Spontaneous bacterial peritonitis (SBP) is a frequent complication to cirrhosis with an unclear long-term prognosis. We aimed to examine its effect on mortality in two independent patient cohorts.
Patients and methodsWe used Danish healthcare data on cirrhosis patients with a first-time paracentesis in 2000–2014 and data from three randomized controlled trials on satavaptan treatment of ascites conducted in 2006–2008. We used the Kaplan-Meier method to estimate cumulative mortality, and Cox regression to compare the confounder-adjusted mortality hazard for patients with vs. without SBP.
ResultsIn the Danish Healthcare Cohort, we included 1.282 patients of whom 133 (10.4%) had SBP. The SBP patients’ cumulative 4-month mortality was 51.2% (95% CI: 43.0–59.9%) vs. 34.7% (95% CI: 32.0–37.6) in those without SBP. The SBP patients’ confounder-adjusted mortality hazard was 1.54-fold higher (95% CI: 1.18–2.00) in the four months after paracentesis, but was not increased thereafter (confounder-adjusted mortality hazard 1.02, 95% 0.72–1.46). In the satavaptan trial data of 1,198 cirrhosis patients with ascites, the 93 patients with SBP had a cumulative 4-month mortality of 38.6% (95% CI: 29.3–49.7) compared with 11.4% (95% CI: 8.5–15.2) in those without. The SBP patients’ confounder-adjusted mortality hazard ratio was 3.86 (95% CI: 2.44–6.12) during the first four months, and was 1.23 (95% CI: 0.54–2.83) thereafter.
ConclusionsIn both cohorts of patients with cirrhosis, an SBP episode had a high short-term mortality compared to patients without SBP, and had no lasting effect on the long-term mortality.
Spontaneous bacterial peritonitis (SBP) is a complication to cirrhosis patients with ascites that affects approximately 10% at hospital admissions [1]. It can present with abdominal discomfort or signs of infection, but may also be clinically silent [2]. The SBP diagnosis is defined by an ascites fluid sample with > 250 polymorphonuclear (PMN) cells per µL [1]. Identification of SBP prompts immediate empirical antibiotic treatment and in those with renal impairment or jaundice also administration of human albumin [3]. Before the advent of these measures, the 1- year mortality of SBP exceeded 70% [4], but it has decreased since, and is currently expected to be 30–50% [5]. However, estimates on cirrhosis patients’ cumulative mortality following an SBP episode are based on small and selected patient cohorts [6–10]. Only one population-based study has compared the impact of SBP on the mortality with a relevant control group without SBP and assessed the impact of SBP over time [11], but their SBP definition was based on diagnosis codes rather than the ascites leukocyte count. Their findings indicate that SBP could be a temporary phenomenon with no lasting effect on the mortality rate. Yet, it is widely believed that SBP is a marker of a permanent aggravation of cirrhosis [1,5]. Valid estimates on the cumulative mortality following an SBP episode, and a clarification of whether SBP is a permanent or a temporary complication may clarify clinical decisions and benefit patient counselling.
We examined the short-and long-term effect of SBP on mortality in two large, independent, and differently constructed patient cohorts.
2Methods2.1Setting and data sources2.1.1Danish healthcare dataDenmark's 5.6 million residents benefit from universal tax-funded access to work-up and treatment at general practitioners and hospitals. The Danish National Patient Registry (NPR) is a nationwide registry that covers non-psychiatric hospital admissions since 1977 and emergency room and outpatient visits since 1995. The data includes admission and discharge dates along with up to 20 diagnoses. The diagnoses are coded in accordance with the International Classification of Disease edition 10 (ICD-10) from 1994, and the ICD-8 before that [12–14]. The NPR also holds data on surgical procedures coded in accordance with the Nordic Classification of Surgical Procedures (NCSP).
The LABKA database holds data on the results of biochemical tests from blood and fluid samples analyzed at hospitals in the Danish Central and Northern Region, including samples obtained at general practitioners. The database was set up in 1995 at Aarhus University Hospital (then Aarhus County Hospital) and data from an increasing number of hospitals was gradually added until 2011 when the database became complete. The data includes the date of each sample and the result coded according to the Nomenclature for Properties and Units (NPU), and the unit of the result [15].
The Danish Central Office of Civil registration keeps track of all Danish residents’ vital status, including the date of death or emigration. It also issues the unique ten digit personal identifier that enables time-true individual level linkage across the NPR, the LABKA database, and the civil registration system [16].
2.1.2Satavaptan trial dataFrom July 2006 to December 2008, three multicenter randomized trials evaluated the clinical efficacy of satavaptan in ascites treatment. A total of 1,198 patients were randomized to satavaptan or placebo according to the same core protocol. The trials had different target populations: patients with diuretic-manageable ascites (n = 462), patients managed with diuretics and occasional therapeutic paracentesis (n = 496), and patients with diuretic-resistant ascites managed primarily with therapeutic paracentesis (n = 240) [17]. The three trials excluded patients with a functioning transjugular intrahepatic systemic shunt, patients with variceal bleeding, and patients with spontaneus bacterial peritonitis in the 10 days before randomization. Additional exclusion criteria were: serum creatinine > 150 µmol/L, serum potassium > 5.0 mmol/L, serum sodium > 143 mmol/L, serum bilirubin > 150 µmol/L, international normalized ratio > 3.0, platelets < 30,000/mm3, neutrophils < 1,000/mm3, systolic arterial pressure < 80 mmHg or orthostatic hypertension, hepatocellular carcinoma beyond the Milan criteria, need for medication with QT-interval prolonging drugs or drugs with potent modification of the cytochrome P450 3A pathway.
All three trials were intended to run for 52 weeks, but the second and third trial were terminated prematurely due to futility. During the trials, all patients were seen every 4 weeks at their hepatology department. At these visits, patients underwent clinical examination, had standard blood tests analyzed, all current medications and their indications were recorded, and all patients underwent paracentesis when needed. All clinical events during follow-up were recorded, and all patients were followed up for death until one year after inclusion in the trial.
2.2Study cohorts2.2.1Danish healthcare cohortWe included all patients with a first-time diagnosis for unspecified cirrhosis (ICD–8: 57.192, 57.199; ICD–10: K74.6) or alcoholic cirrhosis (ICD–8: 57.109; ICD–10: K70.3, K70.4) in the NPR from 1977 to 2014 and a subsequent first-time paracentesis with a leukocyte count in the LABKA database (see appendix for NPU codes). We defined the date of the first-time paracentesis as the index date. To ensure we studied first time and not recurrent SBP, we excluded those with a procedure code for a paracentesis (NCSP: KTJA10x or KJAA00x) more than one day before the index date according to the NPR. We also excluded those with a diagnosis for ascites (ICD-10: R18.x) made at a hospital discharge, an outpatient visit, or an emergency room visit before the index date according to the NPR. To ensure we studied spontaneous bacterial peritonitis and not peritonitis secondary to surgery, we excluded patients that underwent intra-abdominal surgery (NCSP: KJxxxxx) except paracentesis within 30 days before the index date. However, we allowed that patients underwent endoscopic or catheter-based procedures (see Supplementary material for procedure codes) during the 30-day time-period before the index date.
We divided patients with a first-time paracentesis according to ascites PMN cell count (see Appendix 1 for NPU codes). We defined those with an ascites PMN cell count ≥250 cells per µL as spontaneous bacterial peritonitis (SBP) patients, and those with a PMN cell count < 250 per µL as not having SBP. We followed these patients from the index date to death or end of follow-up on 1 January 2015 whichever occurred first. We defined comorbidity with the CirCom score based on diagnoses recorded in the NPR up to two years before the index date [18]. We also used ICD-10 codes recorded before the index date to classify the patients’ cirrhosis etiology and to identify those diagnosed with hepatocellular carcinoma (HCC) (see Table 1 for ICD–10 codes).
Demographic and clinical characteristics for patients with and without spontaneous bacterial peritonitis (SBP) in the Danish Healthcare Cohort and the Satavaptan Trial Cohort (interquartile ranges in parenthesis). Brackets denotes diagnosis codes according to the International Classification of Disease, Edition 10 (ICD–10).
| Danish Healthcare Cohort (1,282) | Satavaptan Trial cohort (464) | |||
|---|---|---|---|---|
| SBP (N = 133) | No SBP (N = 1149) | SBP (93) | No SBP (371) | |
| Median age (years) | 58 (51–66) | 58 (51–65) | 58 (51–63) | 57 (51–63) |
| Sex (% males) | 73.7 | 71.2 | 74.2 | 72.5 |
| Comorbidity (% Circom score>0) | 13.5 | 13.0 | - | - |
| HCC [ICD–10: C22.0] (%) | 6.8 | 4.2 | - | - |
| MELD (median)⁎ | 13.8 (7.1–22.6) | 13.6 (8.5–18.6) | 13.0 (9–16) | 12.5 (9–15) |
| Albumin (g/L, median) | 23 (19–27) | 24 (20–28) | 32 (28–35) | 33 (29–33) |
| Sodium (mmol/L, median) | 132 (129–136) | 133 (129–137) | 134 (132–137) | 135 (132–139) |
| Ascites protein content (g/L, median) | 15 (8–27) | 11 (7–16) | - | - |
| Cirrhosis etiology⁎⁎: | ||||
| Alcohol-related [K70.x] (%) | 79.0 | 86.3 | 70.3 | 56.5 |
| Chronic viral hepatitis [B18.x] (%) | 9.0 | 6.4 | 32.3 | 26.7 |
| Primary biliary cholangitis [K74.3] (%) | 0.8 | 1.5 | 0.0 | 0.5 |
| Autoimmune hepatitis [K73.2, K75.4] | 1.0 | 0.8 | 1.0 | 1.0 |
| Hemochromatosis [E83.1] | 1.0 | 1.5 | 0.0 | 0.0 |
We excluded patients who had SBP before inclusion and identified patients with an SBP episode during the three randomized trials (defined by an ascites PNM cell count > 250 per µL). We defined the date of the first SBP episode as the index date. We matched up to five patients controls to each patient with SBP on model-of-end-stage-liver-disease (MELD) score, serum sodium, serum albumin, inpatient status at the time of paracentesis (yes/no), refractory ascites (yes/no) (incidence density sampling) [19]. We followed SBP patients and patient controls from the index date to death or end of follow-up, whichever occurred first.
2.3Statistical analysisWe estimated cumulative all-cause mortality with the Kaplan-Meier method in both patient cohorts. First, we estimated the 30-, 90-day, 120-day (4-month) and 1-year cumulative all-cause mortality after the index date for patients with and without SBP. Next, we estimated the cumulative all-cause mortality for the patients in each cohort that were alive 4 months (120 days)) after the index date. In the Danish Healthcare Cohort, we followed these patients until death or end of follow-up. We followed patients in the Satavaptan Trial Cohort until 12 months (365 days) after the index date.
We also used another method—Cox regression—to clarify when the mortality hazard after an SBP episode was similar to and when it was different from patients without SBP. We used this analysis to produce a Schoenfeld residuals plot that visualizes the mortality hazard ratio's interaction with time. It enabled us to examine how the mortality hazard for patients with vs. without SBP changes through follow-up. Finally, we divided follow-up into two time-periods based on inspection on these plots.
In the Danish Healthcare Cohort, we estimated the mortality hazard ratio for patients with SBP compared to those without. In the primary analysis, we adjusted for age, sex, comorbidity, HCC, MELD score, serum sodium, and serum albumin. We used multiple imputation to account for missing data on biochemistry (see Appendix 2 for details). We repeated this analysis without serum creatinine to see whether the missing data on serum creatinine in 20% of the patients affected our results. Finally, we repeated our analyses and restricted them to patients with a diagnosis for alcoholic cirrhosis (ICD–10: K70.3 and K70.4).
The SBP patients in the Satavaptan Trial Cohort were matched to patients without SBP on MELD score, serum sodium, serum albumin, inpatient status at the time of paracentesis (yes/no), and refractory ascites (yes/no). We used stratified Cox regression to estimate the mortality hazard ratio for patients vs. those without SBP. Each stratum consisted of one SBP patient and his or her matched cohort members.
2.4Ethical approval statementAccording to Danish law, studies based on Danish healthcare registers do not require approval from an ethical committee. The three satavaptan trials were approved by ethical committee in each of the countries were trial participants were recruited.
2.5Patient consent statementInformed consent is not mandatory for data based on the national patient registry according to Danish law. All trial participants in the three trials concerning the clinical efficacy of satavaptan treatment of ascites in cirrhosis patients gave informed consent.
3Results3.1Danish Healthcare cohortWe included 1282 patients that underwent paracentesis from 2000 to 2014 of whom 133 (10.4%) had SBP. Those with SBP had almost the same age, sex, biochemistry and comorbidity composition as those without (Table 1a). Moreover, they were more likely to have cirrhosis from viral hepatitis and less likely to have alcohol-related cirrhosis (Table 1a). SBP patients’ 4-month cumulative all-cause mortality was significantly higher at 51.2% (95% CI: 43.0–59.9%) compared with 34.7% (95% CI: 32.0–37.6) in patients without SBP (Table 2 and Fig. 1).
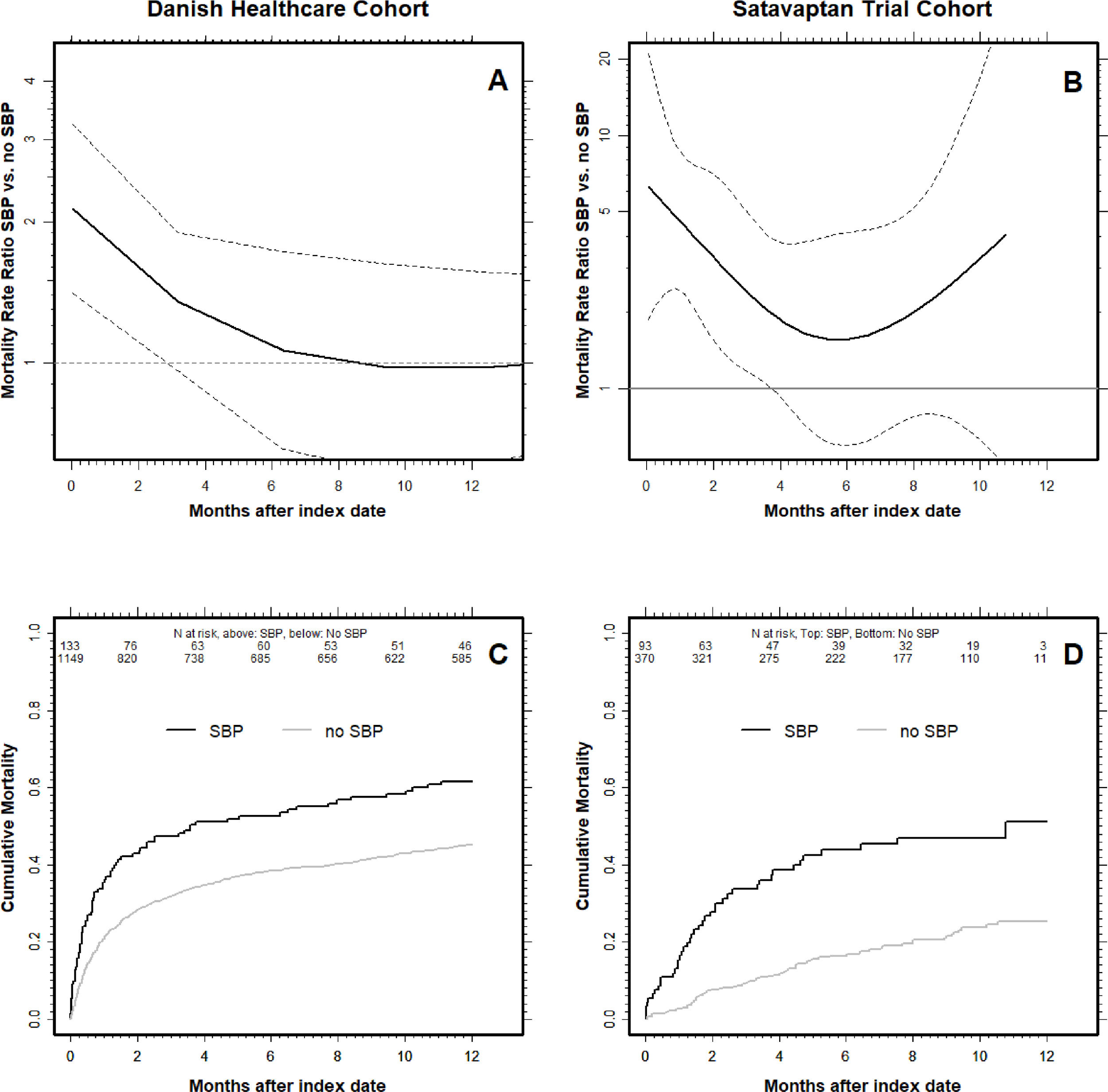
Cumulative mortality after first-time paracentesis in Danish healthcare cohort and the satavaptan trial cohort.
Panel A & B show the mortality hazard ratio with respect to time since first paracentesis for patients with spontaneous bacterial peritonitis (SBP) at the index date compared to those without SBP. The mortality hazard ratio is highest in the first weeks after patients’ first paracentesis (i.e., the ‘index date’) and then declines over time. The dotted lines are 95% CI's, and when they cross the HR = 1, the difference in mortality hazard between SBP-patients and those without is no longer statistically significant. Note the logarithmic scales on the y-axis and that the y-scale differ between the two patient cohorts. Panel C & D show the cumulative all-cause mortality for patients with and without SBP. The number at risk are written underneath the top x-axis for SBP patients (top) and no SBP (bottom). Danish Healthcare Cohort: A & C panels. Satavaptan Trial Cohort: B & D panels.
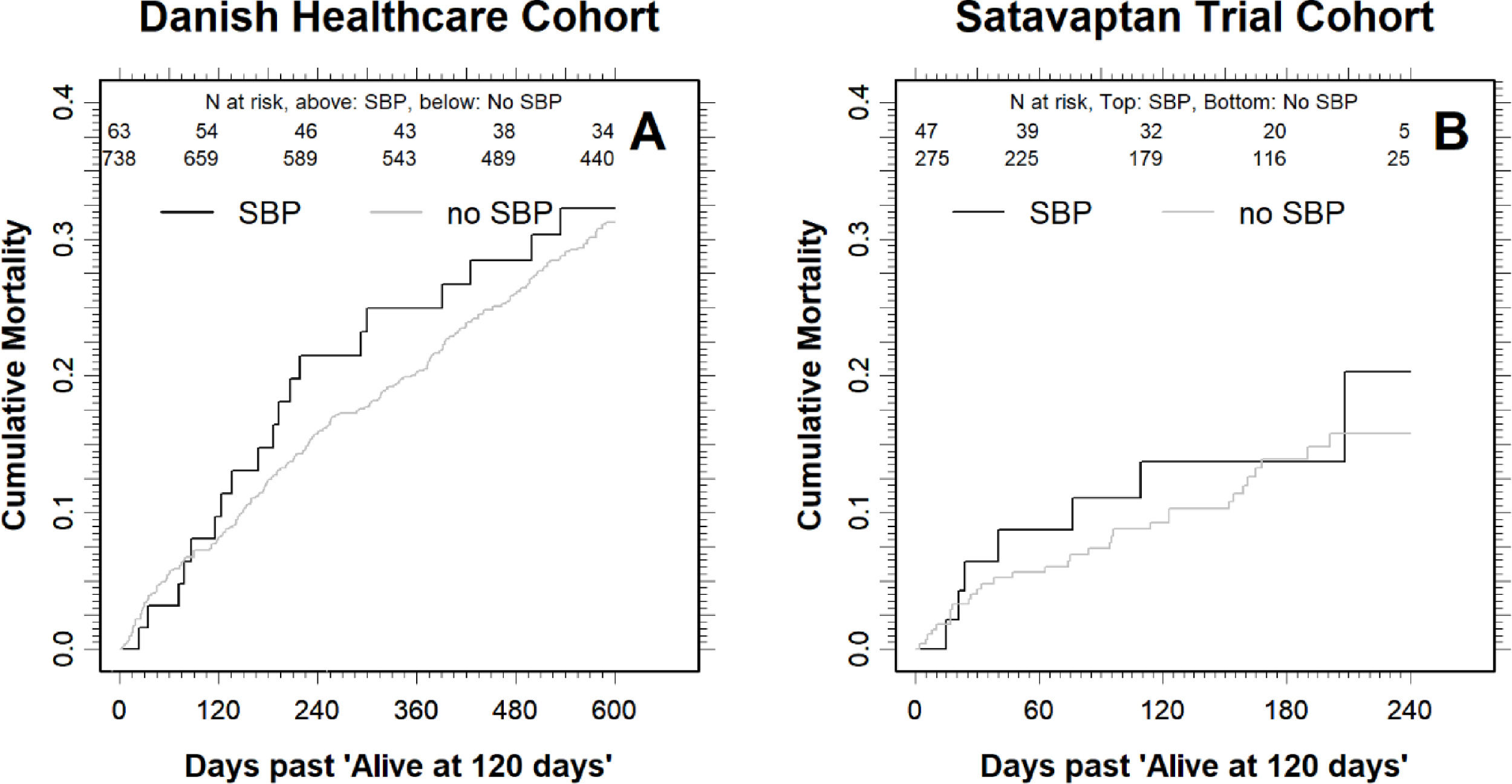
The difference in mortality was smaller for the patients that were alive four months after the index date: For those with SBP, the cumulative all-cause mortality was 21.5% (95% CI: 13.1–34.1) one year (12 months) after the index date, compared with 16.0% (95% CI: 13.5–18.9) for those without SBP (Fig. 2). Moreover, the cumulative all-cause mortality was similar two years after the index date at 32.3% (95% CI: 21.9–45.9) for those with SBP compared with 31.7% (28.4–35.3) in those without (Fig. 2).
Cumulative all-cause mortality for the patients that were alive four months (120 days) after the index date in the Danish Healthcare Cohort (A) and the Satavaptan Trial Cohort (B). Note that the scale on the x-axis differs between the two patient cohorts. The number at risk are written underneath the top x-axis for SBP patients (top) and no SBP (bottom).
The Cox regression analysis revealed a similar pattern. In the time-period beginning at the index date and lasting until four months (120 days) after, we found a mortality hazard that was 1.54-fold higher (95% CI: 1.18–2.00) for SBP patients than for those without SBP (Fig. 1). In the time-period beginning 4 months after the index date the mortality hazards were similar for patients with and without SBP (mortality hazard ratio 1.02, 95% 0.72–1.46). Our results were similar when we repeated our analysis controlling for only HCC, comorbidity, sodium, and albumin indicating a minimal impact of the 20% of patients without a measurement of serum creatinine (see Supplementary material for details). All these results were essentially unaltered when we restricted our analyses to patients with alcoholic cirrhosis.
3.2Satavaptan trial cohortWe included 93 patients with SBP and 371 matched patients without SBP. The age, sex, and biochemical profiles were similar (Table 1b), but alcoholic etiology was more prevalent among those with SBP. Patients with SBP had a four-month cumulative all-cause mortality of 38.6% (95% CI: 29.3–49.7), whereas it was 11.4% (95% CI: 8.5–15.2) in patients without SBP.
In comparison, when we followed the patients alive four months (120 days) after the index date until 12 months after index date, the difference in mortality was much smaller: The cumulative all-cause mortality for those with SBP was 20.3% (9.0–41.7) compared with 15.8% (95% CI: 11.2–21.8) in those without SBP (Fig. 2). The higher cumulative all-cause mortality during the first four months after an SBP episode was reflected in a 3.86-fold higher (95% CI: 2.44–6.12) mortality hazard (Fig. 1b). By contrast, the mortality hazard ratio for patients with SBP vs. those without was 1.23 (95% CI: 0.54–2.83) in the period beginning four months after the index date.
4DiscussionThis study examined the prognosis of SBP in two differently constructed cohorts of cirrhosis patients. One population-based and one based on trial data. The results were broadly similar: We found a markedly higher cumulative mortality and mortality hazard in the first four months following an SBP-episode compared to patients without SBP, but not in the time-period beginning four months after the SBP episode.
The strength of our study is its foundation in two detailed, independent, and large datasets. In the Danish Healthcare Cohort, we believe that the high positive predictive value of a diagnosis for cirrhosis or alcoholic cirrhosis of about 85% [20], was further increased by the fact that we only included patients with a diagnosis for cirrhosis that underwent paracentesis. In addition, the average ascites-protein content was <16 g/, which indicates they were transudates [21]. Finally, all patients in the Satavaptan Trial Cohort had cirrhosis and ascites by inclusion criteria. The association between SBP and death during the first four months after the index date was present even after adjustment for MELD score, serum albumin, and sodium. In the Danish Healthcare Cohort, we also adjusted for comorbidity and HCC. However, data on the hepatic venous pressure gradient, nutritional status and medication use (diuretics and non-selective betablockers) would have strengthened our conclusion. Moreover, the mortality hazard ratio obtained from the Satavaptan Trial Cohort of 1.20 in the period starting four months after the SBP episode, does suggests a remote chance, that we could have reached a different conclusion in a larger dataset with more precisely estimated hazard ratios. On the other hand, there is no such evidence in the Danish Healthcare data, because the mortality hazard ratio essentially is 1.00. Finally, we addressed missing data with appropriate methods [22].
Our 30-day mortality estimate from the Danish Healthcare Cohort of 35% in SBP patients resembles the 30-day mortality of 32% found by Bac in 2003 [7], but slightly higher than the 21% found by Melcarne et al. [9,11]. They are lower than those found in the original paper describing SBP by Kerr et al. [4]. Since the Danish Healthcare Cohort is the hitherto largest population-based patient material with detailed clinical information [23], our estimates provide a sound basis for the prognostic impact of SBP.
The different cumulative mortality following an SBP episode in the Danish Healthcare Cohort and the Satavaptan Trial Cohort might appear incoherent. However, the difference is likely rooted in the fact that we examined mortality in a population-based patient material of cirrhosis patients and in a cohort of trial-recruited cirrhosis patients, respectively. The latter had fewer comorbidities and the necessary compliance to participate in a randomized controlled trial. Moreover, we acknowledge that neither of the two patient cohorts were collected with the intention to answer the research question of this study. Importantly, both datasets were collected prospectively. Finally, since the results of two such different patient cohorts agree on the time dependent mortality hazard after an SBP episode, it provides a strong argument for its validity.
We assume that securing the PMN cell count in both cohorts went hand-in-hand with a subsequent administration of intravenous antibiotics to those with SBP. However, the lack of data on in-hospital care prevents us from confirming our assumption, and that is a limitation. Recommendations for the long-term clinical management of SBP patients also include the possibility for antibiotic prophylaxis [5]. Therefore, it is also a limitation that we were unable to present data on quinolone antibiotics prescription after discharge.
Our results shed a new light on the consequences of decompensated cirrhosis, but do not motivate changes in the classification of the clinical course of cirrhosis. Concerning liver transplantation, the added 15–25% in mortality in those with SBP compared with those without SBP occurred within four months, which indicate that this time-window is when patients with SBP will profit the most from liver transplantation compared to patients without SBP. Moreover, this time-window suggests that patient with SBP may benefit from close clinical attention and care beyond the usual 5-day course of intravenous antibiotics. Our results parallel those of Hung et al., although they defined SBP by diagnosis codes rather than ascites PMN cell count [11]. Altogether, we suggest investigating a practice, where considerations about liver transplantation and antibiotic prophylaxis in patients alive four months after an SBP episode rely on current clinical status, rather than on the previous SBP episode per se. Nevertheless, a previous SBP episode should not hinder liver transplantation in an otherwise well-suited candidate.
5ConclusionsIn conclusion, we found that from one-third to half of cirrhosis patients with ascites and SBP die within 4 months, which is markedly more than in those with the same clinical characteristics but without SBP. However, for those surviving these four months, their SBP episode had no further negative impact on their mortality compared with those without SBP in the past. Thus, an SBP episode was not a marker of generally advancing cirrhosis, but rather reflected a temporary but severe complication.
The authors thank Sanofi-Aventis R&D for providing access to the complete trial database from the satavaptan phase III trials in cirrhotic ascites and for permission to perform the analyses included in this publication.