The activation of hepatic stellate cells (HSCs) is the main cause of liver fibrosis. The beneficial effects of fibroblast growth factor (FGF) 19 on liver fibrosis were recently reported. The S. miltiorrhiza as well as S. miltiorrhiza derived bioactive chemical components has shown prominent antifibrotic effects in liver fibrosis but the mechanism is still not fully understood. We aimed to investigate the bioactive compounds derived from S. miltiorrhiza which exerts antifibrotic effects in HSCs via regulating FGF19.
Materials and methodsFGF19 level in culture media was determined by enzyme-linked immunosorbent assay. Cell proliferation was measured by Cell Counting Kit-8 assay. Further, mRNA and protein expressions were assessed by quantitative polymerase chain reaction and western blotting, respectively. Knocking down of FGF receptor 4 (FGFR4) by transfection with siRNA was used to confirm the role of FGF19/FGFR4 signaling.
ResultsUsing the human HSC cell line LX-2, we screened several natural products and found that bioactive compounds isolated from Salvia miltiorrhiza, particularly salvianolic acid B, strongly upregulated FGF19 secretion by LX-2 cells. We further showed that salvianolic acid B inhibited lipopolysaccharide (LPS)-induced HSC proliferation and activation. LPS treatment may also reduce the mRNA and protein levels of FGF19 and its receptor FGFR4. Salvianolic acid B treatment restored the impaired expressions of FGF19 and FGFR4. Finally, FGFR4 knockdown abolished the antifibrotic effects of salvianolic acid B in the LPS-induced HSC activation model.
ConclusionsSalvianolic acid B prevented LPS-induced HSC proliferation and activation by enhancing antifibrotic FGF19/FGFR4 signaling.
Liver fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) in the liver in response to injuries. Many factors including chronic viral infection, alcohol abuse, and nonalcoholic steatohepatitis (NASH) may cause liver fibrosis. Activated hepatic stellate cells (HSCs) are considered the main source of ECM in fibrotic liver [1,2]. Currently, there are very limited options for the treatment of liver fibrosis. Therefore, exploring the mechanisms underlying HSC activation and identifying novel drugs that can prevent HSC activation are important for improving therapeutic efficiency among patients with chronic liver diseases.
The fibroblast growth factor (FGF) family is a large family of proteins that play crucial roles in development and metabolism. Among 22 known FGFs, the FGF19 subfamily members, including FGF19, FGF21, and FGF23, lack a heparin-binding domain; therefore, they cannot bind to heparin sulfate, which allows them to easily enter circulation as endocrine hormones [3]. FGF19 (FGF15 in mouse) is mainly produced in the intestine in response to bile acid (BA). The activation of farnesoid X receptor (FXR) by liver-derived BA strongly induces the secretion of FGF19, which subsequently travels back to the liver through the portal vein and binds to the FGF receptor 4 (FGFR4)/β-Klotho complex on hepatocytes to reduce BA synthesis [4]. The key enzymes required for BA synthesis such as Cyp7a1 and Cyp8b1 are the downstream targets of FGF19 in the liver [4,5].
Some recent studies showed that serum FGF19 level was correlated with liver damage and cholestasis [6–8]. Moreover, patients with decreased FGF19 level showed more severe hepatic inflammation and fibrosis [9], whereas a negative correlation was found between serum FGF19 level and fibrosis in patients with alcoholic hepatitis [10]. The beneficial effect of FGF19 in liver fibrosis was further confirmed by several reports by examining direct FGF19 treatment in liver fibrosis animal models [11–14]. Importantly, FGF19 analog treatment significantly improved liver fibrosis in patients with NASH and primary sclerosing cholangitis during phase II clinical trials [15,16]. However, whether FGF19 can be directly produced by HSCs in an autocrine manner and whether drugs that regulate its secretion can be used to treat liver fibrosis remain unknown.
The dried root of Salvia miltiorrhiza Bunge (Lamiaceae) has been used to treat various diseases in China for many years as a very commonly used traditional Chinese medicine (TCM) [17]. S. miltiorrhiza has shown prominent antifibrotic effects in liver fibrosis models [18–20]. Several bioactive chemical components such as salvianolic acid B and tanshinone IIA have been isolated from S. miltiorrhiza. Several previous studies explored the antifibrotic potential of these S. miltiorrhiza derived bioactive chemical components including salvianolic acid B. Smad2/3, NF-κB and Angiotensin II signaling has been proposed to be the targets of these compounds [21–24]. However, the antifibrotic effects of these compounds have not been fully elucidated. In this study, we tested the antifibrotic effects of bioactive compounds derived from S. miltiorrhiza in human HSC cell lines and examined the potential role of FGF19 in the beneficial effects of these compounds.
2Materials and methods2.1Cell culture and treatmentLX-2 cells were obtained from the cell bank of the Chinese Academy of Science (Shanghai, China) and were cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1% penicillin–streptomycin solution (Solarbio, Beijing, China). The cells were cultured in a humidified incubator with 5% CO2 at 37 °C. The cells were treated with 100 ng/mL lipopolysaccharide (LPS, from Sigma, St. Louis, MO, USA, Cat. No: L2880), salvianolic acid B, tanshinone IIA, baicalin, puerarin, and saikosaponin (Aladdin, Shanghai, China) at different concentrations for the indicated times shown in figures. The same volume of DMSO was used as the vehicle control.
2.2Cell proliferation assayLX-2 cells (1 × 104 cells/well) were seeded into 96-well plates and treated with salvianolic acid B, tanshinone IIA, baicalin, puerarin, and saikosaponin at 37 °C for 72 h. Cell proliferation was determined using the Cell Counting Kit-8 assay according to the manufacturer’s instructions (SAB, Nanjing, China). Optical density value was measured at a wavelength of 450 nm.
2.3RNA isolation and quantitative reverse transcription polymerase chain reaction (RT-qPCR)Total RNA from LX-2 cells was extracted using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA was reverse transcribed into cDNA, using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The SYBRGreen quantitative polymerase chain reaction (qPCR) master mix (Thermo Fisher Scientific) was used to quantify cDNA on the ABI 7300 real-time PCR system (Applied Biosystem, Foster City, CA) in a 20-µl PCR reaction. qPCR was performed using the following settings: predenaturation at 95 °C for 10 s, denaturation at 95 °C for 10 s, and 40 elongation cycles at 60 °C for 30 s. The expression level of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalize results. The 2−ΔΔCt method was performed to calculate relative expression levels. The PCR primer sequences used are listed below:
FGFR4
Primer F 5′-CCCTCGAATAGGCACAGTTAC-3′
Primer R 5′-GCCTCCAATGCGGTTCTC-3′
Transforming growth factor-β (TGF-β)
Primer F 5′-CGTGGAGGGGAAATTGAGG-3′
Primer R 5′-GCCATGAGAAGCAGGAAAGG-3′
α-Smooth muscle actin (α-SMA)
Primer F 5′-GACGAAGCACAGAGCAAAAG-3′
Primer R 5′-ACAGCACCGCCTGGATAG-3′
Collagen1a1 (COL1A1)
Primer F 5′-GAGGCATGTCTGGTTCGG-3′
Primer R 5′-TGGTAGGTGATGTTCTGGGAG-3′
GAPDH
Primer F 5′-AATCCCATCACCATCTTC-3′
Primer R 5′-AGGCTGTTGTCATACTTC-3′
2.4Hydroxyproline content measurementHydroxyproline content was measured using a hydroxyproline assay kit (Jiancheng, Nanjing, China) according to the manufacturer’s instructions.
2.5Enzyme-linked immunosorbent assay (ELISA)FGF19 level in culture medium was measured using a human FGF19 ELISA kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions.
2.6Western blottingCells were lysed with RIPA buffer (Beyotime, Shanghai, China) on ice for 30 min. Protein level was measured using a BCA protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). A total of 20 µg protein was applied to each lane and separated using 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then, the separated proteins were transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% milk at room temperature for 1 h. The membranes were incubated with primary antibodies diluted in 5% milk at 4 °C overnight. The primary antibodies used in this study included the following: α-SMA (Cell Signaling Technology, Danvers, MA, USA), COL1A1 (Cell Signaling Technology), TGF-β (Abcam), FGFR4 (Abcam), FGF19 (Abcam), and GAPDH (Proteintech, Rosemont, IL USA). The membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Beyotime) at room temperature for 1 h. Enhanced chemiluminescence chromogenic substrate (Thermo Fisher Scientific) was used to visualize protein bands.
2.7RNA interferenceCells were transfected with 50 nM small interfering RNA (siRNA) targeting FGFR4 (siFGFR4) or negative control siRNA (siNC) using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. The siRNA sequences are listed below:
siFGFR4-1: 5′-GCAGAAUCUCACCUUGAUUUU-3′
siFGFR4-2: 5′-CCAGGUAUACGGACAUCAUUU-3′
siFGFR4-3: 5′-GCGUCCACCACAUUGACUAUU-3′
siNC: 5′-CAGUACUUUUGUGUAGUACAA-3′
2.8Plasmid constructionTo overexpress FGFR4, the full-length Homo sapiens FGFR4 coding sequence (CDS) was synthesized by GENEWIZ according to the guidelines of the National Center for Biotechnology Information database (NM_002011.5). FGFR4 CDS was cloned into the pcDNA3.1 vector (Addgene). LX-2 cells were transfected with the construct using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions.
2.9ImmunofluorescenceLX-2 cells cultured on the coverslips were fixed with 1% paraformaldehyde solution containing 0.05% of Triton X-100 for 5 min at room temperature. Fixed cells were stained with phalloidin-TRITC solution at 100 μg/mL for 2 h at room temperature. Nuclei were stained with 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI, Beyotime Biotech.). The images were obtained using a BX51 OLYMPUS microscope.
2.10Statistical analysisData are presented as mean ± standard deviation. Statistical analyses were performed using Graphpad Prism (San Diego, CA) for all experiments. Data from multiple groups were compared with one-way ANOVA. Differences between two groups were analyzed using Student’s t-test. P-value of <0.05 was considered to indicate a statistically significant difference.
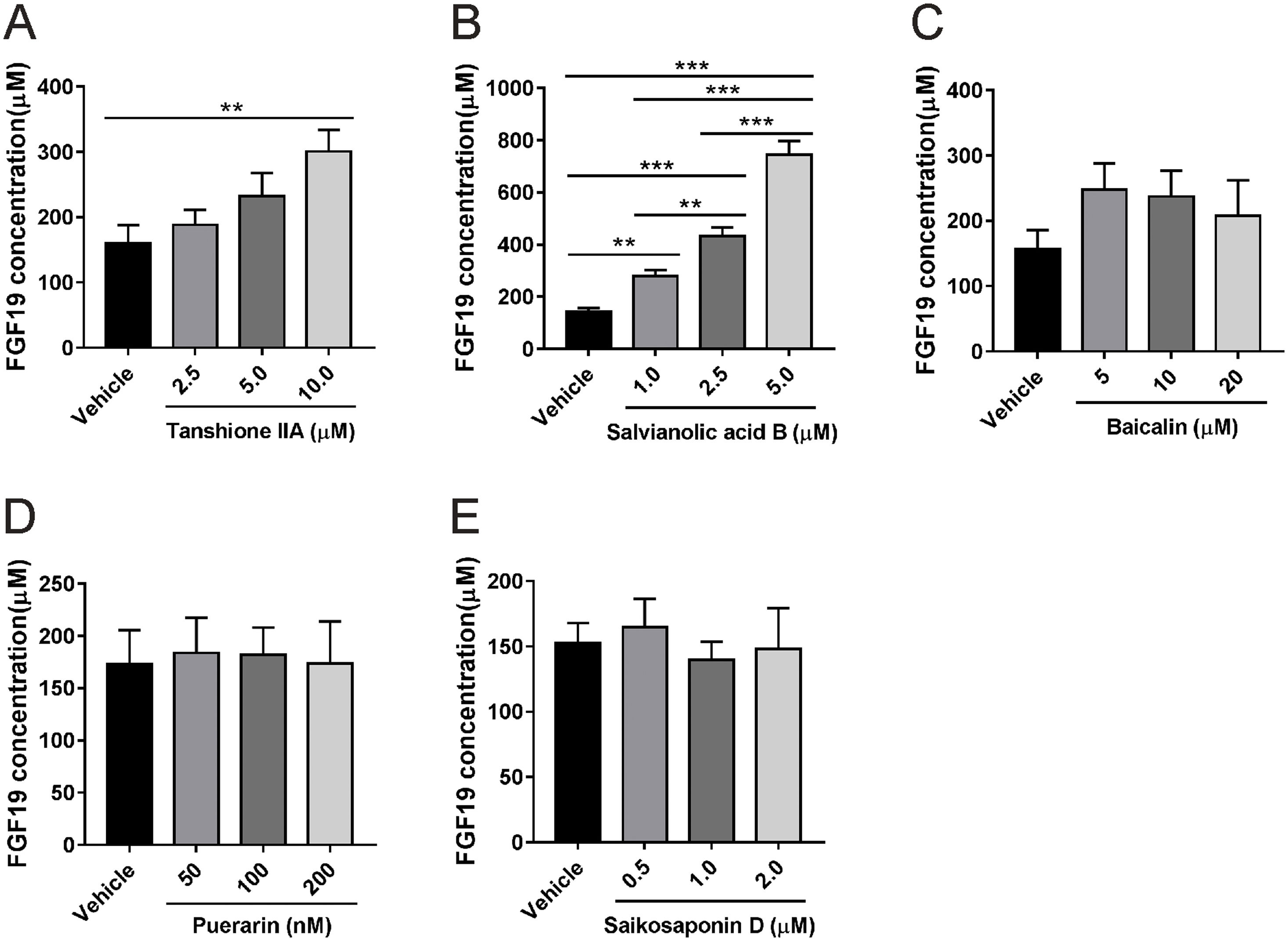
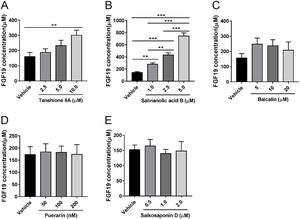
3Results3.1Salvianolic acid B promotes FGF19 secretion by LX-2 cellsWe first tested whether HSCs can secrete FGF19 using the human HSC cell line LX-2. Cells were cultured for 24 h, and approximately 170 µM FGF19 was detected in the culture medium (Fig. 1A), which indicated that HSCs can secrete FGF19 in an autocrine manner. LX-2 cells were treated with two bioactive compounds derived from S. miltiorrhiza, salvianolic acid B and tanshinone IIA. Both compounds increased FGF19 secretion in a dose-dependent manner (Fig. 1A and B). In contrast, several other antifibrotic natural products isolated from other TCM such as baicalin [25], puerarin [26], and saikosaponin D [27] had no effects on FGF19 secretion by LX-2 cells (Fig. 1C–E). Interestingly, salvianolic acid B showed more potent effects on FGF19 secretion by LX-2 cells than the other tested compounds. Therefore, salvianolic acid B was selected for the following experiments. To evaluated the potential cytotoxicity effects of salvianolic acid B on LX-2 cells, we checked cell viability by using MTT assay. As shown in Fig. S1A, 1.0–5.0 µM did not show any cytotoxicity effects. In addition, LX-2 cells showed normal morphology with 1.0–5.0 µM salvianolic acid B treatment (Fig. S1B).
Effects of several natural products on fibroblast growth factor (FGF) 19 secretion by LX-2 cells. LX-2 cells were treated with different concentrations of (A) tanshione IIA, (B) salvianolic acid B, (C) baicalin, (D) puerarin, and (E) saikosaponin D as indicated. Culture media were collected after 24 h, and FGF19 level was measured by enzyme-linked immunosorbent assay. Values represent mean ± standard deviation (n = 5). **P < 0.01, ***P < 0.001.
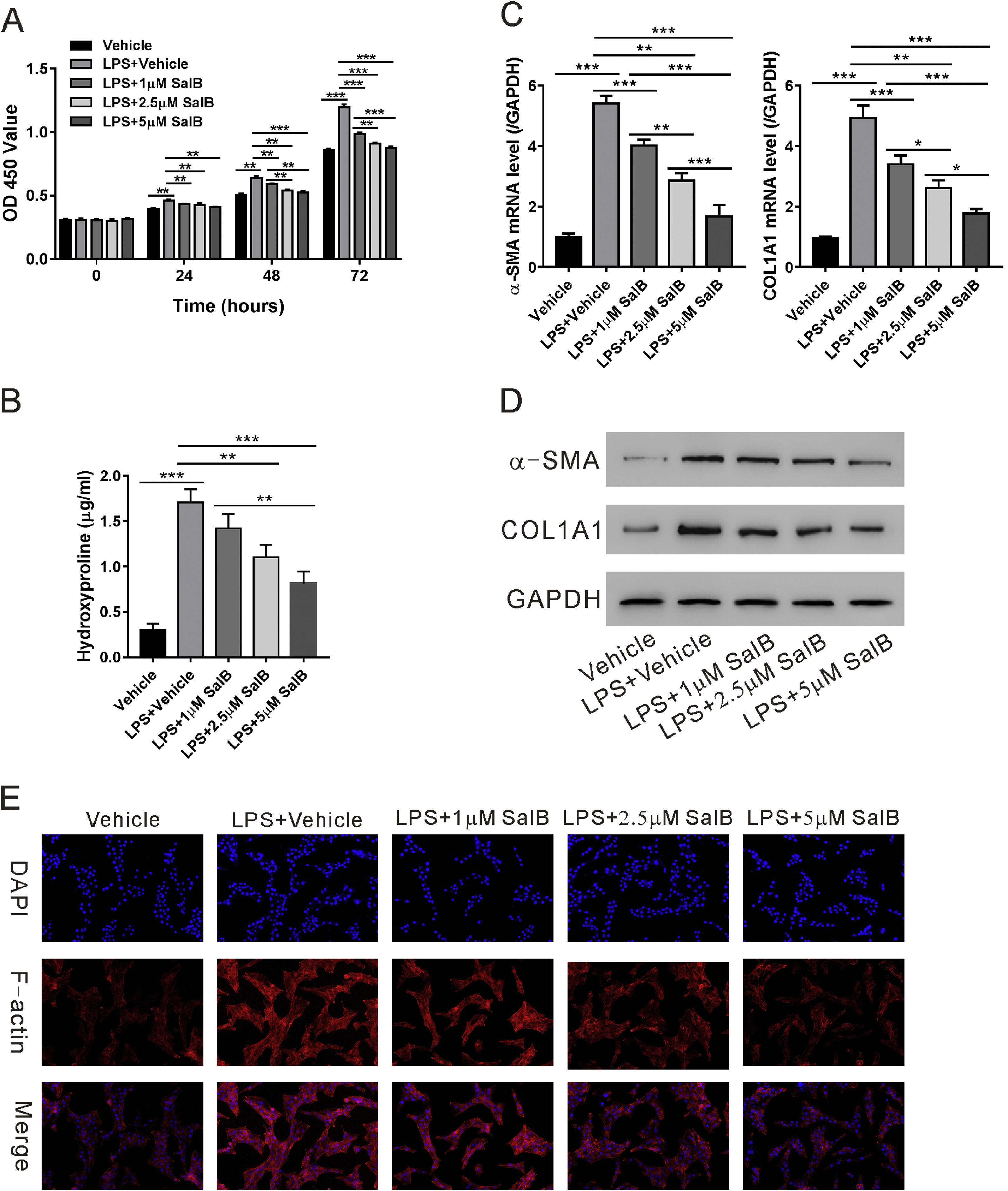
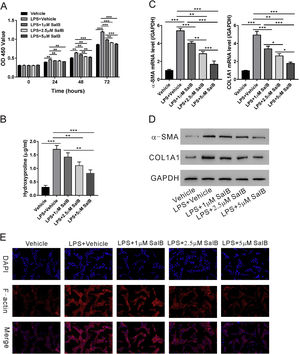
We evaluated the effects of salvianolic acid B on LPS-induced HSC proliferation and activation. As shown in Fig. 2A, LX-2 proliferation significantly increased in the presence of LPS and the cotreatment with LPS and salvianolic acid B significantly blocked LPS-induced proliferation of LX-2 cells in a dose-dependent manner. LPS also considerably increased the hydroxyproline content of LX-2 cells, a marker of HSC activation. Treatment with salvianolic acid B substantially reduced LPS-induced increase in hydroxyproline content in a dose-dependent manner (Fig. 2B). Consistently, other markers for HSC activation, such as α-SMA and COL1A1 were reduced in both mRNA and protein levels with salvianolic acid B treatment (Fig.2C-D). Previous study indicated that most of the actin F filaments were depolymerized in quiescent LX-2 cells while in activated LX2 cells, actin F filaments were fully polymerized [28]. So, we checked actin F filaments by immunofluorescence. As shown in Fig. 2E, LPS significantly increased polymerization of actin F filaments in LX-2 cells, while much less polymerization of actin F filaments were observed in the presence of salvianolic acid B. These data confirmed that salvianolic acid B can effectively block the activation and proliferation of LX-2 cells.
Salvianolic acid B inhibits LPS-induced activation and proliferation of LX-2 cells. LX-2 cells were treated with different concentrations of salvianolic acid B, as indicated, with or without 100 ng/mL LPS. (A) OD450 values were measured 0, 24, 48, and 72 h after treatment using Cell Counting Kit-8 reagents. (B) Hydroxyproline level was measured using a hydroxyproline assay kit. mRNA (C) and protein (D) levels of α-SMA and COL1A1. (E) F-actin immunofluorescent staining. Values represent mean ± standard deviation (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
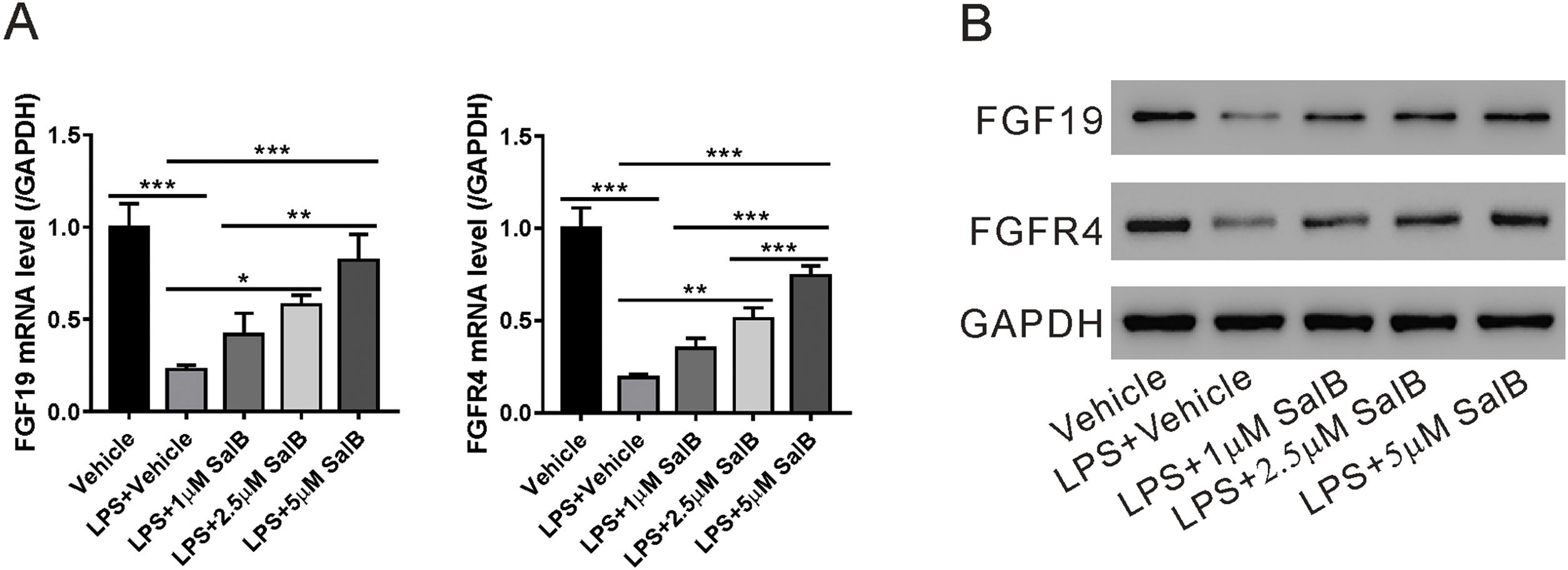
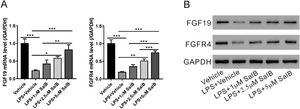
As shown in Fig. 1A, LX-2 cells can secrete FGF19; therefore, we explored whether LPS-induced HSC activation would affect FGF19 secretion. As shown in Fig. 3A and B, LPS treatment considerably reduced both FGF19 mRNA and protein levels. Moreover, the mRNA and protein levels of FGFR4, the FGF19 receptor, decreased in the presence of LPS. These data suggested that FGF19/FGFR4 signaling was greatly impaired during LX-2 activation. When treated with different doses of salvianolic acid B, LPS-induced decrease in mRNA and protein levels of both FGF19 and FGFR4 were partially or almost fully restored (Fig. 3A and B).
Salvianolic acid B restores LPS induced FGF19 and FGFR4 downregulation. LX-2 cells were treated with different concentrations of salvianolic acid B, as indicated, with or without 100 ng/mL LPS. (A) Fibroblast growth factor (FGF19) and FGF receptor 4 (FGFR4) mRNA levels were measured by quantitative reverse transcription polymerase chain reaction. (B) FGF19, FGFR4, and glyceraldehyde 3-phosphate dehydrogenase protein levels were measured by western blotting. Values represent mean ± standard deviation (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
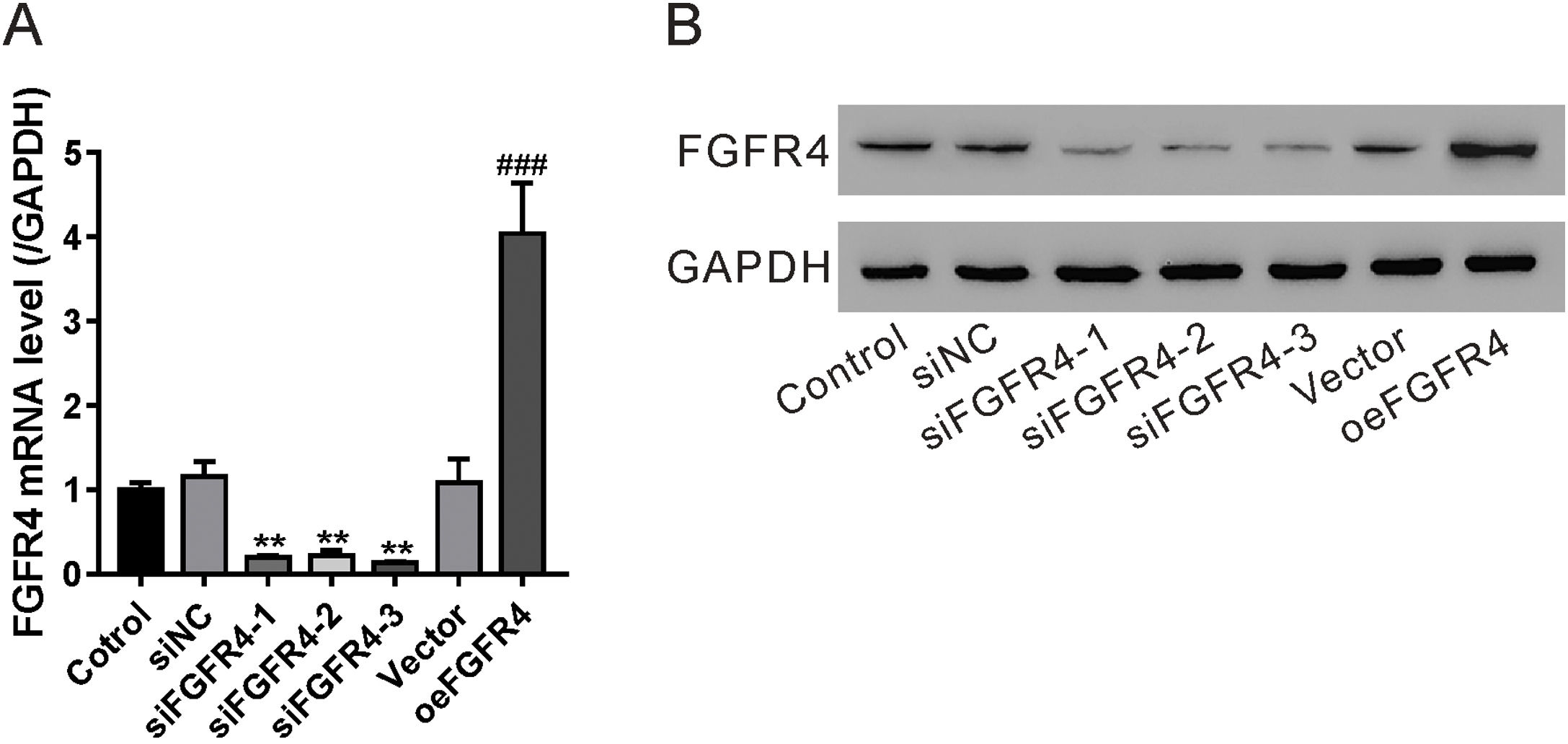
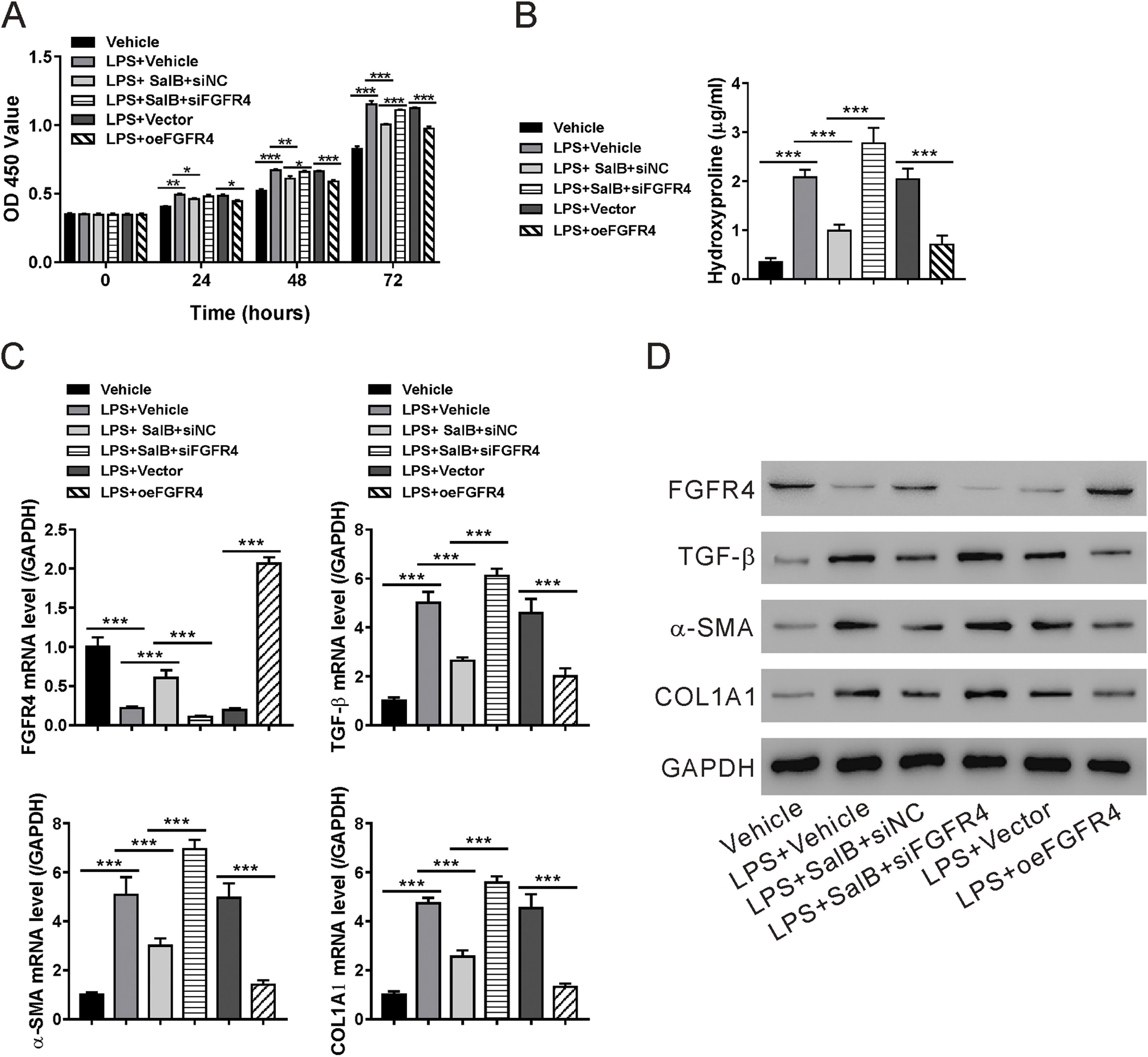
To determine whether the antifibrotic effects of salvianolic acid B were mediated by regulating FGF19/FGFR4 signaling, we used siRNA to knock down FGFR4 expression in LX-2 cells. In addition, FGF4R was overexpressed through the transfection of the pcDNA-FGF4R plasmid. Both FGFR4 knockdown and overexpression were verified by examining its mRNA and protein levels in LX-2 cells (Fig. 4A and B). We tested the impact of FGFR4 knockdown on LPS-induced cell proliferation. As expected, salvianolic acid B inhibited LPS-induced LX-2 cell proliferation; however, this inhibitory effect was abolished by FGFR4 siRNA treatment (Fig. 5A). Moreover, FGFR4 overexpression considerably blocked LPS-induced cell proliferation (Fig. 5A). Similar to the cell proliferation data, FGFR4 siRNA treatment abolished the inhibitory effects of salvianolic acid B on HSC activation, as assessed by measuring hydroxyproline level as well as mRNA and protein levels of several HSC activation markers such as TGF-β, α-SMA, and COL1A1 (Fig. 5B–D). In contrast, FGFR4 overexpression substantially blocked LPS-induced HSC activation (Fig. 5B–D). These data strongly supported the critical role of FGF19/FGFR4 signaling in LPS-induced HSC proliferation and activation. We also demonstrated that the antifibrotic effects of salvianolic acid B are mediated by the regulation of FGF19/FGFR4 signaling.
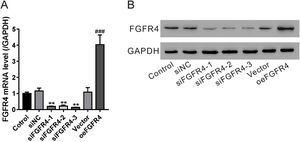
Validation of fibroblast growth factor FGF receptor 4 (FGFR4) knockdown and overexpression. LX-2 cells were transfected with FGFR4 siRNA (siFGFR4-1, 2, and 3), negative control siRNA (siNC), empty vector (Vector), and FGFR4 overexpression vector (oeFGFR4). After 48 h, (A) mRNA and (B) protein levels of FGFR4 were measured by quantitative reverse transcription polymerase chain reaction and western blotting, respectively. Values represent mean ± standard deviation (n = 5). **P < 0.01 vs siNC group, ##P < 0.01 vs Vector group.
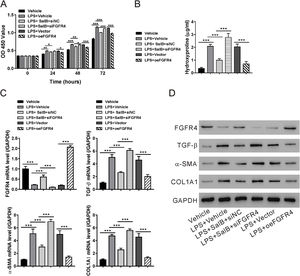
Fibroblast growth factor (FGF19)/FGF receptor 4 (FGFR4) signaling is required for the antifibrotic effects of salvianolic acid B. LX-2 cells were transfected with FGFR4 small interfering RNA (siRNA) (siFGFR4-1, 2, and 3), negative control siRNA (siNC), empty vector (Vector), and FGFR4 overexpression vector (oeFGFR4). The cells were treated with or without LPS and salvianolic acid B. (A) OD450 values were measured 0, 24, 48, and 72 h after treatment with Cell Counting Kit-8 reagents. (B) Hydroxyproline level was measured using a hydroxyproline assay kit. (C) FGFR4, FGF receptor 4 (TGF-β), α-smooth muscle actin (α-SMA), and Collagen1a1 (COL1A1) mRNA levels were measured by quantitative reverse transcription polymerase chain reaction. (D) FGFR4, TGF-β, α-SMA, COL1A1, and GAPDH protein levels were measured by western blotting. Values represent mean ± standard deviation (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
In this study, we found that FGF19 could be produced by HSCs and that FGFR4 was expressed in HSCs. LPS treatment impaired FGF19/FGFR4 signaling by downregulating both FGF19 and FGFR4 expressions. Salvianolic acid B, a bioactive compound isolated from the TCM S. miltiorrhiza, showed a strong ability to enhance FGF19 secretion and to restore FGF19/FGFR4 expression impaired by LPS treatment. Finally, we confirmed that the antifibrotic effects of salvianolic acid B were mediated by restoring FGF19/FGFR4 signaling during LPS-induced HSC proliferation and activation.
As a unique “hormone-like” FGF, FGF19 has been reported to exert strong regulatory activity during energy metabolism and BA homeostasis [29]. Enterocytes of the terminal ileum are considered a major source of FGF19 [30]. In response to BA, the nuclear receptor FXR is activated and translocated to the FXR-responsive element in the intron area of FGF19 to initiate FGF19 transcription [31]. Some other mechanisms are also involved in the regulation of FGF19 expression including sterol regulatory element-binding protein 2, vitamin D receptor, and retinoid X receptor [32,33]. We found that FGF19 can also be secreted by HSCs (Fig. 1A–E). Interestingly, LPS treatment significantly blocked FGF19 secretion by HSCs (Fig. 3A–B). A previous report showed that LPS might reduce FXR expression [34], which could explain why LPS treatment was associated with decreased FGF19 secretion.
FGF19 binds to the FGFR4/β-Klotho complex, with its major target being the liver [35]. A recent report suggested that HSCs were also targeted by FGF19 [14]. Our study confirmed that FGFR4 is expressed on HSCs (Fig. 3A and B). We also showed that enhanced FGF19/FGFR4 signaling, mediated by FGFR4 overexpression, blocked HSC proliferation and activation (Fig. 4A–D). These data not only support the antifibrotic role of FGF19/FGFR4 signaling proposed in previous reports [12,13,16] but also provide evidence for the direct role of FGF19 in HSC proliferation and activation, which may prevent the development of liver fibrosis.
TCM drugs have been used to treat liver fibrosis for many years, and the efficacy of several TCM drugs has been tested for the treatment of chronic liver diseases associated with fibrosis or cirrhosis [36]. Many TCM drugs for fibrotic liver disease contain S. miltiorrhiza as a major ingredient [36]. The antifibrotic effects of S. miltiorrhiza have been well-documented in several mouse and rat liver fibrosis models induced by different factors including chemicals, parasites, and iron overload [18–20,37–41]. Several mechanisms have been proposed to explain the antifibrotic effects of S. miltiorrhiza and its extracts. Treatment with S. miltiorrhiza possibly decreases the production of TGF-β, a potent activator of HSCs [20,40]. S. miltiorrhiza can also alleviate oxidative stress in liver fibrosis models [39]. Some other studies have attributed the beneficial effects of S. miltiorrhiza against liver fibrosis to the regulation of autophagy and nature killer cells [19,41]. Here, we identified a novel mechanism that can explain the antifibrotic effects of S. miltiorrhiza. Both salvianolic acid B and tanshinone IIA, two of the most important bioactive compounds derived from S. miltiorrhiza, showed strong activity in promoting FGF19 secretion (Fig. 1A and B). We further tested the effects of salvianolic acid B, which showed a better ability to promote FGF19 secretion than tanshinone IIA, using an LPS-induced HSC proliferation and activation model. Our result confirmed that the antifibrotic effects were mediated by FGF19/FGFR4 signaling using FGFR4 siRNA (Fig. 4A–D).
Chronic inflammation in the liver was considered as a major driving force for liver fibrosis. Many inflammatory cells, including Kupffer cells, neutrophils and T cells as wells as cytokines derived from these cells, such as TGF-β, IL-1 and TNF-α were critical in supporting HSC activation and ECM production [42]. Interestingly, salvianolic acid B showed prominent anti-inflammatory effects in several LPS induced inflammatory organ damage models [43–45]. Although whether the anti-inflammatory effects of salvianolic acid B contribute to the regulation of FGF19/FGFR4 signaling in HSCs in vivo is not clear, the inhibition of inflammation will be helpful to prevent fibrogenesis and improve the resolution of liver fibrosis in chronic liver diseases.
5ConclusionSalvianolic acid B could promote FGF19 secretion by the HSC cell line LX-2. The increased FGF19 expression and activation of FGF19/FGFR4 signaling were necessary for salvianolic acid B-mediated decrease in LPS-induced LX-2 cell proliferation and activation. Our study detailed a potential mechanism for the antifibrotic effects of S. miltiorrhiza.AbbreviationsECM extracellular matrix nonalcoholic steatohepatitis hepatic stellate cells Fibroblast growth factors bile acid farnesoid X receptor primary sclerosing cholangitis Traditional Chinese Medicine lipopolysaccharide Cell Counting Kit-8 reverse transcription-quantitative polymerase chain reaction. coding sequence sterol regulatory element-binding protein 2 vitamin D receptor retinoid X receptor nature killer
This study was supported by grants from National Natural Science Foundation of China [81774061], the key subjects construction in the health system of Pudong New Area [PWZxk2017-02], Traditional Chinese Medicine Heritage and Science and Technology Innovation Project of Shanghai [ZYKC2019035] and Special Project of Integrated Traditional Chinese and Western Medicine of Shanghai [ZHYY-ZXYJHZX-201910].
Conflict of interestThe authors declare no conflicts of interest.