. Crucially, in a multicentre study [6], HCC patients satisfying the Milan Criteria upon diagnosis had longer disease-free survival than HCC patients satisfying the Brazil-Milan Criteria only. The difference in disease-free survival can be explained with reference to the latter group's greater recurrence potential. This point is even more strongly supported by the fact that disease-free survival between the latter group and HCC patients not satisfying either Criteria was similar. While the size of the tumour nodule is paramount, we should never ignore the significance of the number of tumour nodules, however small they are.
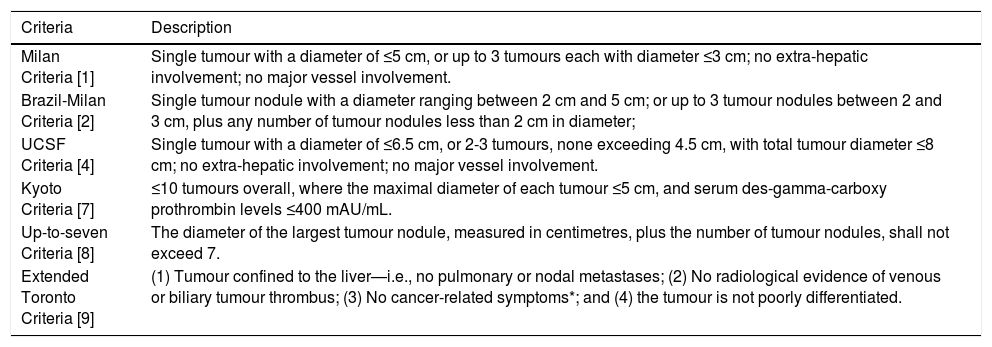
The number of tumour nodules is recognised as a relevant parameter in other criteria, including the University of California in San Francisco (UCSF) Criteria [4], Kyoto Criteria [7], and Up-to-seven Criteria [8]. Details are included in Table 1. The prognostic outcomes of patients satisfying the aforementioned criteria are generally satisfactory. Even though patients satisfying the Up-to-seven Criteria (but not the Milan Criteria) present with worse prognostic outcomes, unlike those satisfying the Brazil-Milan Criteria only, they still present with significantly better disease-free survival than patients falling out of the Criteria (1-year survival: 85.6% vs 65.8%; 3-year survival: 75.6% vs 42.5%; 5-year survival: 75.6% vs 41.7%, p<0.01) [8]. Although the Extended Toronto Criteria do not pay much regard to tumour size or number, this is justified since the Criteria focus on a broad spectrum of factors including tumour differentiation, extrahepatic manifestations (including vascular dissemination), and general health status [9]. Such a holistic approach is not strictly adhered to when applying the Brazil-Milan Criteria in the selection of HCC patients for transplantation. Hence, it warrants the weight of importance given to the number of tumour nodules that this article advocates. Moreover, as the Extended Toronto Criteria determine the degree of differentiation of the tumour, intratumoural heterogeneity and general tumour activity can already be deciphered. This may be a better surrogate marker than the number of tumour nodules, but it may struggle to be adopted in resource-poor settings.
Different Criteria used for determining whether a patient with hepatocellular carcinoma should be treated with liver transplantation.
| Criteria | Description |
|---|---|
| Milan Criteria [1] | Single tumour with a diameter of ≤5 cm, or up to 3 tumours each with diameter ≤3 cm; no extra-hepatic involvement; no major vessel involvement. |
| Brazil-Milan Criteria [2] | Single tumour nodule with a diameter ranging between 2 cm and 5 cm; or up to 3 tumour nodules between 2 and 3 cm, plus any number of tumour nodules less than 2 cm in diameter; |
| UCSF Criteria [4] | Single tumour with a diameter of ≤6.5 cm, or 2-3 tumours, none exceeding 4.5 cm, with total tumour diameter ≤8 cm; no extra-hepatic involvement; no major vessel involvement. |
| Kyoto Criteria [7] | ≤10 tumours overall, where the maximal diameter of each tumour ≤5 cm, and serum des-gamma-carboxy prothrombin levels ≤400 mAU/mL. |
| Up-to-seven Criteria [8] | The diameter of the largest tumour nodule, measured in centimetres, plus the number of tumour nodules, shall not exceed 7. |
| Extended Toronto Criteria [9] | (1) Tumour confined to the liver—i.e., no pulmonary or nodal metastases; (2) No radiological evidence of venous or biliary tumour thrombus; (3) No cancer-related symptoms*; and (4) the tumour is not poorly differentiated. |
*According to the Extended Toronto Criteria, cancer-related symptoms include: (1) weight loss over 10 kg and/or (2) an increase in the Eastern Cooperative Oncology Group score of ≥1 point over a period of 3 months. Patients are required to present with a performance status of 0.
Following this theory, there are two points to be made. Since performing liver transplantation on HCC patients with more tumour nodules may be less efficacious due to the higher risk of disease recurrence, the maximum benefit of limited donor livers can be reaped by offering them to HCC patients manifesting fewer tumour nodules, since this is indicative of a more favourable overall pathological profile. Moreover, HCC patients with more tumour nodules may be initiated on systemic therapy (e.g. sorafenib and levatinib) early rather than wait for a donor liver. Clinical outcomes are expected to be improved. Even though systemic therapy can serve as an adjunct to other bridging therapies such as transarterial chemoembolisation (TACE) and radiofrequency ablation (RFA), the potentially vast differences in pathological profiles between in-Milan and in-Brazil/Milan (who are out-Milan) patients do not justify liver transplantation and its incumbrances, such as the risk of rejection, issues with wound healing, and the burden of lifelong immunosuppression. This is particularly true in the COVID-19 era, where liver supply remains scarce, and patients having received liver transplantation are at risk of more severe COVID-19 [10]. Medical professionals should therefore be more prudent in organ allocation.
Ultimately, adhering to the Brazil-Milan Criteria may bring hope, but is ultimately infeasible in clinical practice due to the over-inclusion of HCC patients who may not reap the fullest benefit from liver transplantation. While current medical opinion that the Milan Criteria may be overly restrictive is veritable, other criteria acknowledging both the size and number of tumour nodules, or adopting a more holistic approach, should be used. In light of the aforementioned theoretical reasons, and findings from the latest medical literature, the applicability of the Brazil-Milan Criteria is ripe for re-examination and re-evaluation.
Author contributionACN is responsible for all aspects of production of the manuscript.