The major hepatological consequence of HCV infection is the progression to cirrhosis and its potential complications. Several factors have been clearly shown to be associated with fibrosis progression rate: duration of infection, age, male sex, consumption of alcohol, HIV coinfection and low CD4 count. As age and duration of infection increases, the risk of fibrosis increases and the impact of treatment (IFN) decreases. In conclusion, fibrosis progression has a progressive acceleration, sex, age and consumption of alcohol are strongly involved in this progression; the possibility to assess with nonagressive biochemical markers the fibrosis stage will probably allow in the future to identify other factors related to fibrosis progression.
Mortality associated with chronic hepatitis C is usually due to the development of cirrhosis and its potential complications: haemorrhage, hepatic insufficiency and primary liver cancer.1-6
Current understanding of HCV infection has been advanced by the concept of liver fibrosis progression7,8(Figures 1-3). Fibrosis is the deleterious but variable consequence of chronic inflammation. It is characterized by the deposition of extra-cellular matrix component leading to the distortion of the hepatic architecture with impairment of liver microcirculation and liver cell functions. HCV is usually only lethal when it leads to cirrhosis, the last stage of liver fibrosis. Therefore, an estimate of fibrosis progression represents an important surrogate endpoint for evaluation of the vulnerability of an individual patient and for assessment of the impact of treatment on natural history.
Progression of liver fibrosis in patients with chronic hepatitis C. Using the median fibrosis progression rate, in untreated patients, the median expected time to cirrhosis is 30 years (Intermediate fibroser). 33% of patients have an expected median time to cirrhosis of lessthan 20 years (Rapid fibroser). 31% will progress to cirrhosis in morethan 50 years, if ever (Slow fibrose)
Activity and fibrosis are two major histologic features of chronic hepatitis C which are included in different proposed classifications.9-12 One of the few validated scoring systems is called the METAVIR scoring system.11,12 This system assesses histologic lesions in chronic hepatitis C using two separate scores, one for necro-inflammatory grade (A for Activity) and another for the stage of fibrosis (F). These scores were defined as follows; Stages of fibrosis (F) (Figure 1): F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with rare septa, F3 = numerous septa without cirrhosis, F4 = cirrhosis. Grade for activity (A): A0 = no histologic activity, A1 = minimal activity, A2 = moderate activity, A3 = severe activity. The degree of activity was assessed by integration of the severity of the intensity of both piecemeal (periportal) necrosis and lobular necrosis as described in a simple algorithm.12 The intra-and inter-observer variations of this METAVIR scoring system are lower than those of the widely used used Knodell scoring system.9,10 For METAVIR fibrosis stages there is an almost perfect concordance (kappa = 0.80) among pathologists. The Knodell scoring system has a non-linear scale. There is no stage 2 for fibrosis (range 0-4) and the activity grade ranges from 0 to 18 with the sum of periportal necrosis, intralobular and portal inflammation grades. The modified Histological Activity Index is more detailed with 4 different features and continuous grades and the modified fibrosis staging includes 6 stages.
Activity grade, which represents the necrosis feature, is not a good predictor of fibrosis progression.7 In fact fibrosis alone is the best marker of ongoing fibrogenesis.13 So far there is no study demonstrating clearly that activity grades are predictive of fibrosis progression independently of fibrosis stage.14 Fibrosis stage and inflammatory grade are correlated but for one third of patients, there is discordance. Clinician should not take a “significant activity” as a surrogate marker of “a severe disease”. The clinical hallmarks of major necrosis and inflammation i.e. severe acute hepatitis and fulminant hepatitis are finally very rare in comparison to hepatitis B. Even in immunologically compromised patients there are very few acute flareups in patients with chronic hepatitis C.
The dynamic view of fibrosis progressionFibrosis stage summarizes the vulnerability of a patient and is predictive of the progression to cirrhosis7(Figure 2) There is a strong correlation for fibrosis stages, almost linear, with age at biopsy and duration of infection. This correlation was not observed between activity grades.
The model of fibrosis progression from infection to complications estimated key numbers of HCV natural history from literature and our database: The median time from infection (F0) to cirrhosis (F4) is 30 years. The mortality at 10 years for cirrhosis is 50%. The transition probability per year from non-complicated cirrhosis to each of the complications is around 3%.
Because of the informative value of fibrosis stage there is an interest for clinician to assess the speed of the fibrosis progression. The distribution of fibrosis progression rates suggests the presence of at least 3 populations: one population of “rapid fibrosers”, a population of “intermediate fibrosers” and one population of “slow fibrosers” (Figure 3) Therefore the expressions of a mean (or median) fibrosis progression rate per year (stage at the first biopsy/ duration of infection) and of a mean expected time to cirrhosis does not signify that the progression to cirrhosis is universal and inevitable. Using the median fibrosis progression rate, in untreated patients, the median expected time to cirrhosis is 30 years; 33% of patients has an expected median time to cirrhosis of less than 20 years and 31% will progress to cirrhosis in more than 50 years, if ever (Figure 3)
Limitations of any estimate of fibrosis include (i) the difficulty in obtaining paired liver biopsies, (ii) the necessity for large numbers of patients to achieve statistical power and (iii) the sample variability in fibrosis distribution. Because the time elapsed between biopsies is relatively short (usually between 12 to 24 months), the number of events, (transition from one stage to another) is rare. Therefore the comparisons between fibrosis progression rates requires a large sample size to observe significant differences. The slope of progression is difficult to assess because there is no large database with several biopsies. Therefore the real slope is currently unknown and even if there is a linear relationship between stages and age at biopsy or duration of infection, other models are possible.15 Furthermore liver biopsy has its own limit to assess liver fibrosis. Although it is considered as the gold standard to score fibrosis, its value is limited by sample variability. At least a 15-mm length biopsy is mandatory to accurately assess fibrosis.
Factors associated with fibrosis progressionFactors associated and not associated with fibrosis are summarized in Table I. Several factors have been clearly shown to be associated with fibrosis progression rate:4,7,16-18 duration of infection, age, male gender, consumption of alcohol, HIV coinfection and low CD4 count. The progression from infection to cirrhosis depends strongly on sex and age.4,7
Factors associated or not with fibrosis progression.
| Associated in uni and multivariate analysis | Not sure | Not associated |
|---|---|---|
| Age at infection | Necrosis | Last serum viral load |
| Duration of infection | Inflammation | Genotype |
| Age at biopsy | Hemochromatosis heterozygote | Mode of infection |
| Consumption of alcohol > 50 g per day | Cigarette consumption | DR antigens |
| HIV coinfection | Steatosis | Liver viral load |
| CD4 count < 200/mL | Body Mass Index | HCV-HVR1 complexity |
| Male gender | Moderate alcohol consumption | |
| Fibrosis stage | Glucose intolerance |
The role of ageing in fibrosis progression could be related to higher vulnerability to environmental factors, especially oxidative stress, to reduction in blood flow, in mitochondria capacity, or in immune capacities.19
The effect of age on fibrosis progression is so important that it is impossible to assess any rate of fibrosis without taking into account the age at infection.7,32,57,67 The estimated probability of progression per year for men aged between 61 and 70 years was 300 times greater than that for men aged between 21 and 40 years.4
In patients infected before 20 years of age, there were either very slow or no events during the first 30 years. In those aged 20 to 30 years and those 30 to 40 years, there was a clear increase of the slopes after 30 years of infection. In those aged 40 to 50 years, there was a clear increase in slopes versus younger ages after 10 years of infection. And after 50 years of age, there were steep rates of fibrosis progression for all stages of fibrosis,61(Figure 6)
Probability of fibrosis progression to F4 according to age at infection. A total of 2313 patients were included in the F4 analysis including 729, 165, and 32 patients still at risk at 20, 30 and 40 years infection duration, respectively. Whatever the stage, there were higher probabilities of fibrosis progression according to the age at infection (p < 0.001). First line represents 754 patients infected before the age of 21 years. Second line represents 851 patients infected between 21 to 30 years. Third line represents 348 patients infected between 31 to 40 years. Fourth line represents 211 patients infected between 41 to 50 years. Fifth line represents 149 patients infected after the age of 50 years.
Recently we have assessed the potential of Markov modelling for quantifying fibrosis progression in HCV patients according to cofactors and assessing treatment impact, even for data as sparse as two biopsies per patient. Fibrosis progression was modelled as a time-homogeneous Markov process through three stages: F0+F1, F2 and F3+F4. Data from 287 patients (including them 236 with known time of infection) who had had two biopsies were used to build the model, which was applied separately to patients receiving interferon alpha (IFN) (n = 185) and untreated patients (n = 102). Age and duration of infection were found to be significant independent cofactors of progression.
GenderIn the male gender, there was an increase in the slope of fibrosis progression compared to females for F3 and F4, independent of age at infection and of alcohol consumption. Differences were greater after duration of 20 years of infection. However the role of body mass index as a confounding variable as well as metabolic factors must be investigated.57,63
The female gender is associated to 10 times less rapid progression to cirrhosis than male whatever the age.18 Oestrogen modulates fibrogenesis in experimental injury. Oestrogen blocks proliferation and fibrogenesis by stellate cells in primary culture. Oestrogen could be modifying the expression of transforming growth factor and other soluble mediators. Recently, a study has been done to evaluate the influence of pregnancies, oral contraceptives, menopause, and hormone replacement therapy on liver fibrosis progression in HCV-infected women. Through multivariate analyses, the rate of fibrosis progression was higher in post-menopausal (p = 0.05) and nulliparous (p = 0.02) women, and was associated with high histology activity index (p < 0.001). Prior use of oral contraceptives had no significant influence. Among post-menopausal women, the rate of fibrosis progression (± SE) was lower in women who received hormone replacement therapy as compared to untreated patients (0.099 ± 0.016 vs 0.133 ± 0.006 METAVIR units/year; p = 0.02), and was similar to that of pre-menopausal women (0.093 ± 0.012 METAVIR units/ year; p = NS).58
AlcoholThe role of alcohol consumption has been established for daily doses greater than 50 grams per day7,16 progression after 10 years of infection to stages of fibrosis F2, F3 and F4. For lower doses there are discordant results with even preliminary studies suggesting a protective effect of very small doses. Alcohol consumption is difficult to quantify and conclusions must be prudent. However it seems from these studies that influence of alcohol is independent from other factors, weaker compared with age, and is exerted only at toxic levels of intake.
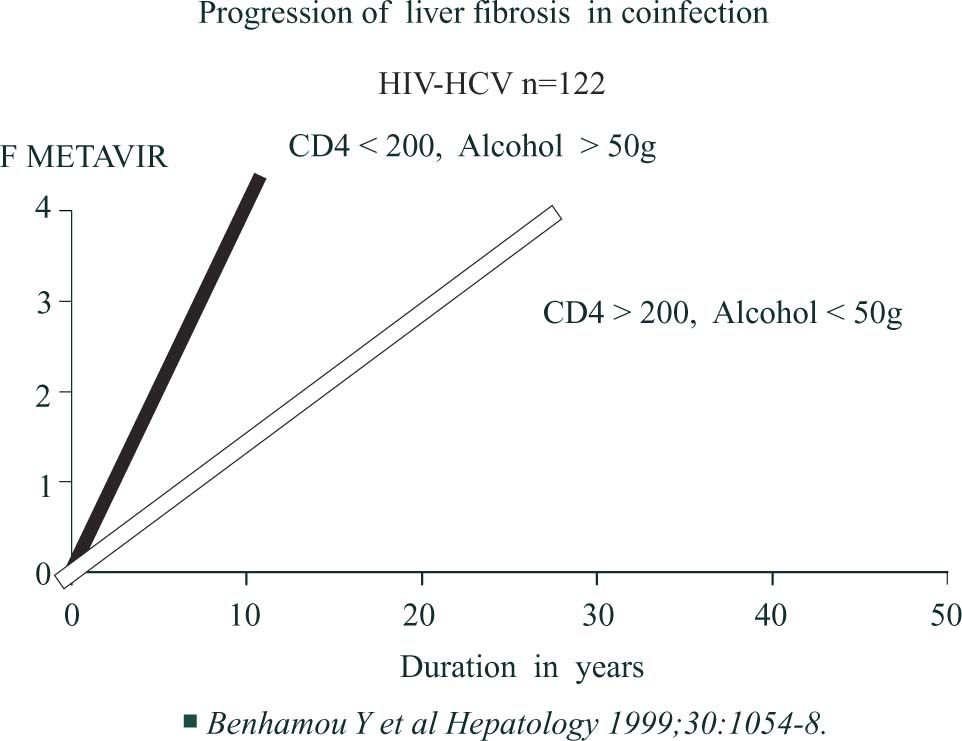
HIV coinfectionSeveral studies have demonstrated that patients coinfected with HCV and HIV have a faster fibrosis progression rate than controls even before the occurrence of marked decline in CD4 cell count63 and after taking into account age, sex and alcohol consumption17,20(Figure 4a) An An HIV-infected patient with less than 200 CD4 cells/μL and drinking more than 50 g of alcohol daily has a median expected time to cirrhosis of 16 years versus 36 years for an HIV-infected patient with more than 200 CD4 cells/μL, drinking 50 g or less of alcohol daily (Figure 4b)
We identified by multivariate analysis identified 4 independent predictors of progression to cirrhosis: absence of protease inhibitor therapy (relative risk [RR] = 4.74, 95% confidence interval [CI], 1.34-16.67), heavy alcohol
consumption (> or = 50 g daily) (RR = 4.71, 95% CI, 1.92-11.57), low CD4 cell count (< 200/microL) (RR = 2.74, 95% CI, 1.17-6.41), and age at HCV contamination (> or = 20 years) (RR = 2.37, 95% CI, 1.04-5.38). This study suggested that protease inhibitor therapy might not accelerate progression to HCV-related cirrhosis. Furthermore, chronic use of antiretroviral therapy containing PI together with reduction of alcohol consumption and maintenance of high CD4 count could have a beneficial impact on liver fibrosis progression in HIV/HCV coinfected patients.64
Viral factorsViral factors such as genotype, viral load at the time of the biopsy, and quasi species are not associated with fibrosis.7,21,22 There are very few studies for the following factors and more studies with high sample size are needed: fluctuations of HCV RNA, intrahepatic cytokines profiles, HLA class genotype, C282Y heterozygote hemochromatosis gene mutation, and cigarette consumption. About genotypes, there was no statistically significant overall difference between HCV genotypes in the incidence of cirrhosis at 20 years. There was clearly no association between genotype 1 (1b or 1a) with fibrosis progression, even at 40 years. In fact, slower slopes in patients infected with genotype 1b in comparison to genotype 3 was observed. The role of specific steatosis related to genotype 3 is suspected for this increase in fibrosis progression.69 HCV viral load had no effect. Because it is impossible to perform a longitudinal study with repeated liver biopsies and repeated viral load determinations, we do not know if viral load varies36 and if a high viral load at the beginning of infection could be predictive of fibrosis thereafter. However, even for F1 in the early years, there was no difference in the slopes according to the viral load.57
SmokingAbout hepatotoxicity of cigarette smoke a recently study has shown that smoking was related to increased fibrosis and activity scores in age-adjusted (P = 0.09 and P = 0.05 respectively) and multivariate analyses (P = 0.03 and P = 0.04 respectively).65
Risk of fibrosis in patients with normal transaminasesPatients with repeated normal serum transaminases activity have lower fibrosis progression rate than matched control patients with elevated transaminases23(Figure 5) However there is still 15 % of these patients with moderate or high fibrosis progression rates. Therefore, we recommend assessing fibrosis stage, by liver biopsy or biochemical markers68 in these PCR positive patients. If the patient has septal fibrosis or portal fibrosis with a high fibrosis rate a treatment should be considered.
Histological activityHistological activity is not always associated with fibrosis progression.7,14,47,57 Differences in slopes were graphically observed between grade A0 and higher grades only after duration of 20 years of infection. Sampling error and intra-observer discordances are possible causes of false negative results. Fibrosis stage is always a better predictor of fibrosis progression than the activity grade.7
Mode of infectionIn study with big sample size, mode of infection and fibrosis progression. We observed significantly more patients without cirrhosis at 20 years among intravenous (IV) drug users (95%) in comparison to transfused patients (89%) which was probably related to a younger age at infection.57
Steatosis and metabolic factorsSteatosis occurs in more than 50% of patients with chronic hepatitis C and is associated with increased hepatic fibrosis. In many of these patients the pathophysiology of steatosis appears to be the same as for patients with non-alcoholic fatty liver disease-that is, related to visceral adiposity and obesity.62 This suggest that increasing body mass index has an important role in the pathogenesis of steatosis in the chronic hepatitis C. Weight loss in patients with chronic hepatitis C may be associated with a reduction in steatosis and abnormal liver enzymes and an improvement in fibrosis, despite the persistence of the virus. Weight reduction may provide an important adjunct treatment strategy for patients with chronic hepatitis C.66,67 Recently we have confirmed that genotype 3 HCV infection was associated with significantly more steatosis than other genotype, with lower cholesterol levels and that this “viral” steatosis disappeared in sustained responders. Therefore any studies on steatosis in patients with chronic hepatitis C must separate the genotype 3 population from other patients.
Impact of treatment on fibrosis progressionBetter knowledge of factors associated with fibrosis progression has permitted to better assess the impact of treatment on fibrosis progression.
According to the Markov age-dependent modelling, a ten-year increment in duration of infection increased the risk of progression by 32% for IFN-treated patients and by 51% for untreated patients. The course of a series of 1000 IFN-treated and 1000 untreated patients was simulated over 5 years according to the initial stage of fibrosis and age and duration of infection at diagnosis. IFN treatment decreased the risk of progression to F3+F4 by a factor of 4.8, for subjects aged 40 years, infected for 10 years, and in F0+F1 at diagnosis. As age and duration of infection increased, the risk of fibrosis increased and the impact of IFN treatment decreased.
We pooled individual data from 3010 naÏve patients with pre-treatment and post-treatment biopsies from 4 randomized trials.63 Ten different regimens combining standard interferon, PEG interferon, and ribavirin were compared. The impact of each regimen was estimated by the percentage of patients with at least 1 grade improvement in the necrosis and inflammation (METAVIR score), the percentage of patients with at least 1 stage worsening in fibrosis METAVIR score, and by the fibrosis progression rate per year. Necrosis and inflammation improvement ranged from 39% (interferon 24 weeks) to 73% (PEG 1.5 μg/kg + ribavirin >10.6mg/kg/day; P < 0.001). Fibrosis worsening ranges from 23% (interferon 24 weeks) to 8% (PEG 1.5 m g/kg + ribavirin >10.6mg/ kg/day; P < 0.001). All regimens significantly reduced the fibrosis progression rates in comparison to rates be fore treatment. The reversal of cirrhosis was observed in 75 patients (49%) of 153 patients with baseline cirrhosis.
Six factors were independently associated with the absence of significant fibrosis after treatment: baseline fibrosis stage (odds ration [OR] = 0.12; P < 0.0001), sustained viral response (OR = 0.36; P < 0.0001), age < 40 years (OR = 0.51; P < 0.001), body mass index < 27 kg/m2 (OR = 0.65; P < 0.001), no or minimal baseline activity (OR = 0.70; P = 0.02), and viral load < 3.5 millions copies per milliliter (OR = 0.79; P = 0.03).
In conclusion, fibrosis progression is mostly regular from stage to stage, with progressive accelerations, and that the main factors associated with fibrosis progression are age and daily alcohol consumption equal to or greater than 50 gr. The progression of fibrosis begins to accelerate at 50 years of age, whatever the duration of infection. Metabolic factors as glucose intolerance, steatosis and overweight are probably important independent factors. The possibility to assess with non-aggressive biochemical markers68 (Bismut et www.biopredictive.com) the fibrosis stages will probably allow in the future to identify other factors related to fibrosis progression.