Introduction: The course of HBV infection and the outcome of interferon alpha (IFNα) therapy of patients with chronic hepatitis B, is determined by the antiviral immune response of the host. The aim of the study was to investigate 1) the correlation between IL-6 and IL-12 serum levels and biochemical and histopathological changes in children with chronic hepatitis B, 2) predictive value of pre-treatment serum levels of these cytokines in patients treated with interferon alpha and 3) changes in serum levels of these cytokines after interferon alpha treatment. Methods: Serum levels of IL-6, IL-12 (heterodimer p70) and IL-12 (heterodimer p70 & p40 subunit) were determined by specific ELISA in 39 children with chronic hepatitis B on the first and the last day of IFNα therapy. Results: Serum levels of IL-6, IL-12 (p70) and IL-12 (p70&p40) were respectively within the following ranges of values: 0-1.7 pg/mL, 3.0-85.1pg/mL, 93.7-442.7 pg/mL and they showed no correlation with biochemical and histopathological changes. The pre-treatment cytokines levels in patients who responded and those who did not respond to IFNα therapy did not differ statistically. There was no statistical difference between the end and pre-treatment cytokines levels in both groups. Conclusions: Serum levels of IL-6 and IL-12 do not reflect the inflammatory activity of hepatitis and have no predictive value of positive response to the IFNα therapy in children with chronic hepatitis B. Serum IL-6 and IL-12 levels at the end of INFa treatment do not inform of their role in immunological changes which take place while inhibition of HBV replication or virus clearance.
The course of an infection with non-cytopathogenic hepatitis B virus (HBV) is determined by the antiviral immune response of the host. Specific immunological response - cellular and humoral, non-specific inflammatory response and inhibition of viral replication with no cytolytic effect, contribute to the viral clearance. In all these mechanisms cytokines-polypeptides of a wide spectrum of inflammatory, metabolic and immunologic regulatory properties-are involved. Recent studies proved interleukin 6 (IL-6) and interleukin 12 (IL-12) to be the most important ones.
IL-6, involved in T and B cell regulation, inflammation response, regeneration and fibrogenesis plays a crucial role in the pathogenesis of liver diseases. Elevated serum levels of IL-6 were reported in adults with chronic autoimmune hepatitis, alcoholic liver cirrhosis, primary biliary cirrhosis, metabolic liver diseases and chronic viral hepatitis B and C. Some studies in adults showed correlation between serum IL-6 levels and serum activity of transaminases.1-9 Advanced fibrosis in these patients also confirmed IL-6 relationship with increased intercellular matrix deposition and stimulation of collagen production.1,2,5,8 Spontaneous IL-6 production by peripheral blood mononuclear cells (PBMC) was reported to be increased in chronic hepatitis B. Mitogen-induced IL-6 production was significantly higher in patients, who seroconverted to anti-HBe, than in patients with HBeAg presence.4 These findings may suggest IL-6 participation in HBeAg/HBeAb seroconversion and inhibition of HBV replication.
Increased expression of IL-6 in adults with chronic hepatitis B, was detected not only in PBMC trapped-in liver tissue, but also in endothelial and Browicz-Kupfer cells. This might suggest the involvement of locally produced IL-6 in the mechanism of inflammation and liver injury. IL-6 expression was especially increased in the areas of focal necrosis and it correlated with histopathological grading. By reverse transcription polymerase chain reaction (RT-PCR) m-RNA for IL-6 was also detected in hepatocytes mainly of periportal region in patients with chronic hepatitis B.10,11
IL-12, stimulating NK cells activity, cytotoxic T lymphocytes proliferation and interferon production, plays an important role in the pathogenesis of HBV infection. Recent studies showed that IL-12 is the dominant cytokine, which specifically promotes antigen-dependent Th1 cell differentiation, suppresses Th2 function and may contribute to the viral clearance.12,13 Biologically active IL-12 is a heterodimer, composed of two subunits of 40 (p40) and 35 (p35) kD coded by independent genes. Overproduction of p40 was proved, but the biological significance of this phenomenon has not been elucidated yet.
Interferon alpha (IFNα) is one of the few accepted treatments for chronic hepatitis B. However, still unsatisfactory efficacy of this treatment and its side effects especially in children, oblige to constant verification of indications to IFNα therapy and search for predictive factors of positive response to this treatment. Typical serum cytokine profile could allow to select a group of patients prone to positive response of IFNα therapy. The group of paediatric patients with immature immunological system in whom exogenous IFNα may cause distinct, serious side effects, seem to be the most important. The aim of the study than was to investigate 1) the correlation between interleukin 6 and 12 serum levels and biochemical and histopathological changes in children with chronic hepatitis B, 2) the prognostic value of pre-treatment serum levels of these cytokines in patients treated with interferon alpha and 3) changes in serum levels of these cytokines after interferon alpha treatment.
Material and methodsThe following study was the clinical series of patients including 39 children (27 boys and 12 girls), aged from 2.8 to 17.1 years (mean 8.5 ± SD = 3.6, median 8), with chronic hepatitis B. The inclusion criteria were
- •
serum HBsAg and HBeAg presence for 6 months prior to the study,
- •
increased serum transaminases activity,
- •
inflammation changes in liver biopsy specimens.
The probable age of the moment of HBV infection ranged from 2.5 to 16.0 years (mean 8.3 ± SD = 3.5, median 7.9) and the duration of HBV infection ranged from 0.9 to 7.2 years (mean 3.6 ± SD =1.9, median 3.7). Those data were estimated on the bases of anamnesis and epidemiological investigation.
The exclusion criteria were: co-infection with HCV, HDV, chronic autoimmune hepatitis and metabolic chronic liver diseases.
IFNα (interferon α – 2b Intron A, Shering-Plough) was given subcutaneously in the dose of 3.0 mega units (MU)-three times a week for 20 weeks - total dose 180 MU. Positive response to the IFNα therapy was defined as serum transaminases activity normalization and HBeAg/HBeAb seroconversion (partial response) and HBsAg/HBsAb seroconversion (full response).
Serum levels of IL-6 and IL-12 were determined on the first and the last day of INFa therapy. Patients did not demonstrated any symptoms of infection on physical examination. None of the patients had received immunosuppressive or immunomodulatory treatment within one year before INFα therapy. Serum samples for cytokines detection were collected and stored in -80° C. IL-6, IL-12 (p70) and IL-12 (p70&p40) levels were determined in serum samples by ELISA with Endogen Inc. kites. Each sample was tested in duplicate. Serum levels of IL-6 and IL-12 in healthy subjects are within the following ranges of values: for IL-6 0-149 pg/mL, for IL-12 (p70) 0-7.9 pg/mL, for IL-12 (p70&p40) 39-170 pg/mL (manufacturer’s references 5.10, 14-17). Sensitivity of the assays for IL-6 was < 1 pg/mL, for IL-12 (p70) < 3pg/mL and for IL-12 (p70&p40) < 5pg/mL.
Biochemical liver tests included ALT activity, prothrombin index (INR), bilirubin and total protein, albumin, gamma globulin levels. Serum ALT (normal values 0-40 IU/L) activity before IFNα treatment were 59 – 374 IU/L (mean 135 ± SD = 77). Increased bilirubin level was detected in 1 child, elevated INR in 3 patients. Total protein, albumin and gamma globulin levels were within values of normal ranges.
Liver biopsy was performed in all patients with biochemical and serological markers of chronic hepatitis B from 1 to 3 weeks prior to IFNα therapy. Histological “grading” and “staging” numerical scores of chronic hepatitis were used to evaluate inflammation and fibrosis in liver specimens.18,19
The results of the study were statistically analysed using Statistical v.5.77. Differences of various groups were analysed using the Kruskal-Wallis test. Correlation analysis was done by the Spearman test. P-value < 0,05 was considered statistically significant.
The research was carried out with each patient’s informed consent and Ethics Committee approval.
ResultsBaseline levels of IL-6 and IL-12.Baseline serum IL-6 levels in studied group of 39 children with chronic hepatitis B before IFNα treatment were 0-1.7 pg/mL (mean 0.5 ± SD = 0.5pg/mL, median 0.1 pg/mL).
IL-12 (p70) was evaluated in 15 of 39 patients and Il-12 (p70&p40) in 24 patients. The pre-treatment serum levels of IL-12 (p70) were 3-85.1 pg/mL (mean 24.4 ± SD = 22.2 pg/mL, median 15.4 pg/mL) and IL-12 (p70&p40) were 93.7-442.7 pg/mL (mean 211.8 ± SD = 106.3 pg/mL, median 283.6 pg/mL). No statistically significant correlation was found between serum levels of particular cytokines and the age of patients, the probable age of the moment of HBV infection and the duration of HBV infection in the studied group (Spearman RSs > 0.05, P > 0.05).
Serum ALT activity before IFNα therapy was below 100 IU/L in 16 (41%) patients, between 100 and 200 IU/L in 17 (44%) and over 200 IU/L in 6 (15%) children. There was no statistically significant correlation between serum IL-6, IL-12 (p70) and IL-12 (p70&p40) levels and ALT activity in the studied group of children (Spearman RSs> 0.05, P > 0.05).
Minor severity of inflammation was dominant on histopathological findings of liver specimens. In 35 (90%) patients grade I and II was found and in only 4 (10%) children grade III. There was no statistically significant correlation between serum IL-6, IL-12 (p70) and IL-12 (p70&p40) levels and severity of necroinflamatory changes in liver biopsy specimens (Spearman RSs> 0.05, P > 0.05).
In the studied group of children with chronic hepatitis B, no features of fibrosis in liver specimens were found in 23 (59%) patients, in 14 (36%) children stage I was observed and in 2 children stage II. No statistically significant correlation was found between serum levels of particular cytokines and extent of fibrosis in liver tissue (Spearman RSs> 0.05, P > 0.05).
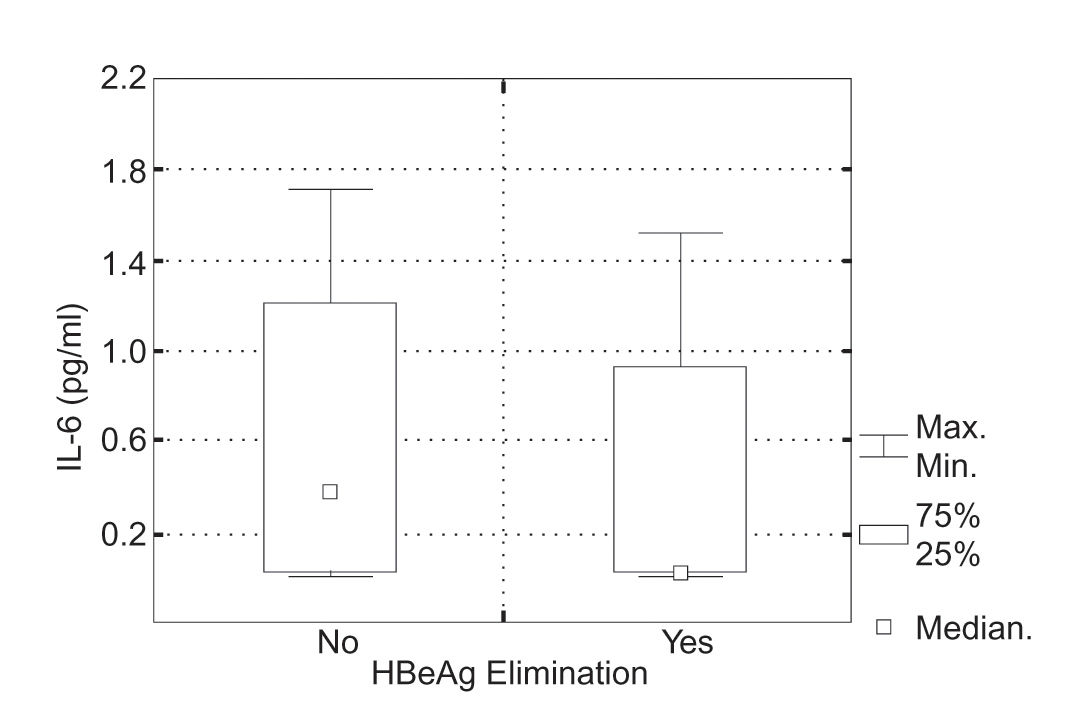
IL-6 and IL-12 serum levels and response to IFNα therapy.Positive response to the IFNα therapy defined as serum normalization and HBeAg/HBeAb seroconversion was achieved in 14 (36%) of 39 patients; in 6 of 39 children seroconversion HBsAg/HBsAb was observed. Initial serum Il-6 levels in the group of patients, who responded to the IFNα therapy were 0-1.5 pg/mL (mean 0.3 ± SD = 0.2 pg/mL, median 0.1 pg/mL) and in the group of children, who did not 0-1.7 pg/mL (mean 0.6 ± SD = 0.6 pg/mL, median 0.4 pg/mL). There was no statistically significant difference in serum Il-6 levels in particular groups of patients (Kruskal-Wallis test, P = 0.083) (Figura 1).
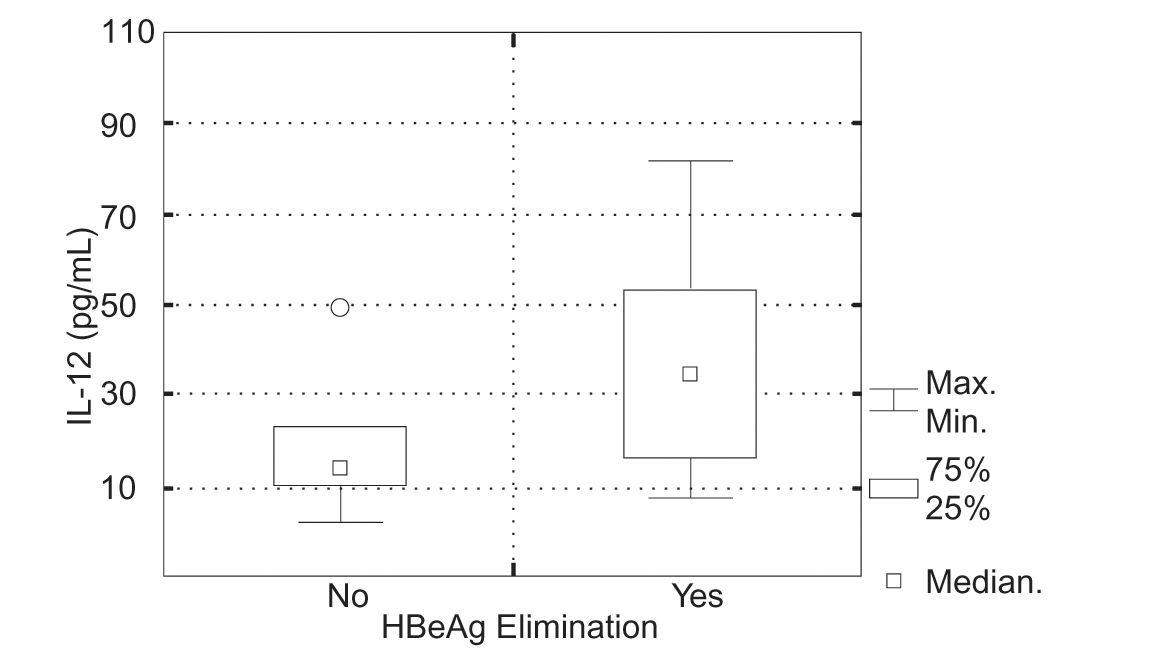
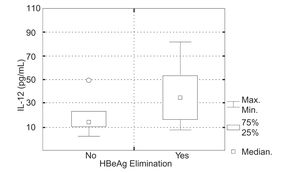
In 5 (34%) of 15 children in whom IL-12 (p70) was evaluated, positive response to the IFN a therapy was achieved. Initial serum IL-12 (p70) levels in the group of patients, who responded to the IFNα therapy were 8.5-85.1 pg/mL (mean 39.0 ± SD = 30.6 pg/mL, median 32.7 pg/mL) and in the group of children, who did not 3.049.7 pg/mL (mean 17.1 ± SD = 13.1 pg/mL, median 13.9 pg/mL). There was no statistically significant difference in serum IL-12 (p70) levels in particular groups of patients (Kruskal-Wallis test, P = 0.111) (Figura 2).
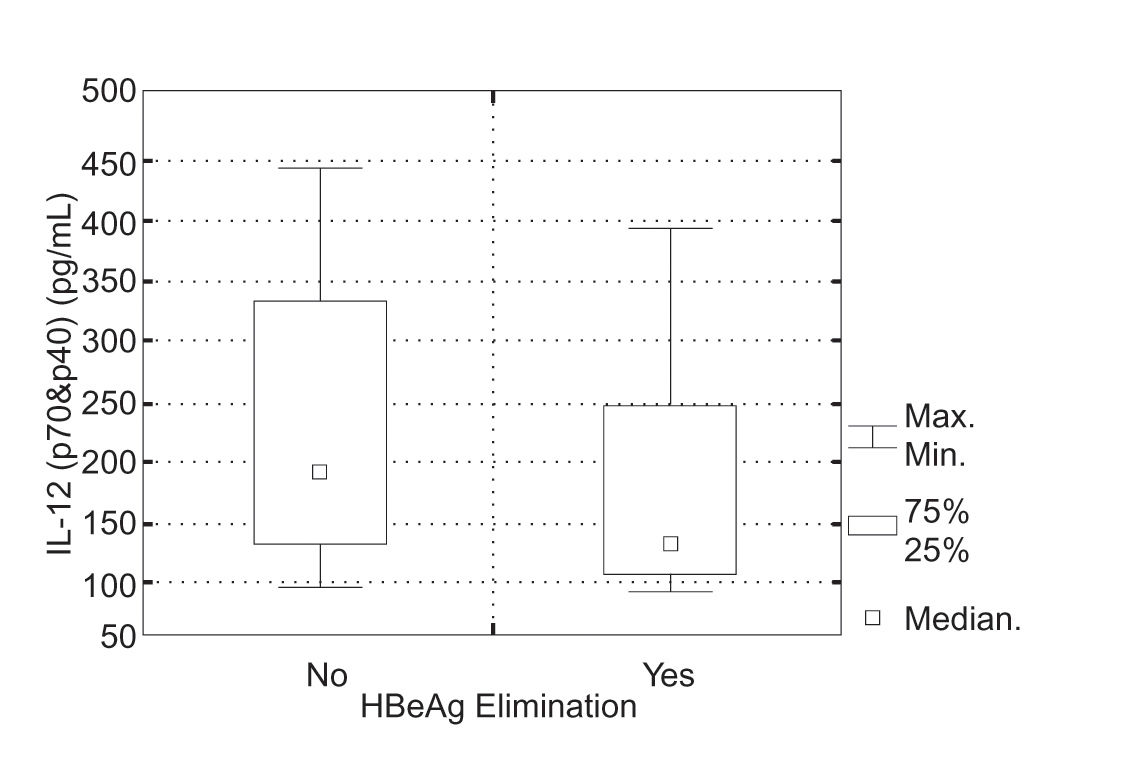
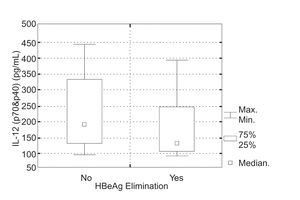
In 9 (38%) of 24 children in whom IL-12 (p70&p40) was evaluated, positive response to the INF a therapy was achieved. Initial serum IL-12 (p70&p40) levels in the group of patients, who responded to the IFNα therapy were 93.7-395.4 pg/mL (mean 181.4 ± SD = 104.2 pg/mL, median 32.7 pg/mL) and in the group of children, who did not 94.6-442.7 pg/mL (mean 229.9 ± SD = 106.8 pg/mL, median 198.2 pg/mL). There was no statistically significant difference in initial IL-12 (p70&p40) serum levels in particular groups of patients (Kruskal-Wallis test, P = 0.189) (Figura 3).
Post-treatment serum levels of IL-6 and IL-12.Serum levels of IL-6, IL-12 (p70) and IL-12 (p70&p40) after IFNα therapy were respectively within the following ranges of values: 0-3.2 pg/mL (mean 0.5 ± SD = 0.7 pg/mL, median 0.1 pg/mL), 1.6-79.5 pg/mL (mean 25.7 ± SD = 23.2 pg/mL, median 20.1 pg/mL), 28.5-379.8 pg/mL (mean 189.1 ± SD = 91.0 pg/mL, median 161.7 pg/mL) and they did not differ from pre-treatment values (P > 0.05).
For the purpose of evaluation the changes of the IL-6 and IL-12 profiles in each patient of analysed groups of responders and non-responders to IFNα therapy, the parameter delta (A) IL-6, IL-12 (p70), IL-12 (p70&p40) was introduced. Delta (A) IL-6, IL-12 (p70), IL-12 (p70&p40) is the difference between post-and pre-treatment serum levels of particular interleukins in the same patient. No statistically significant difference was found in the group of patients, who responded to IFNα therapy and children, who did not (P > 0.05).
DiscussionThe studies on the role of IL-6 in immunological processes accompanying the HBV infection are difficult due to the pleiotropic activity of IL-6 and the complex mechanisms of its production and degradation.
Some published data showed correlation between IL-6 serum levels and inflammatory changes in the liver. Kakum et al. reported elevated serum levels of IL-6 in adults with chronic active hepatitis B, while it was within normal ranges in patients with chronic persistent hepatitis B. Remarkable elevation of serum IL-6 was also observed while seroconversion HBeAg/HBeAb with accompanying ALT increase. In patients with chronic active hepatitis B correlation between serum IL-6 levels and serum ALT activity was noted. However no statistically significant correlation between serum IL-6 levels and biochemical parameters of liver function (bilirubin level, INR, total proteins and albumins levels), was observed.4,5,20 In the present study serum IL-6 levels were low with no correlation with biochemical parameters of liver function and grading on histopathology assessment. Low serum levels of IL-6 in children with chronic hepatitis B were also noted by some other authors.3,6,7 Probably it is so, because of mild necro-inflammatory changes in liver of children with chronic hepatitis B. In the studied group, only 15% of children demonstrated ALT activity higher than 200 IU/L. The bilirubin and INR was elevated in few patients and in the same time the total proteins and albumins levels were within normal ranges in all patients. Mild necro-inflammatory changes in liver of children with chronic hepatitis B are also noted by some other authors.21-27 In the studied group, 90% of children demonstrated grade I and II of the inflammatory activity and no correlation between serum Il-6 levels and grading was noted. The results of this research show that serum IL-6 levels do not reflect inflammatory processes in the liver particularly when it is chronic and of the low grade. However significant increase of serum IL-6 levels were noted in patients with acute hepatitis B with higher risk of fulminant course.5,28
IL-6 like platelet derived growth factor (PDGF), transforming growth factor ß (TGF-ß), tumour necrosing factor (TNF-α) were reported to play a role in liver fibrogenesis.29,30 Serum levels of IL-6 in adults with chronic liver disease like chronic hepatitis B and C, autoimmune and metabolic liver diseases, were higher in cirrhotic to non-cirrhotic patients irrespective of aetiology.2,8,14 In the studied group of children fibrosis on the histopathological assessment of the liver was of the low extent and showed no correlation with serum Il-6 levels. Minor fibrosis in children with chronic hepatitis B was also proved by other authors probably because of the short duration of the disease.7,23-27 The risk of cirrhosis was reported to be the higher the longer was the duration of the HBV infection.31,32
In our opinion low IL-6 serum levels and lack of correlation with biochemical and histopathological parameters of the chronic hepatitis may be also explained by the impairment of the cytokines production in HBV infected patients. HBV is a highly hepatotropic infectious agent, however recent studies shows that it may also infect immunocompetent cells. The HBV replication was detected in peripheral blood mononuclear cells (PBMC), marrow and spleen. In the HBV infected PBMC, reduced production of INFß, INFy, TNF a, Il-1 and Il-2 was observed either in adults or in children.15,35 Decreased IL-6 production may be a result of either lowered IL-1 and IL-2 production or viral inhibition of gen for IL-6 expression. Muller et al. reported decreased IL-6 production in the cultures of PBMC of patients with chronic hepatitis B to the contrary to increased lL-6 production in PBMC of patients with other chronic liver diseases.15,35
Low serum levels of IL-6 and no correlation with biochemical and histopathological markers of chronic hepatitis B in the studied group of patients may be also caused by paracrine activity of IL-6, its dominant production in situ and its very short half-time (T1/2). In some chronic inflammatory entities like in chronic arthritis, significant differences in concentration of IL-6 in serum and in situ are reported. Serum presence of IL-6 is probably a result of its diffusion to the circulatory system.36
Studies on pre-treatment serum levels of IL-6 as prognostic factor in the INFα therapy are very few and conducted on small groups of patients. Gregorio et al. reported higher serum levels of IL-6 in patients, who responded to the IFNα therapy (7 patients) than in those, who did not (4 patients).4 In the studied group of children with chronic hepatitis B, positive response to IFNα treatment was achieved in 36% of patients and correlation between serum ALT activity and response to the IFNα therapy was observed - results comparable with other authors.23,39,40 Due to IL-6 activity in inflammatory processes in hepatitis and its elevated serum levels in adults with acute and chronic hepatitis B, relationship between its serum levels and response to the IFN a therapy was analysed. However, probably because of mild necro-inflammatory changes, pre-treatment serum levels of IL-6 were low and they did not differ in the group of children, who responded to the INFα therapy and those, who did not and thus they showed no prognostic value.
In the therapy of chronic hepatitis B IFNα shows not only direct antiviral activity, but it also plays an important role in the mechanisms regulating production of some cytokines like IL-1, IL-2 and TNF, which may influence IL-6 production.24,41-44 In the studied group of children serum IL-6 levels after IFNα therapy did not differ significantly to the pre-treatment levels. There are few reports on the influence of the exogenous IFNα in patients with chronic hepatitis B on the IL-6 production and its role in immunological changes which take place while inhibition of HBV replication or virus clearance. Pawlowska et al. reported increase in serum IL-6 levels on the 6th week of IFNα therapy in adults, but not in children with chronic hepatitis B. On the end of treatment serum IL-6 levels were slightly increased irrespective of the response to the IFNα therapy.6 Gregorio et al. however reported the increase in serum IL-6 levels only in the group of patients, who responded to the INFα therapy; multiple determinations of IL-6 serum levels showed significant increase in the moments of seroconversion HBeAg/HBeAb during IFNα treatment.4 Recognition of dynamics of IL-6 serum levels changes during IFNα therapy may contribute to better understanding of its role in immunological changes which take place while inhibition of HBV replication or virus clearance.
Recent studies has suggested that IL-12 is one of the most important cytokines in the immune defense against intracellular pathogens. Few studies in adult patients report on its role in the course of HBV infection. IL-12 is the dominant cytokine, which specifically promotes Th0 cell differentiation to Th1 cells, suppresses Th2, enhances IFN production and stimulates T and NK cells cytotoxity and that is why it might play a crucial role in the course of HVB infection, its final outcome and in the response to IFNα therapy.12,13 Studies on the regulation of proliferation and cytokine production after antigenic stimulation in PBMC from chronically HBV-infected adult patients proved IL-12 to be a critical factor that determines the efficacy of the immune response against HBV.53,54 In the studied group and as some other authors report in patients with chronic hepatitis B, serum IL-12 levels were elevated. However they did not correlate with biochemical and histopathological markers of chronic hepatitis B. Serum IL-12 levels can not inform than about the inflammatory activity in the liver probably because of its paracrine manner of action and its dominant production in situ.
Due to IL-12 activity in immunological mechanisms, which determine HBV clearance, serum IL-12 levels were evaluated in the group of children, who seroconverted to anty-HBe and group of patients, who did not respond to the IFNα therapy. They did not differ significantly so these results suggest that serum IL-12 levels cannot be a prognostic factor of the IFNα treatment. Due to different results in the studies on adult patients with chronic hepatitis B and no other reports on children, further scientific investigations may be interesting.
Biologically active IL-12 is a heterodimer, composed of two subunits of 40 (p40) and 35 (p35) kD. In the studied group of children both IL-12 (p70) and IL-12 (p70&p40) were evaluated by ELISA with the monoclonal antibody application. Serum levels of IL-12 (p70&p40) were higher than IL-12 (p70) levels. This fact confirmed overproduction of subunit p40 reported by some other authors.46,54 Results of this study concerning IL-12 (p70) and IL-12 (p70&p40) levels were analogous, so it may suggest that biological activity of IL-12 as heterodimer and its subunit p40 is similar.
Both our and others studies - many a time being at variance with each other - regarding the cytokines serum levels in patients with chronic hepatitis B, suggest that IL-6 and IL-12 serum levels seem to be not reliable parameters informing about the inflammatory activity and extend of fibrosis in the liver or probable positive response to the IFNα therapy. It is possible that it is due to the distinctions in children’s immunological system and different course of HBV infection in this group of patients, biological activity of those cytokines, complex mechanisms of their production and their paracrine manner of action.
ConclusionsThe present study indicates that serum IL-6, IL-12 (p70) and IL-12 (p70&p40) levels 1) show no correlation with biochemical and histopathological markers of hepatitis and 2) seem to be of poor prognostic value of positive response to the INF α therapy in children with chronic hepatitis B. Serum IL-6 and IL-12 levels at the end of interferon alpha treatment do not inform of their role in immunological changes which take place while inhibition of HBV replication or virus clearance.