The oncogene diencephalon/mesencephalon homeobox 1 (DMBX1) is widely overexpressed in a variety of human cancers. The present study aimed to analyze the expression and clinical importance of DMBX1 in nonneoplastic tissues and tumor tissues from patients with hepatocellular carcinoma (HCC).
Materials and methodsDMBX1 expression in HCC and adjacent nontumor tissues was analyzed using immunohistochemical staining. Chi-square tests were applied to compare DMBX1 expression between the tumors and the adjacent normal tissues. We explored the correlation of DMBX1 expression with clinicopathological factors and its effect on the prognosis of HCC. Finally, we investigated the role of DMBX1 in HCC via knockdown experiments, which analyzed changes in cell invasion, cell proliferation and epithelial-mesenchymal transition (EMT) biomarkers (E-cadherin, N-cadherin, vimentin). The mRNAs that were coexpressed with DMBX1 in HCC, based on the TCGA cohort (n = 366), were obtained from the cBioPortal database.
ResultsThe average score for DMBX1 expression was significantly different (P < 0.001) between HCC and paired adjacent nontumor tissues, and DMBX1 expression correlated with hepatitis B virus (HBV) infection, tumor size, metastasis, and tumor node metastasis (TNM) stage (P < 0.05). A multivariate Cox regression analysis identified significant correlations of DMBX1 expression with tumor metastasis, TNM stage, and tumor capsule. Moreover, Kaplan-Meier survival analysis revealed an association between DMBX1 overexpression and shorter overall survival of patients with HCC (P < 0.05). In HCC cell lines, silencing DMBX1 markedly inhibited migration, proliferation and EMT markers. The mRNAs that were negatively (R ≤ −0.25, n = 1094) or positively (R ≥ 0.25, n = 2906) coexpressed with DMBX1 mRNA were selected for further Gene Ontology enrichment analysis, and the results revealed that the predicted functions of DMBX1 in HCC support the in vitro experimental results.
ConclusionsOur data provide evidence that DMBX1 overexpression is associated with HCC metastasis and poor prognosis, suggesting that DMBX1 represents a therapeutic target in HCC.
Hepatocellular carcinoma (HCC) ranks fifth in the global incidence of malignant tumors, and the number of deaths resulting from HCC accounts for 8.2% of all cancer-related deaths [1]. HCC is not easily diagnosed or classified at an early stage due to the lack of characteristic symptoms and the paucity of clinically specific markers for serodiagnosis [2]. Thus, the disease is usually diagnosed at an advanced stage, and late-stage HCC is extremely difficult to treat [3]. HCC results in very high health care costs and has a poor prognosis [4]. The mechanism associated with the occurrence and development of HCC has not yet been clearly elucidated.
Diencephalon/mesencephalon homeobox 1 (DMBX1) is located on chromosome 1p33 and encodes a member of the bicoid subfamily of homeodomain-containing transcription factors [5]. Transcription factors regulate the mRNA transcription rate, and many of them function as oncogenes or tumor suppressor genes in cancer [6–8]. DMBX1 is a member of the homeobox family and was first identified in 2002 [9]. Subsequent studies have identified important roles for DMBX1 in growth [10], the central nervous system [11–13], postnatal survival, limb development [13], and the activity of agouti-related protein [14]. DMBX1 was recently shown to modulate the cell cycle and differentiation of stem cells during retinal and midbrain development [15–17]. However, the role of DMBX1 modifications in HCC has not been reported in the literature.
In the present study, DMBX1 expression was analyzed in 90 paired HCC and adjacent nontumor tissues. We concluded that DMBX1 was upregulated in HCC tissues. DMBX1 expression was significantly associated with distant metastasis in patients with HCC and resulted in a poor prognosis. In cell and database experiments, we also verified that DMBX1 could promote the aggressiveness of hepatocellular carcinoma. Based on our results, DMBX1 potentially represents a clinical biomarker or therapeutic target in HCC.
2Materials and methods2.1Tissue microarrayHuman HCC tissues and matched noncancerous tissues for a tissue microarray were purchased from Superchip (Shanghai, China). The Human Ethics Committee approved the experiments, and consent was obtained from all subjects for the publication of their data. In the current study, all 90 tissues were obtained from individuals with HCC. The expression of DMBX1 in the tissue microarray slides was detected using immunohistochemistry (IHC).
2.2Cell cultureThe HCC cell lines MHCC97H, HepG2, SMMC7721, Huh7, and Hep3B and the nontumorigenic hepatocyte cell line LO2 were purchased from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). HCC cells were cultured with RPMI 1640 (Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Calabasas, CA) and 1% penicillin/streptomycin (Euroclone, Pero, Italy). All cells were placed in an incubator at 37 °C with an atmosphere containing 5% CO2 and sufficient humidity.
2.3siRNA transfectionCells that had adhered to the wells of the plate and reached 30–40% confluence were transfected with DMBX1 or negative control (NC) siRNAs (RiboBio Technologies, Guangzhou, China) using Lipofectamine 2000™ (Thermo Fisher Scientific, Carlsbad, CA, USA) according to the manufacturer’s instructions. The conditions used for siRNA transfection are listed below. Twenty picomoles of RNA was dissolved in EP tube A containing 50 µL of Opti-MEM (Invitrogen, Groningen, Germany), and 2 µL of Lipofectamine 2000 was mixed with 50 µL of Opti-MEM in EP tube B. After incubation for 5 min, the contents of tubes A and B were mixed, incubated for 20 min at room temperature, and added to a 24-well plate. Four hundred microliters of Opti-MEM was added to the 24-well plate, and the total amount per well was 500 µL. After incubation for 8 h, the medium was replaced with complete medium and incubated for another 48 h.
2.4Quantitative real-time PCRThe amplification system was composed of 1 µL of the cDNA template, 0.4 µL each of upstream and downstream PCR primers (5 µM), 5 µL of the SYBR GREEN polymerase mixture (Thermo Fisher Scientific, Paisley, UK), and 3.2 µL of DEPC-treated water (Thermo Fisher Scientific, Waltham, MA) for a total volume of 10 µL. The amplification conditions were an initial denaturation at 95 °C for 30 s and 40 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. Fluorescence signals were collected at the end of each cycle extension, and a DNA amplification curve was created. After 40 cycles, a melting curve analysis was performed at 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s, and the fluorescence signals were recorded during the heating process from 60 °C to 95 °C. The following primer sequences were used: DMBX1, sense 5′-CTTGGAGGCCCGTTATGGTTC-3′ and antisense 5′-CTGGGTAGTGAGTCTTCTGGA-3′; and GAPDH, forward 5′-GGGAGCCAAAAGGGTCAT-3′ and reverse 5′’-GAGTCCTTCCACGATACCAA-3′. The 2−ΔΔCT method was used to analyze the RT-qPCR data, and the values are presented as log2 fold changes.
2.5Western blottingRIPA lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China) was used to extract total cellular proteins, and the Bradford method was used to determine the protein concentration. Twenty micrograms of protein samples was mixed with 2 × SDS loading buffer at a 1:1 ratio, heated at 95 °C for 10 min, incubated on ice for 2 min, and separated on 12% SDS-PAGE gels. After electrophoresis, the proteins on the gel were transferred to a PVDF membrane. After the PVDF membrane was incubated with the blocking solution for 1 h, it was then incubated with primary antibodies (Supplementary Table 1) overnight at 4 °C. The membranes were then washed and incubated with HRP-labeled secondary antibodies (Supplementary Table 1) and detected using an ECL chemiluminescence detection kit (Beyotime, Shanghai, China).
2.6Wound-healing assayA wound-healing assay was used to assess cell migration. Cells were seeded in a 6-well plate and grown to 100% confluence; the monolayer was then scratched with a pipette tip (at time 0 h). The medium was replaced to remove the cell debris, and the cells were cultured in the presence of 10 μg/mL mitomycin C to block cell proliferation. Pictures of the wound areas were taken at 48 h after scratching.
2.7Cell counting kit 8 (CCK-8) assayCCK-8 assay was used to measure cell proliferation. Transfected cells were cultured in a 96-well plate with complete medium. The CCK-8 cell proliferation assay kit (Dojindo, Kumamoto, Japan) was used 24, 48, and 72 h after seeding. A microplate reader (GloMax-multi microplate reader, Promega, USA) was used to determine the proliferation in each well by measuring the absorbance at 450 nm.
2.8Colony formation assayOne thousand stably transformed cells were seeded into 6-well plates (Corning, NY, USA). After 15 days of incubation, the cell colonies were fixed with 5% paraformaldehyde (Sigma Biochemicals, Poole, UK) and stained with a 3% crystal violet solution (Beyotime, Shanghai, China). Cell colonies were counted under an Olympus microscope (Olympus, Tokyo, Japan) and analyzed using ImageJ software.
2.9Cell invasion assaysForty thousand HCC cells were suspended in 100 μL of medium (without FBS) and inoculated into the upper chamber (BD, Bedford, MA), which was inserted in a 24-well plate, and 500 μL of complete medium was added to the lower chamber of the inserts. The chamber was coated with Matrigel (Corning Life Sciences, Tewksbury, MA) and diluted medium without FBS at a ratio of 1:8. After 48 h of incubation, the cells that penetrated the filter were fixed with 3% paraformaldehyde, stained with 0.5% crystal violet, and photographed under a microscope (Nikon, Tokyo, Japan).
2.10Immunohistochemical (IHC) stainingThe immunohistochemical staining and scoring methods used were performed as previously reported. The expression of DMBX1 protein in cells was assessed semiquantitatively by multiplying the staining intensity and the number of positives. The percentage of cells was scored under an upright microscope (Olympus, Milan, Italy), and the dyeing intensity and brightness were scored as follows: the criteria for staining intensity were 0 points for no staining, 1 point for light yellow staining, 2 points for brownish-yellow staining, and 3 points for yellowish-brown staining. The criteria for the percentage of positive cells among 100 cells per field were 0 for <5% positive cells, 1 for 5–25% positive cells, 2 for 25–75% positive cells, and 3 for over 75% positive cells. The staining intensity score was multiplied by the percentage of positive cells to obtain the final score of each stained section; a score of 0–1 was designated as negative (-) staining, 2–4 was designated as low expression, and 6–9 was regarded as high expression.
2.11Statistical analysisStatistical analyses were performed with SPSS 13.0 software. The quantitative data from experiments with biological replicates are expressed as the mean ± standard error of the mean (SEM). Student's t-test, Mann-Whitney U test and analysis of variance (ANOVA) followed by Dunnett's T3 test analyses were used to analyze the differences in counting data between groups. Pearson's correlation efficiency analysis was also used. Survival analysis was performed with Kaplan-Meier and log-rank tests. The difference was considered statistically significant at P < 0.05.
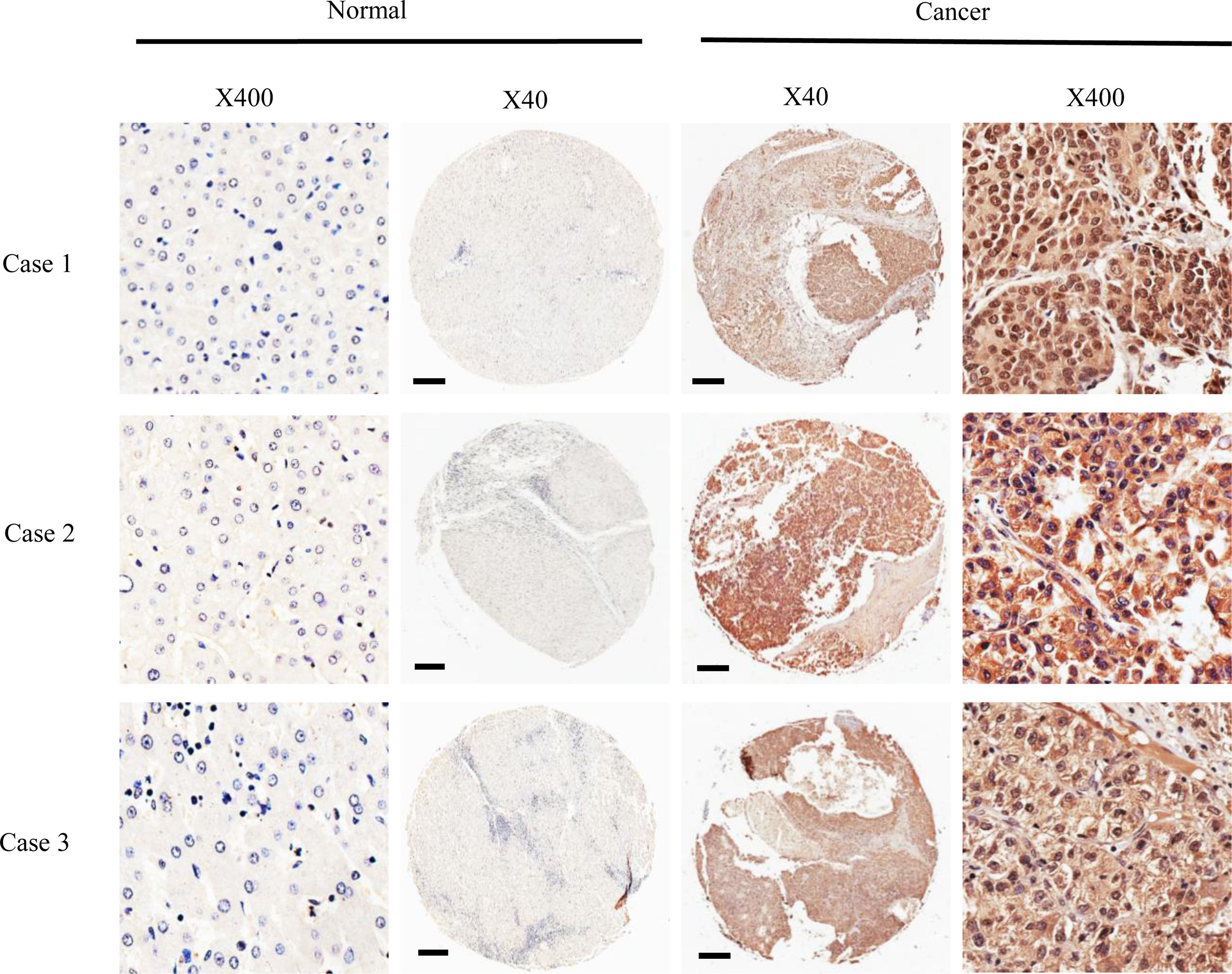
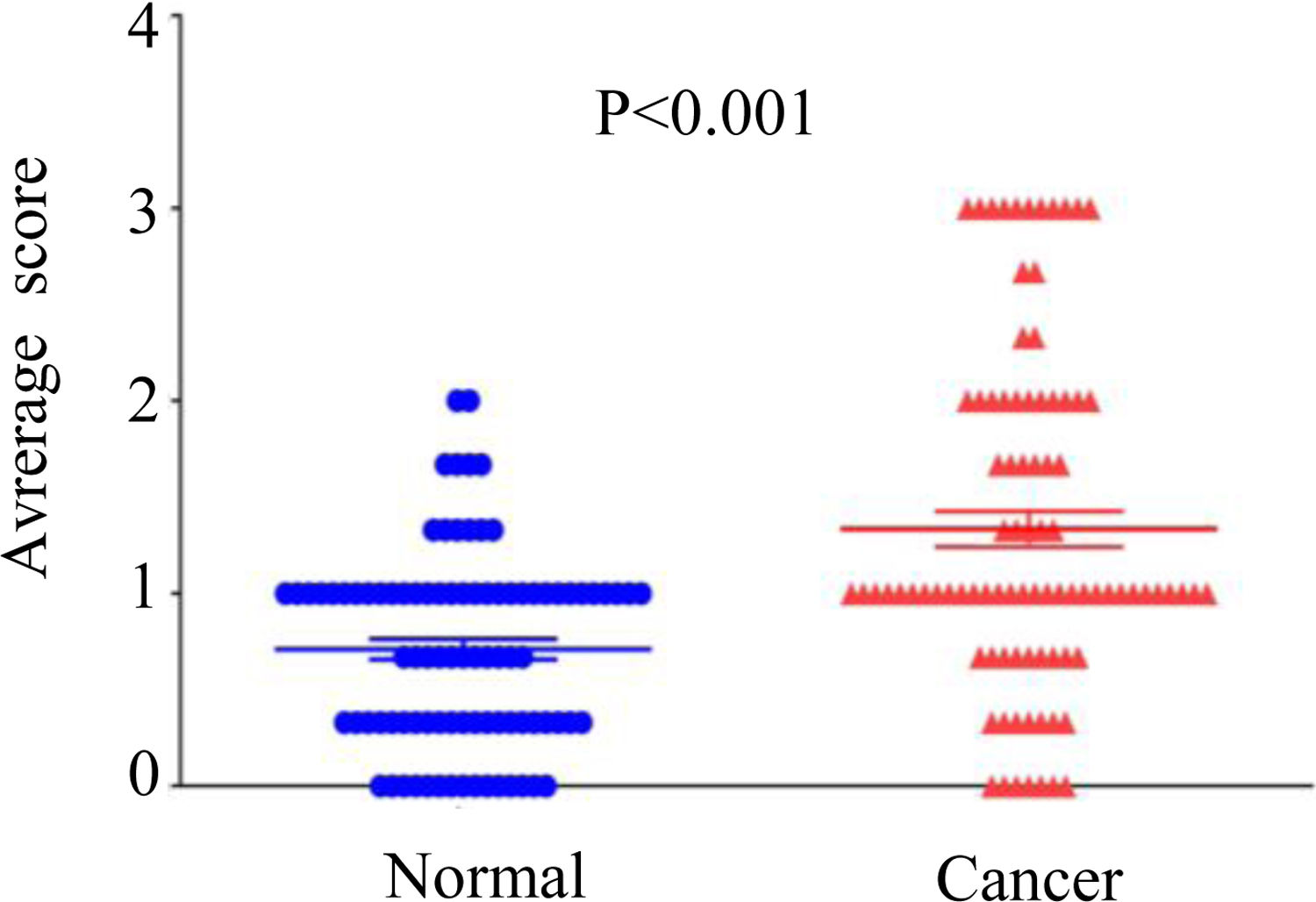
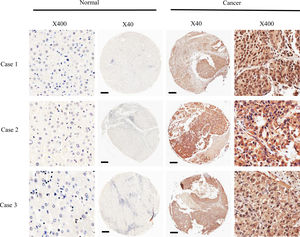
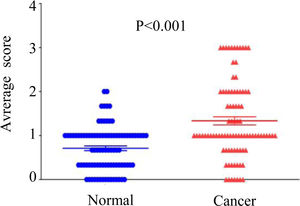
3Results3.1DMBX1 expression differs between tumor and adjacent nontumor tissuesNinety paired HCC and adjacent nontumor samples were stained using IHC to assess the expression of the DMBX1 protein. As shown in Fig. 1, DMBX1 was mainly localized in the cytoplasm and nucleus. Semiquantitative scoring of IHC staining revealed significantly higher DMBX1 expression in the HCC tissues than in the adjacent noncancerous tissues (Fig. 2).
The 90 patients with HCC were divided into two groups according to the DMBX1 protein expression level. We collected the clinicopathological characteristics of the patients with HCC, such as age, sex, hepatitis B virus (HBV) infection status, serum alpha-fetoprotein (AFP) level, tumor size, tumor metastasis, portal vein tumor thrombus (PVTT), tumor number, liver cirrhosis, tumor capsule and tumor node metastasis (TNM) stage. As summarized in the characteristics presented in Table 1, DMBX1 expression was positively correlated with HBV infection, tumor size, metastasis and TNM stage (P < 0.05). DMBX1 expression was not obviously correlated with the other clinicopathological characteristics of patients with HCC, namely, age, sex, AFP, PVTT, liver cirrhosis, tumor number, or tumor capsule (P > 0.05) (Table 1). The multivariate logistic regression analysis identified associations between DMBX1 expression and tumor metastasis, TNM stage, and tumor capsule (P < 0.05) (Table 2).
DMBX1 protein expression and the clinicopathological parameters of HCC.
| Characteristics | low level of DMBX1expression number | high level of DMBX1expression numbe | χ2 | p-Value |
|---|---|---|---|---|
| (n = 32) | (n = 58) | |||
| Age(years) | 0.57 | 0.45 | ||
| <60 | 25 | 49 | ||
| ≥60 | 7 | 9 | ||
| Gender | 0.000 | 0.606 | ||
| Male | 27 | 49 | ||
| Femal | 5 | 9 | ||
| HBV infection | 4.005 | 0.045* | ||
| + | 18 | 20 | ||
| − | 14 | 38 | ||
| AFP | 0.254 | 0.615 | ||
| <400 | 17 | 34 | ||
| ≥400 | 15 | 24 | ||
| Tumor size# | 4.314 | 0.038* | ||
| <5cm | 20 | 23 | ||
| ≥5cm | 12 | 35 | ||
| Metastasis | 9.336 | 0.002* | ||
| No | 24 | 24 | ||
| Yes | 8 | 34 | ||
| PVTT | 3.258 | 0.058 | ||
| No | 28 | 41 | ||
| Yes | 4 | 17 | ||
| Tumor number | 2.399 | 0.121 | ||
| Solitary(1) | 29 | 45 | ||
| Multiple(≥2) | 3 | 13 | ||
| Liver cirrhosis | 0.364 | 0.546 | ||
| No | 9 | 13 | ||
| Yes | 23 | 45 | ||
| TNM Stage | 5.556 | 0.018* | ||
| Ⅰ/Ⅱ | 27 | 35 | ||
| Ⅲ/Ⅳ | 35 | 23 | ||
| Tumor capsule | 2.091 | 0.148 | ||
| No | 5 | 17 | ||
| Yes | 17 | 41 |
Univariate and multivariate analyses of different prognostic factor in 90 patients HCC.
| Features | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%) P-value | HR (95%) | P-value | ||
| Age(years) | 1.066 (0.414–2.742) | 0.895 | ||
| <60 | ||||
| ≥60 | ||||
| Gender | 1.957 (0.891–4.299) | 0.095 | ||
| Male | ||||
| Femal | ||||
| HBV infection | 0.620 (0.317–1.211) | 0.162 | ||
| + | ||||
| – | ||||
| AFP | 0.803 (0.411–1.571) | 0.522 | ||
| <400 | ||||
| ≥400 | ||||
| Tumor size# | 0.553 (0.282–1.082) | 0.084 | ||
| <5cm | ||||
| ≥5cm | ||||
| Metastasis | 0.338 (0.159–0.719) | 0.005* | 0.406 (0.178–0.925) | 0.032* |
| No | ||||
| Yes | ||||
| PVTT | 0.041 (0.089–0.953) | 0.041* | 0.450 (0.129–1.576) | 0.212 |
| No | ||||
| Yes | ||||
| Tumor number | 0.559 (0.132–2.367) | 0.430 | ||
| Solitary(1) | ||||
| Multiple(≥2) | ||||
| Liver cirrhosis | 0.927 (0.455–1.887) | 0.835 | ||
| No | ||||
| Yes | ||||
| TNM Stage | 0.312 (0.110–0.886) | 0.029* | 0.432 (0.191–0.975) | 0.043* |
| Ⅰ/Ⅱ | ||||
| Ⅲ/Ⅳ | ||||
| Tumor capsule | 0.475 (0.231–0.978) | 0.043* | 0.349 (0.166–0.736) | 0.006* |
| No | ||||
| Yes | ||||
| Expression of DMBX1 | 3.237 (1.395–7.511) | 0.006* | 2.681 (1.036–6.938) | 0.042* |
| Low expression | ||||
| High expression | ||||
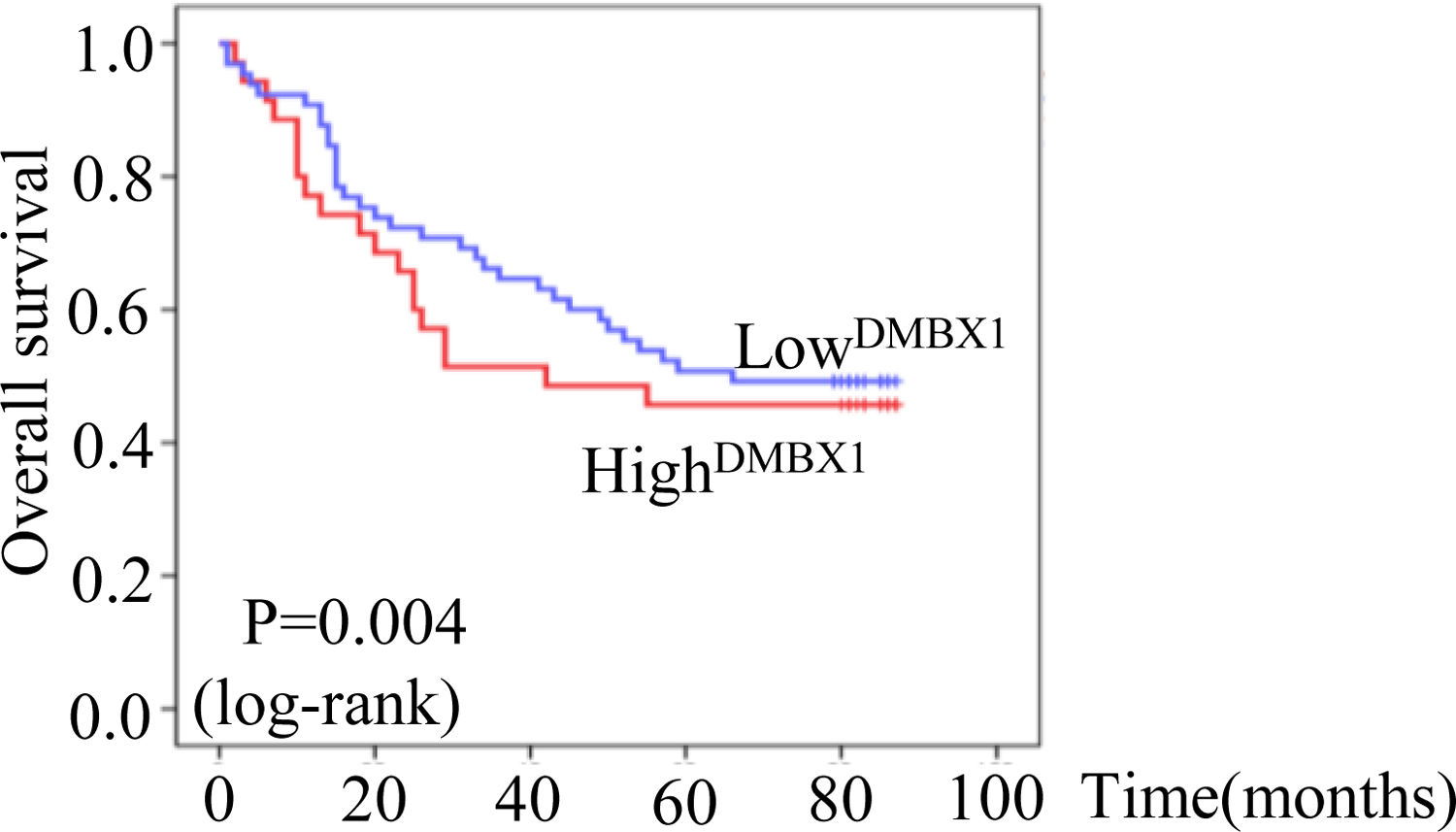
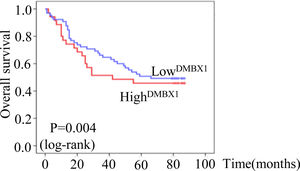
The median survival times of patients with HCC who were positive and negative for DMBX1 expression were 22.4 months (95% confidence interval (CI): 17.780–27.056) and 37.4 months (95% CI: 29.200–45.614), respectively, according to the Kaplan–Meier method. Based on the log-rank test, the survival time of patients with HCC who were positive for DMBX1 expression was significantly shorter than that of patients with HCC who were negative for DMBX1 expression (P < 0.05, Fig. 3), indicating that DMBX1 expression was associated with a poor prognosis of patients with HCC. Therefore, DMBX1 potentially represents a biological tool to evaluate the prognosis of patients with HCC.
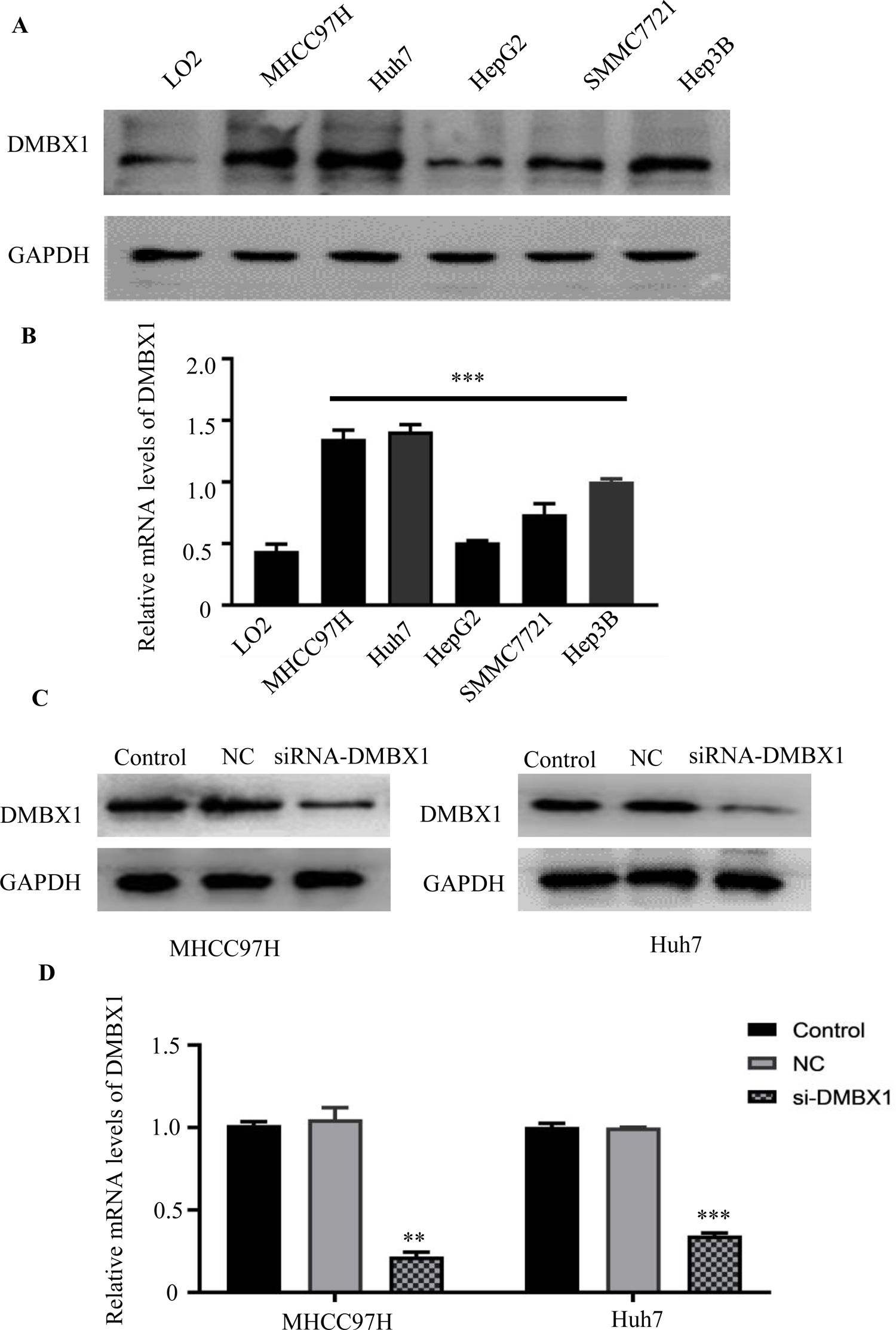
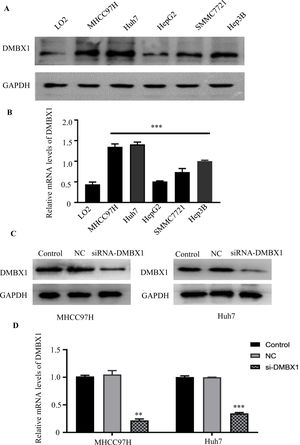
3.4DMBX1 expression in normal liver cells and HCC cell linesThe levels of the DMBX1 protein and mRNA were evaluated in the HCC cell lines MHCC97H, Huh7, HepG2, SMMC7721 and Hep3B and in LO2 normal liver cells using western blotting (Fig. 4A) and RT-qPCR (Fig. 4B), respectively. HCC cell lines expressed DMBX1 at higher levels than LO2 normal liver cells. MHCC97H and Huh7 cells displayed a relatively higher expression of DMBX1 than the other HCC cell lines and were selected for subsequent experiments. Transfection of the DMBX1-targeted siRNA significantly decreased the protein and mRNA expression levels of DMBX1, indicating the silencing effect of the siRNA (Fig. 4C–D).
HCC cells show higher DMBX1 expression than normal liver cells. (A–B) Western blot and RT-qPCR were used to compare the protein and mRNA expression levels, respectively, of DMBX1 in a normal liver cell line (LO2) and four HCC cell lines; the two HCC cell lines with high DMBX1 expression were used for subsequent experiments. (C–D) DMBX1 was knocked down in MHCC97H and Huh7 cells, and decreased expression was confirmed by western blotting and RT-qPCR. The real-time qPCR and western blotting analyses were replicated three times. **P < 0.01, ***P < 0.001. DMBX: diencephalon/mesencephalon homeobox 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC: negative control; siRNA: small interfering RNA.
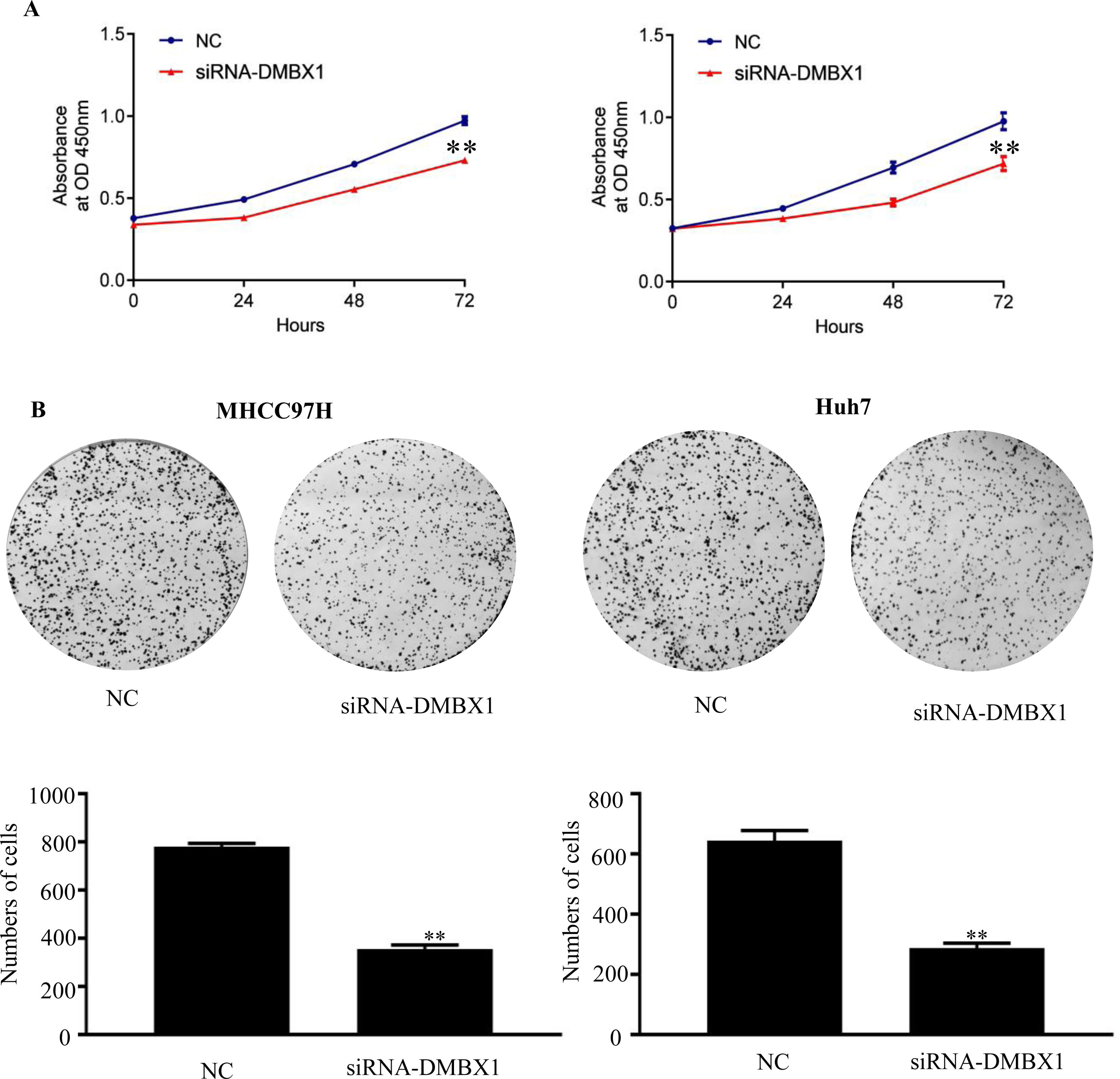
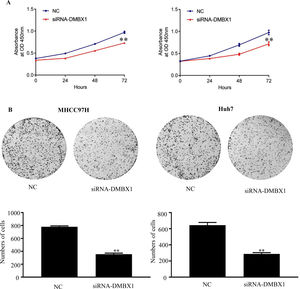
The CCK-8 assay was performed to examine cell growth. Compared with the control group (0 h), the groups at 24, 48 and 72 h after seeding showed significantly reduced cell viability after DMBX1 knockdown (Fig. 5A).
DMBX1 promotes HCC proliferation in vivo. (A) The relative growth rates in DMBX1-knockdown cells were measured using CCK-8 assays in two different HCC cell lines (MHCC97H and Huh7). (B) The results from the colony formation assay showed that DMBX1 knockdown significantly inhibited viability in both HCC cell lines compared with the control cells. **P < 0.01, ***P < 0.001. DMBX1: diencephalon/mesencephalon homeobox 1; NC: negative control; siRNA: small interfering RNA.
A colony formation assay was performed to elucidate the effect of DMBX1 on HCC cell proliferation. DMBX1 silencing prominently inhibited HCC cell proliferation compared with that in the control cells (Fig. 5B).
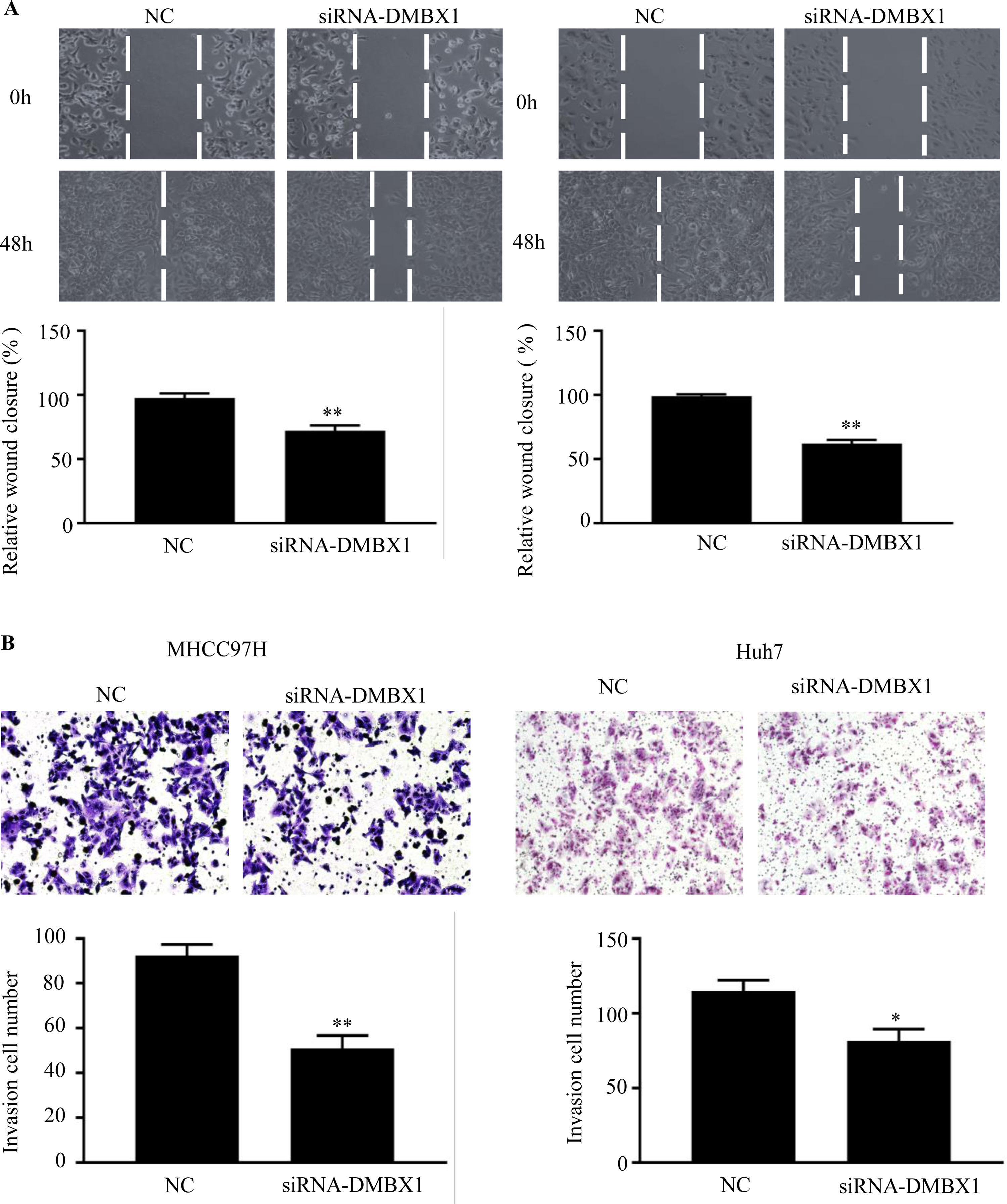
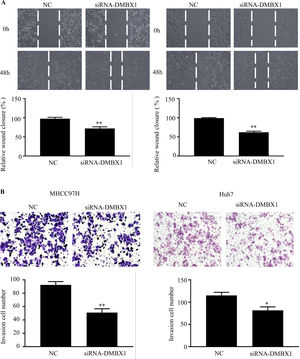
3.6DMBX1 is the key factor regulating cell migration and invasionTo further characterize the migratory activity of DMBX1 in HCC, a wound-healing assay was performed in HCC cells with a DMBX1 knockdown. As shown in Fig. 6A, HCC cell migration was significantly inhibited in the DMBX1-knockdown group compared with the control group (P < 0.05). We performed Transwell invasion assays to further evaluate the effects of DMBX1 on HCC cells. The motilities of MHCC97H and Huh7 cells were markedly reduced after transfection with DMBX1 siRNA compared with that of the control cells (Fig. 6B).
DMBX1 knockdown suppresses the migration and invasion of HCC cells. (A) At 48 h after siRNA-DMBX1 transfection, the cell migration activity was measured by the wound-healing assay. (B) The results from the cell migration assay show that silencing DMBX1 caused a significant decline in cell motility in the two HCC cell lines compared with the control cells. *P < 0.05, **P < 0.01. DMBX1: diencephalon/mesencephalon homeobox 1; NC: negative control; siRNA: small interfering RNA.
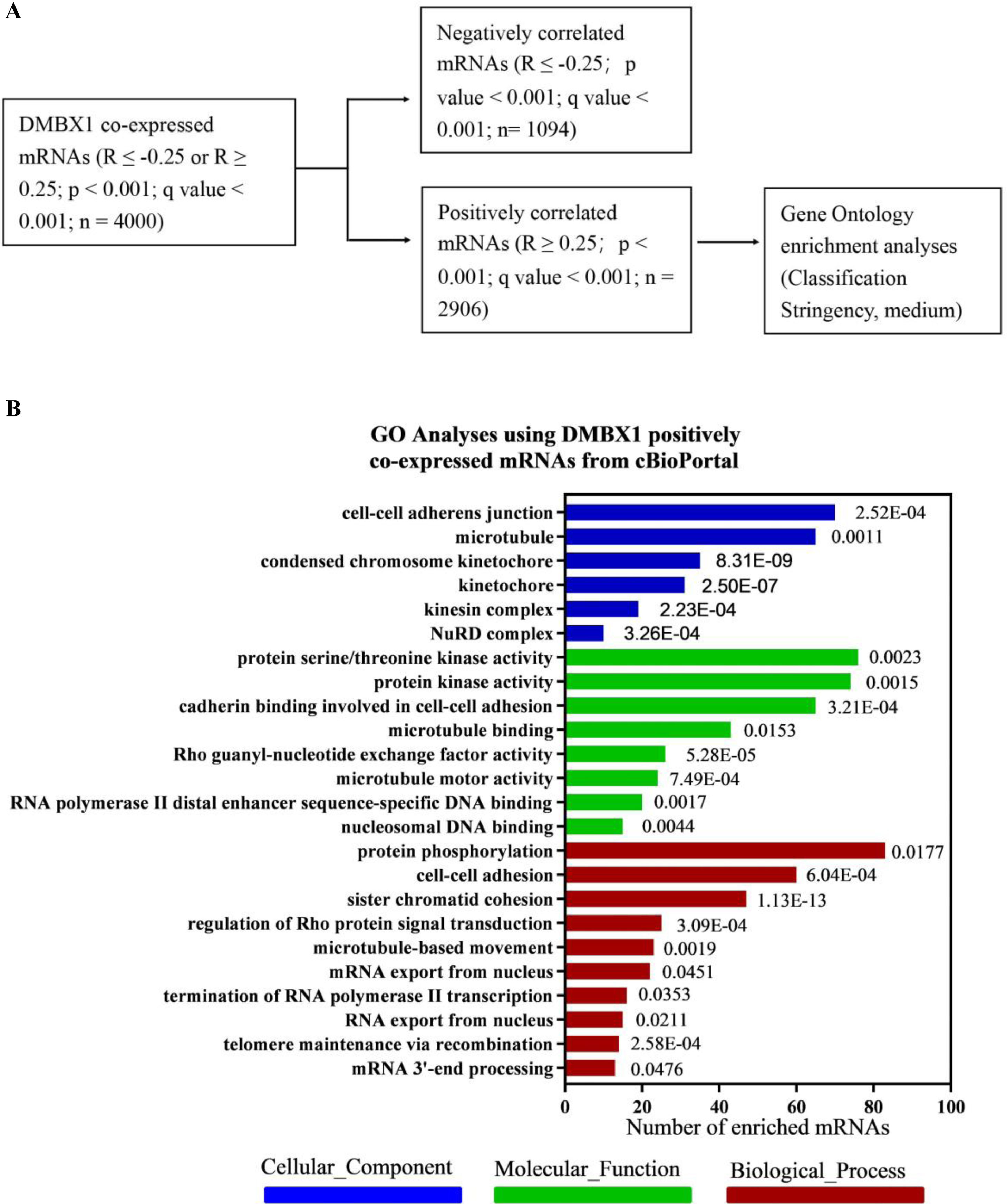
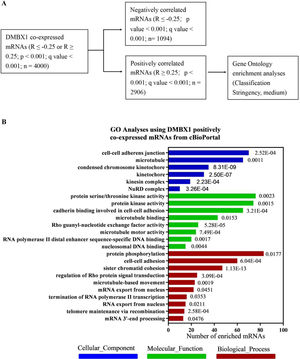
To discover the exact role of DMBX1 in the progression of liver cancer, the mRNAs coexpressed with DMBX1 mRNA in HCC were obtained from cBioPortal, which was extracted from TCGA database. To obtain useful results, after trying a variety of analysis methods, the mRNAs whose expression was positively correlated with DMBX1 were selected for further GO analysis using DAVID Bioinformatics Resources 6.8 (Fig. 7A). As shown in Fig. 7B, many mRNAs were enriched in cell-cell adherens junctions, microtubules, condensed chromosome kinetochores, and kinetochores (cellular components), cadherin binding involved in cell-cell adhesion, microtubule binding, Rho guanyl-nucleotide exchange factor activity, and microtubule motor activity (molecular functions), and cell-cell adhesion, sister chromatid cohesion, regulation of Rho protein signal transduction, and microtubule-based movement (biological processes), which indicates that DMBX1 is significantly related to cellular proliferation and mobility and likely to metastasis in HCC.
A bioinformatics analysis was used to predict the biological function of DMBX1. (A) Selection of the mRNAs whose expression positively correlated with DMBX1 from the mRNAs coexpressed with DMBX1, obtained from cBioPortal (R ≥ 0.25 or ≤ −0.25; P < .001); Gene Ontology (GO) analysis was performed with the selected mRNAs. (B) GO analysis results using the selected DMBX1 positively related mRNAs through online DAVID bioinformatics resources.
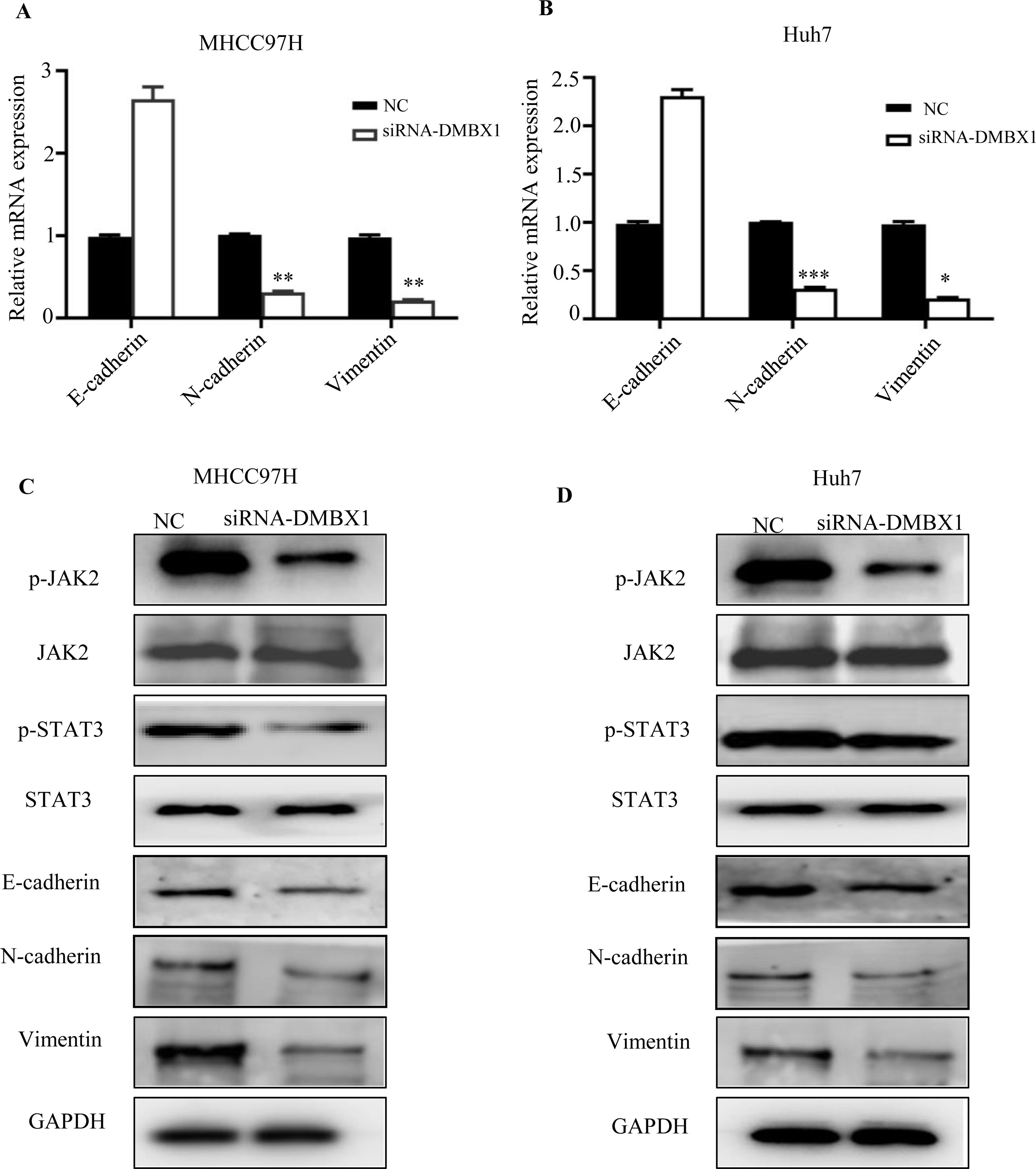
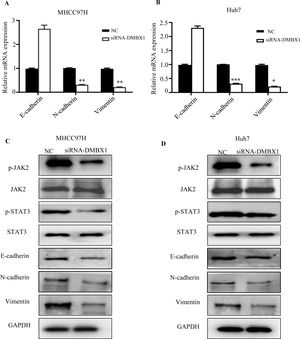
DMBX1 was shown to regulate migration and invasion in HCC. As EMT is an important biological process in cancer cell migration and invasion, we investigated the effect of DMBX1 on the EMT process. Fig. 8A and B show that knocking down DMBX1 significantly decreased the mRNA levels of vimentin and N-cadherin in HCC and significantly increased the mRNA levels of E-cadherin. These data suggest that DMBX1 knockdown suppresses HCC metastasis by blocking EMT.
Loss of DMBX1 expression mitigates EMT activation and JAK/STAT3 signaling in HCC cells. (A–B) RT-qPCR was used to determine the expression levels of E-cadherin, N-cadherin and vimentin to study the effect of DMBX1 knockdown on EMT in MHCC97H and Huh7 cells. (C–D) The expression levels of JAK and STAT3 were measured to investigate the effect of DMBX1 knockdown on the activation status of the JAK/STAT3 pathway in MHCC97H and Huh7 cells. *P < 0.05, **P < 0.01, ***P < 0.001. DMBX1: diencephalon/mesencephalon homeobox 1; NC: negative control; siRNA: small interfering RNA.
Previous studies have reported that STAT3 activation is closely related to EMT in HCC [18–20]. Therefore, we checked whether there is an association between DMBX1 and JAK/STAT3 signaling. The results indicated that the levels of phosphorylated JAK2 and phosphorylated STAT3 were decreased in the DMBX1-knockdown cell lines, while the levels of total JAK2 and STAT3 were unchanged (Fig. 8C–D), suggesting that DMBX1 knockdown reduces JAK/STAT3 signaling in HCC. Therefore, we speculate that the mechanism by which DMBX1 regulates the malignant phenotype of HCC is activation of the JAK/STAT3 signaling pathway to promote EMT.
4DiscussionHCC is one of the most common malignant tumors in Asian countries and remains a major health issue, representing the third most frequent cause of cancer-related deaths worldwide [21]. Multiple genetic and environmental factors are involved in HCC; thus, HCC exhibits familial aggregation and regional and racial characteristics [22,23]. The mechanisms by which these common variants affect susceptibility to HCC remain largely unknown.
As shown in the study by Luo et al. [24], DMBX1 facilitates tumor proliferation and regulates cell cycle progression in lung adenocarcinoma cells. Xu et al. [25] identified DMBX1 as an independent prognostic factor for endometrial carcinoma. In the present study, we aimed to determine the important clinical and biological roles of DMBX1 in HCC and further examined the function of DMBX1 in HCC progression. In the present study, we first analyzed the level of DMBX1 expression in HCC. DMBX1 was upregulated in HCC tissues, and higher DMBX1 expression was associated with HBV infection, tumor size, metastasis, TNM stage and shorter overall survival. Based on these results, we speculated that DMBX1 may be involved in the malignant progression of HCC. We used two HCC cell lines, MHCC97H and Huh7, and then silenced DMBX1 expression in these cells to examine the function of DMBX1 in HCC. In vitro clone formation and invasion assays revealed that the DMBX1 knockdown in two HCC cell lines decreased proliferation and migration. These results suggested that DMBX1 functions as a tumor promoter in HCC, which is consistent with previous studies concerning DMBX1 in other cancers.
Metastasis is the basic biological characteristic of malignant tumors and the main factor affecting the survival of patients. It is a difficult problem and has thus become a focus in cancer research [26]. The most prominent feature of tumor metastasis is tissue specificity, in which different tumors can be formed and metastasize to the same or different parts of the body [27]. The epithelial-mesenchymal transition or transformation (EMT) is a process characterized by the loss of cell adhesion and the enhancement of cell motility. In this study, we found that DMBX1 promoted EMT and enhanced the invasiveness of HCC cells, while silencing DMBX1 inhibited the EMT phenotype and reduced the transformation and metastatic potential of HCC cells in vitro [28]. Cytoskeletal reorganization is a prerequisite for cell movement and antigen invasion. Rho GTPases play an important role in EMT. Specifically, Rho A induces the formation of actin stress fibers and adhesion between cells, while Rac1 stimulates the formation of lamellar lipoproteins [29]. This study used bioinformatics analysis, which found that DMBX1 and Rho have a positive correlation and that a number of mRNAs coexpressed with DMBX1 are enriched in cell-cell adhesion-associated processes, suggesting that DMBX1 is significantly related to EMT. EMT is a well-known process in which epithelial cancer cells acquire the ability to migrate and invade and maintain the original state to form metastatic lesions. Signaling pathways, such as Wnt, Notch, IGF, JAK2/STAT3 and TGF-β, are critical inducers and regulators of EMT [30]. The JAK2/STAT3 pathway also plays a role in the occurrence and development of HCC [31]. In our research, we found that silencing DMBX1 inhibits the activation of JAK2/STAT3. Therefore, our results show that DMBX1 plays a role in HCC by activating the JAK2/STAT3 signaling pathway.
This study is the first to report that DMBX1 expression exerts negative effects on the prognosis of patients with HCC. Because the early diagnosis of HCC is essential for prolonging the overall survival of patients and because the currently available diagnostic methods are limited, DMBX1 is emerging as a novel diagnostic and prognostic biomarker for HCC [32,33]. However, one limitation of this multivariate prognostic analysis is the small sample size. A larger cohort is needed to confirm that DMBX1 is an independent prognostic marker or a biomarker of tumor invasion and metastasis.
5ConclusionIn this study, DMBX1 was found to be expressed at high levels in HCC tissues and cells and exert negative effects on the prognosis of patients with HCC. DMBX1 is significantly related to cellular proliferation and mobility and likely to metastasis of HCC. Thus, DMBX1 could represent a therapeutic target for HCC.
Patient consent for publicationNot applicable.
Authors’ contributionsXiaoting Huang and Leyang Xiang contributed equally to this study.
FundingThis study was supported by grants from the National Natural Science Foundation of China (Nos. 81502342 and 81773354), the Natural Science Foundation of Guangdong Province (No. 2020A1515010208), and the Funds of Guangzhou Medical College, Guangzhou Key Medical Discipline Construction Project, Guangdong Province, China (Nos. 2016C37 and 2015A23).
Ethics approval and consent to participateWritten informed consent was obtained from each patient, and the study was approved by the Ethics Committee of Cancer Hospital Affiliated with Guangzhou Medical University.
Competing interestsThe authors have no conflicts of interest to declare.
Availability of data and materialsThe datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Not applicable.