Dear Editor:
An estimated 80% of the world population uses herbal and supplementary medicines. Herbal toxicity due to herbal products is a rare but well-known adverse event.1 However less is known about the effects of other phytotherapics. Among these is shark liver oil, a popular over-the-counter Asian and Mediterranean folk remedy, which has been in use for over 40 years as both a therapeutic and preventive agent.1
A case of severe acute hepatitis caused by ingestion of shark liver oil capsules is presented. No severe side effects in dosages of 500 mg three times a day prescribed for its immunomodulatory effects have previously been recorded.
A 31-year-old woman was admitted to our Gastroenterology Department. Preliminary physical examination revealed that she was suffering from jaundice, pruritus, new onset malaise and abdominal discomfort. In her medical history she denoted that she had been taking shark liver oil capsules, 2 times a day for two weeks. Her malaise and abdominal discomfort began at the end of the first week of treatment and progressed slowly over a 1-week period, she was also suffering from jaundince and pruritis that began on the tenth day of treatment. She stopped taking shark liver oil capsules and consulted our department.5 She said she had not consumed any alcohol, any herbal or folk remedies, or any other over-the-counter agents. No environmental issues were identified. She had no history of liver disease, elevated liver enzyme levels, jaundice, gastrointestinal bleeding, or ascites; no family history of liver disease; and no other risk factors for liver disease. Vital signs were normal. Physical examination was only remarkable for jaundice of the sclera and skin.
Liver function tests on admission revealed: alanine aminotransferase (ALT) 1,102 U/L (reference range 0-49), aspartate aminotransferase (AST) 908 U/L (0-34), alkaline phosphatase (ALP) 248 U/L (0-270), у-glutamyl transferase (GGT) 265 U/L (0-61), total bilirubin 9.2 mg/dL (0-1.2), direct bilirubin 6.3 mg/dL (0-0.2), and serum albumin 3.5 g/dL (3.4-4.8). These highly abnormal results indicated that the patient was suffering from acute toxic hepatitis.
Results of other biochemical tests, including serum creatinine, blood urea nitrogen, sodium, potassium, cholesterol, and triglycerides, were in normal limits. A complete blood cell count revealed hemoglobin 13.1 g/dL, white blood cell count 4.8 x 103/mm3, and platelet count 140 x 103 cells/mL.2 Differential white blood cell count was normal. Erythrocyte sedimentation rate was 19 mm/h, and prothrombin time was 16.1seconds (10.5-14.5). Urine analysis revealed 2+ bilirubin and urobilinogens, respectively. Creatinine clearance was 102 mL/min, 24 h urinery copper excretion was 18 /U.g/24 h (10-30),3 serum iron and iron binding capacity were normal, and the ferritin level was 303 ng/mL (13400).
Negative results were received from tests for viral hepatitis (hepatitis A virus IgM, hepatitis B virus surface antigen and core antibody, hepatitis C virus by polymerase chain reaction, and cytomegalovirus and Epstein-Barr virus IgM), autoimmune hepatitis (antinuclear antibody, anti-smooth muscle antibody, and serum y-globulin), and Wilson, disease.
In the abdominal ultrasonography, liver parenchyma was homogeneous and the edges were regular, the vertical size of liver was 13 cm and spleen was 13 cm. Liver biopsy was not performed because of the acute-toxic hepatitis situation.
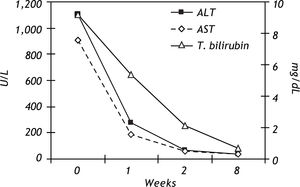
These unusual findings led us to suspect that the recorded consumption of shark liver oil capsules had led to the high toxic levels in the liver. When shark liver oil tablets were no longer taken, an immediate improvement in the patient's condition was seen. Liver enzymes reduced rapidly and were at completely normal levels 8 weeks after the first admission. Based on the labarotory findigs there was a hepatocellular type injury and the uptadated causality assessment in hepapoxicity by drugs and dietary sublements scale (CIOMS) for his association was "probable" (score +7) (Table 1).8 The time course of the liver function tests is presented in figure 1.7-8
Causality assessment in hepatotoxicity by drugs and dietary supplements.
| Type of liver injury | Hepatocellular | Points | |
|---|---|---|---|
| Time of onset of the event | First exposure | Second exposure | - |
| Time from drug intake until reaction onset | 5 to 90 days | 1 to 15 days | +2 |
| < 5 or > 90 days | > 15 days | + 1 | |
| Time from drug withdrawal until reaction onset | < 15 days | < 15 days | + 1 |
| Risk factors | Alcohol | + 1 | |
| Age > 55 years | + 1 | ||
| Course of the reaction | > 50% improvement 8 days | +3 | |
| > 50% improvement 30 days | +2 | ||
| No information | +0 | ||
| Worsening or < 50% improvement 30 days | -2 | ||
| Concomitant therapy | Time to onset incompatible | +0 | |
| Time to onset compatible but with unknown reaction | -1 | ||
| Time to onset compatible but known reaction | -2 | ||
| Role proved in this case | -3 | ||
| None or information not available | +0 | ||
| Exclusion of non drug-related causes: | All causes ruled out | +2 | |
| a) Group I (HBV, HAV, HCV, alcoholism, | |||
| acute recent hypotension history, | The 6 causes of group I ruled out | + 1 | |
| hepatobiliary sonography of liver vessels) | 4 or 5 causes of group I ruled out | +0 | |
| b) Group II [complications of underlying diseases, | Fewer than 4 causes of group I ruled out | -2 | |
| other viral causes (VZV, HSV, CMV, EBV)] | Nondrug cause highly probable | -3 | |
| Previous information on hepatotoxicity | Reaction unknown | +0 | |
| Reaction published but unlabeled | + 1 | ||
| Reaction labeled in product's characteristics | +2 | ||
| Response to re-administration | Doubling of ALT with the drug alone | +3 | |
| Doubling of ALT with the drugs already given | +2 | ||
| at the time of first reaction | |||
| Increase of ALT but less than the first administration | + 1 |
Total points/causality: .0, excluded; 1-2, unlikely; 3-5, possible; 6-8, probable; 8, highly probable. ALT: alanine aminotransferase. CMV: cytomegalovirus. EBV: Epstein-Barr virus. HAV: hepatitis A virus. HBV: hepatitis B virus. HCV: hepatitis C virus. HSV: herpes simplex virus. VZV: varicella zoster virus.
Shark liver oil has been found useful in adjunctive treatment of several types of cancer. Fish oils contain several active compounds that modify cell activity and influence various functions of the body. Shark liver oils are rich in alkylglycerols and squalene, but contain relatively low amounts of n-3 polyunsaturated fatty acids. Virtually all species of sharks are known to have an extraordinary resistance to the growth of tumors. Several reports have referred to an extremely low incidence of cancer in sharks, some even stating that no cases of cancer in sharks have been recorded.3
Shark liver oil has also been found useful in treatment of conditions resulting from inadequate immune response.2-4 Alkylglycerols may control immune response possibly through modification of diacylglycerol (DAG) and platelets to activating factor (PAF) production.6 Alkylglycerols and squalene, enhance antigen presentation and induction of inflammatory response.5,6
Recorded side effects have been negligible.2-4 In small trials, fish oil capsules up to 12 g/d (containing 6 g/d n-3 polyunsaturated fatty acids) have been administered for more than 2 years without serious adverse events.4,6,7 Perhaps the most common symptom causing discontinuation of fish oil supplements is its “fishy taste” following eructation (burping). Although many people have taken shark liver oil, the issue of potential toxicity at the usual doses has not been studied.
However a case of acute toxic hepatitis has been shown to be related to the use of shark liver oil capsules. To our knowledge there have been no other reported cases of this kind.
As a result, when presented with patients who have liver injury with no obvious cause, health care professionals should be vigilant in inquiring about the use of health supplements and alternative medicines, including the use of shark liver oil.