Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, et al. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy. A randomized controlled trial. JAMA 2012; 308(3): 274-82.
CommentsChronic HCV infection leads to cirrhosis and hepatocellular carcinoma and causes more than 300,000 deaths per year. Current treatment is based on a combination of pegylated interferon (Peg-IFN) and ribavirin in combination with oral protease inhibitors of HCV like boceprevir1 and telaprevir,2 which directly inhibits HCV replication and drives progressive infected cell clearance through intricate and only partly understood mechanisms.3 Multidrug resistance represents an increasing problem in the treatment of several diseases. It often appears after prolonged exposure of cells to a single drug and is characterized by cells resistance to structurally unrelated compounds.4 It has been demonstrated that current therapy could eradicate just certain HCV genotypes. In addition, certain limitations associated with current treatment as side effects and poor response leads to the need to search better anti-HCV therapies.
Silybum marianum has been used for over 2000 years to treat a range of liver and gallbladder disorders, including hepatitis, cirrhosis, and jaundice, and to protect the liver against poisoning from chemical and environmental toxins, giving a hepatoprotective and/or antioxidant effects. The active component or complex extract of this plant is Silymarin, which pure compound Silybin, is considered a mixture of several flavonoids. The growing interest in the new activities of Silybin/Silymarin in non-traditional applications is documented by the fact that even before 2006 documented papers in indexed journals and clinical reports have been published on this topic.4
According to resistance mechanisms, we should mention the involving of drug cell depletion by membrane efflux proteins, for example P-glycoprotein (Pgp) in mammalian cells. It is well documented that Silymarin exerts an inhibitory effect of Pgp function,4,5 which suggests its role in resistance mechanisms. On the other hand, it has been demonstrated an antiviral,6,7 anti-inflammatory and immunomodulatory8 effect.
Ahmed-Belkacem, et al. demonstrated that a Silybin mixture is able to inhibit HCV genotype 1b and 2a strain JFH1 replication in a cell culture system mediated partially through polymerase (RdRp) activity inhibition. Moreover, in a viral kinetic modeling study using Legalon® (SIL), a chemically hydrophilized version of Silybin, non-responder patients to Peg-IFN and ribavirin therapy present a blocking in terms of viral production, as well as a higher rate of rapid viral decline than that typically observed during Peg-IFN ± ribavirin treatment.9 This suggests that Silymarin might be useful in combination with Peg-IFN and ribavirin especially in non-responder patients with based therapy.
In this study Freid, et al. assessed the efficacy of increasing doses of Silymarin in patients with chronic HCV genotype 1 infection (frequency 91%).10 However, Fried, et al. did not find significant improvement in serum ALT levels in patients unsuccessfully treated with IFN-based therapy, as compared with placebo group. Indeed, serum HCV RNA levels also remained unchanged during the course of the study, 2 years of enrollment and 1 year of follow-up. Although, it is a rigorous trial and Silymarin doses were standardized before,11 the doses employed were 3 and 5 times higher than the customary doses (Table 1); this should be taken into consideration since the existence of resistant mechanisms involved Silymarin4 is well known.5 It is important to mention that the percentage of participants with serious adverse events was numerically higher with Silymarin treatment (6.9%) than placebo group (1.9%); however these differences were not statistically significant (P = 0.27).10
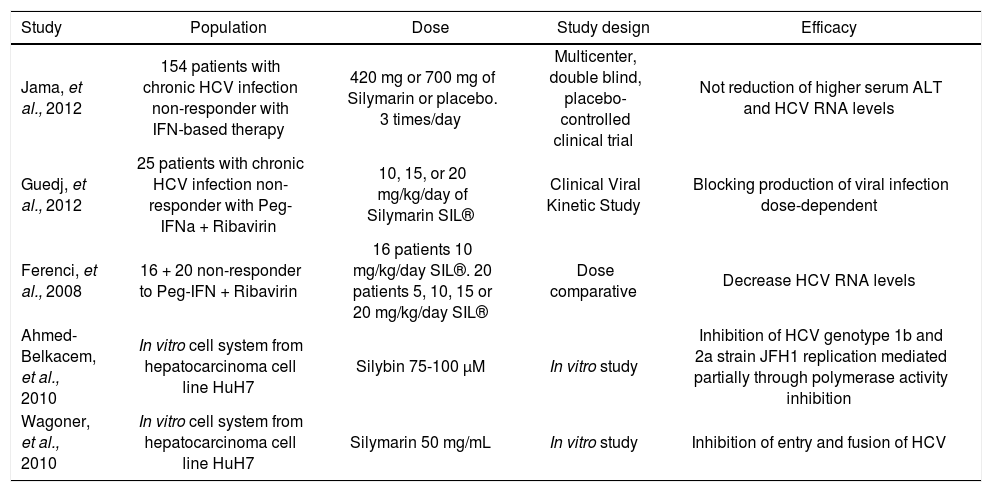
Comparison of studies investigating Silybin/Silymarin effect in chronic hepatitis C.
| Study | Population | Dose | Study design | Efficacy |
|---|---|---|---|---|
| Jama, et al., 2012 | 154 patients with chronic HCV infection non-responder with IFN-based therapy | 420 mg or 700 mg of Silymarin or placebo. 3 times/day | Multicenter, double blind, placebo-controlled clinical trial | Not reduction of higher serum ALT and HCV RNA levels |
| Guedj, et al., 2012 | 25 patients with chronic HCV infection non-responder with Peg-IFNa + Ribavirin | 10, 15, or 20 mg/kg/day of Silymarin SIL® | Clinical Viral Kinetic Study | Blocking production of viral infection dose-dependent |
| Ferenci, et al., 2008 | 16 + 20 non-responder to Peg-IFN + Ribavirin | 16 patients 10 mg/kg/day SIL®. 20 patients 5, 10, 15 or 20 mg/kg/day SIL® | Dose comparative | Decrease HCV RNA levels |
| Ahmed-Belkacem, et al., 2010 | In vitro cell system from hepatocarcinoma cell line HuH7 | Silybin 75-100 μM | In vitro study | Inhibition of HCV genotype 1b and 2a strain JFH1 replication mediated partially through polymerase activity inhibition |
| Wagoner, et al., 2010 | In vitro cell system from hepatocarcinoma cell line HuH7 | Silymarin 50 mg/mL | In vitro study | Inhibition of entry and fusion of HCV |
HCV: hepatitis C virus. Peg-IFNα: pegylated interferon alpha. ALT: alanin transaminase. JFH1: Japan fulminant hepatitis 1. SIL: Silymarin.
On the other hand, there were patients that had failed prior treatment to IFN-based therapy, instead of full dose of Peg-IFN and ribavirin therapy. This could explain the differences in terms of effect/response in comparison to the other studies, which their protocols were similar and consistent with each other (Table 1).
We should highlight the importance of the formulation therapy for analyzing the final effect. Silymarin is composed by the flavonolignans silybin, silydianin, silycristin, and isosilybin, in which its hepatoprotective properties are attributed to silybin, the main constituent (60-70%).12 However, Legalon,® the pharmaceutical form of simylarin contains a specific composition: 52 mg silybin, 22 mg isosilybin, 23 mg silycristin and 28 mg silydianin.13
This could lead to differential effects suggesting potential differential in vivo12 and in vitro3,14 activities among several studies.
Evidence suggests that future clinical trials will be needed in order to assess which patients could benefit from the administration of Silybin/Silymarin concomitantly to Peg-IFN and ribavirin, especially in difficult-to-treat patients previous non-responders to Peg-IFN and ribavirin.7
To sum up, in order to further investigate the controversy of silymarin role in HCV therapy, we suggest that rigorous trials with a consistent pattern of therapy and protocols will need to be assessed. Further in vitro experiments that provide more insights into mechanism(s) of action against HCV are needed to understand the nature of Silybin or Silymarin and bring them into the respective clinical application. This could be supported with the combination of HCV protease or polymerase inhibitors for increasing the effect. We should bear in mind that standardized preparations in terms of composition and purity as well as requisite drug regulations will be indispensable requirements for a successful clinical application.4