Cystic lesions of the liver are common and a major proportion is formed by parasitic cysts and simple cysts. Biliary cystic tumors (BCTs), namely biliary cystadenoma (BCA) and biliary cystadenocarcinoma (BCAC), are rare tumors which usually arise from the intrahepatic biliary tree. BCAs have malignant potential and are difficult to differentiate from BCAC pre-operatively on radiological imaging. Here we have presented 4 patients with BCTs and reviewed the literature pertaining to them.The data of four patients with BCA/BCAC diagnosed and treated at our institute were retrieved from our database and records were reviewed for age, sex, history, imaging, surgery, pathology and follow-up. Mean age of the patients was 53.5 years (range 30-71 years). Two male and two female patients presented with abdominal pain, of which one male patient had pancreatitis at diagnosis. Characteristic features were seen on pre-operative imaging (cystic lesions with internal septations) and biliary communication was identified in the patient with pancreatitis. Three patients were diagnosed with a BCA on final histology, while one patient had a BCAC. Following surgical resection, all the patients are asymptomatic and disease free with a mean follow-up of 24 months (range 10-40 months). In conclusion, BCTs should be suspected in the presence of a well-encapsulated, cystic hepatic lesion with internal septations. Although pre-operative distinction between BCA and BCAC is difficult, the lesion, whenever possible, should be completely resected as long-term outcomes are good, especially with BCA.
Around 5-10% of the populationharbours cystic lesions of the liver.1 The vast majority of non-parasitic cystic lesions of the liver are simple cysts. Other differential diagnoses of non-parasitic cysts include degenerated tumors (primary or metastatic), polycystic liver disease, Caroli’s disease, and biliary cystictumors (BCTs) amongst others. BCTs are exceedingly rare and comprise both benign (with malignant potential) and malignant tumors, specifically biliary cystadenoma (BCA) and biliary cystadenocarcinoma (BCAC). Although theypredominantly originate from the intrahepatic biliary system, a small proportion may arise from the extrahepatic biliary tree. They are slow growing tumors and can be difficult to differentiate radiologically from other cystic lesions of the liver. BCTs are postulated to originate as a result of proliferation of ectopic embryonic tissue nests which are otherwise involved in the development of the gall bladder.2 However, half of them contain endocrine cells and probably arise from intrahepatic peribiliary glands.3 Because of the malignant potential of a cystadenoma, and because of the difficulty in differentiating a benign cystadenoma from its malignant counterpart, only total ablation is considered appropriateas any other treatment is associated with a high likelihood of recurrence. The prognosis associated with these tumors is good and recurrence is uncommon. Here we have presented a case series of four patients with BCTs- three patients with a biliary cystadenoma and one with a cystadenocarcinoma- and have discussed the clinical presentation, pathology, radiological features, surgical management and prognosis of these tumors.
Material and MethodsA retrospective review of case records identified 3 patients with a biliary cystadenoma and 1 patient with a biliary cystadenocarcinoma. The records were reviewed for age and sex of the patient, presenting complaints, findings on pre-operative imaging, operation performed, diagnosis on final histopathology and details of follow-up.
Liver resection was performed either as an open procedure or laparoscopically. Open liver resection was done using a reverse L-shaped incision, and laparoscopic liver resection was carried out with the help of 5 ports. In the laparoscopic procedures, the specimen was retrieved by enlarging the infra-umbilical 11 mm port.
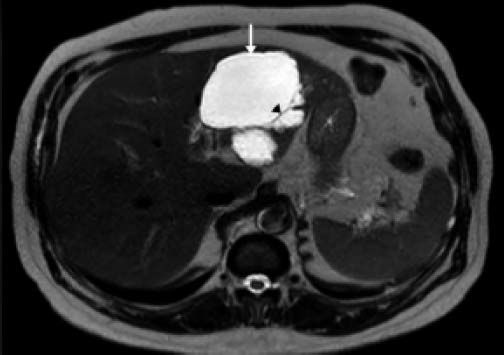
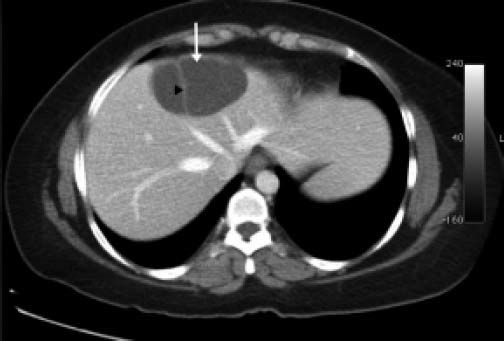
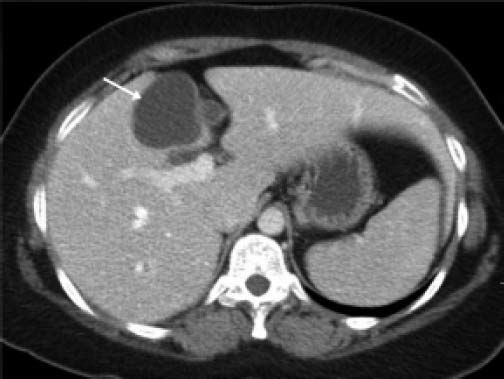
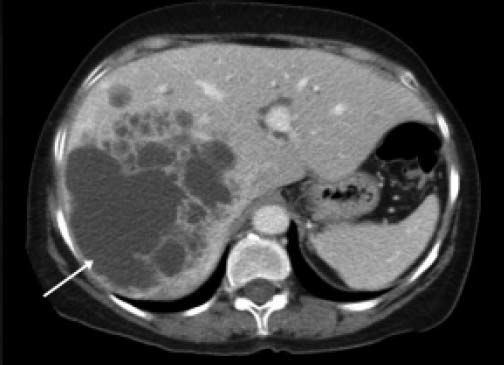
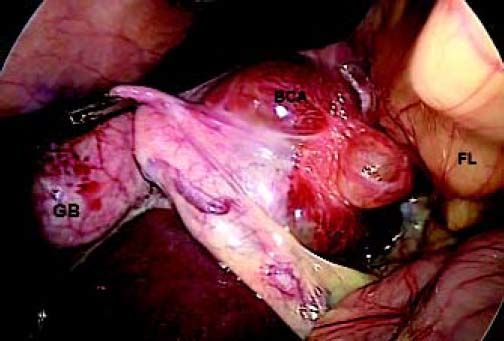
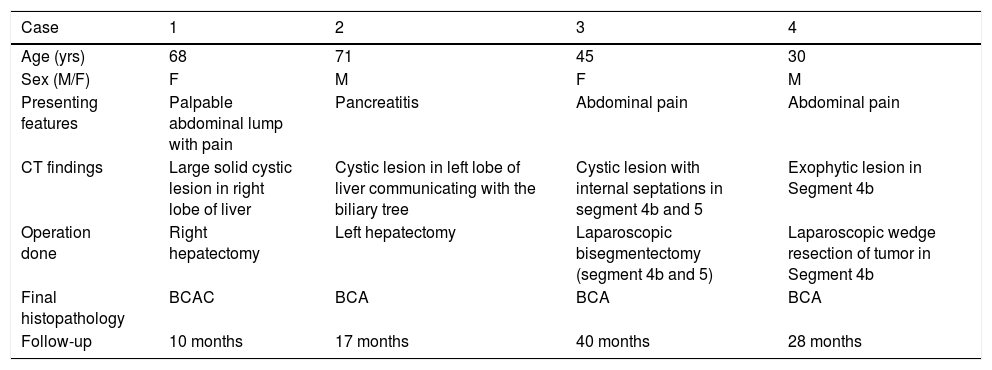
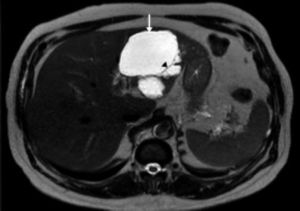
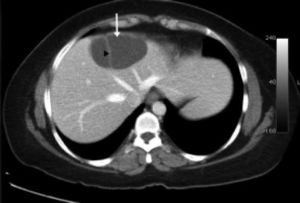
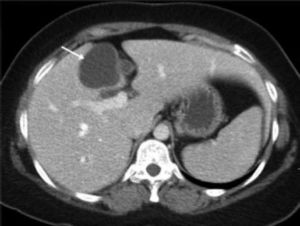
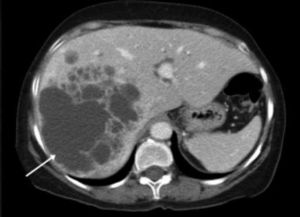
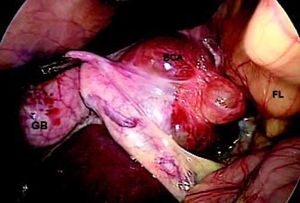
ResultsA summary of the characteristics of the four patients reviewed is shown in table 1. The mean age of the patients was 53.5 years (range 30-71 years). There were 2 male and 2 female patients. All the 4 patients presented with abdominal pain. While three of them presented mainly with right upper quadrant discomfort and occasional pain, one patient presented with signs and symptoms of pancreatitis. Computed tomography (CT) of the abdomen of the patient who presented with pancreatitis showed a cystic lesion in the left lobe of the liver which appeared to be communicating with the biliary tree. This was confirmed with magnetic resonance imaging (MRI) of the liver (Figure 1). The other patients had characteristically large cystic lesions in either the right or left lobe of the liver with internal septations (Figures 2-4). They, however, did not have any biliary communication. Two patients had lesions in segment 4b which was amenable to laparoscopic resection. One of them underwent a wedge resection, whereas the other had a formal bisegmentectomy (segments 4b and 5). Intra-operative photograph of the laparoscopic bisegmentectomy, prior to excision of the tumor (the CT scan of which is shown in figure 3) in segment 4b, is shown in figure 5. An open right hepatectomy was performed in one patient with a large lesion in the right lobe (Figure 4) and a left hepatectomy was done for another patient. Histopathological examination revealed a BCA (Figure 6) in three of our patients and a BCAC in one (patient whose CT scan is shown in figure 4). The mean follow-up of the 4 patients is 24 months (range 10-40 months). There have been no recurrences in the follow-up period. A follow-up CT-abdomen of the patient who underwent a right hepatectomy (and later found to have BCAC) is shown in figure 7.
Summary of the characteristics of the four patients reviewed.
| Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age (yrs) | 68 | 71 | 45 | 30 |
| Sex (M/F) | F | M | F | M |
| Presenting features | Palpable abdominal lump with pain | Pancreatitis | Abdominal pain | Abdominal pain |
| CT findings | Large solid cystic lesion in right lobe of liver | Cystic lesion in left lobe of liver communicating with the biliary tree | Cystic lesion with internal septations in segment 4b and 5 | Exophytic lesion in Segment 4b |
| Operation done | Right hepatectomy | Left hepatectomy | Laparoscopic bisegmentectomy (segment 4b and 5) | Laparoscopic wedge resection of tumor in Segment 4b |
| Final histopathology | BCAC | BCA | BCA | BCA |
| Follow-up | 10 months | 17 months | 40 months | 28 months |
BCA: biliary cystadenoma. BCAC: biliary cystadenocarcinoma.
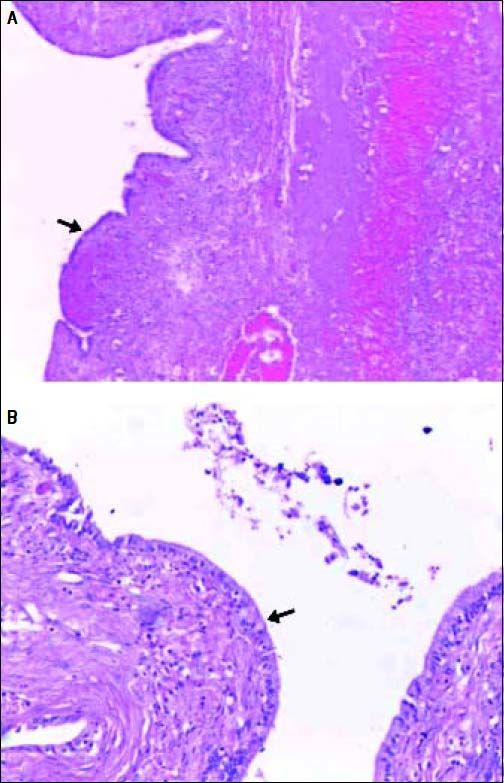
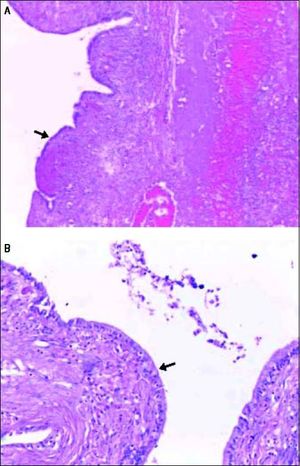
Histopathological examination of the BCA seen in figure 5 showing hematoxylin and eosin stained sections of the cyst wall lined by columnar epithelium (arrows) under 20x (A) and 40x (B) magnification.
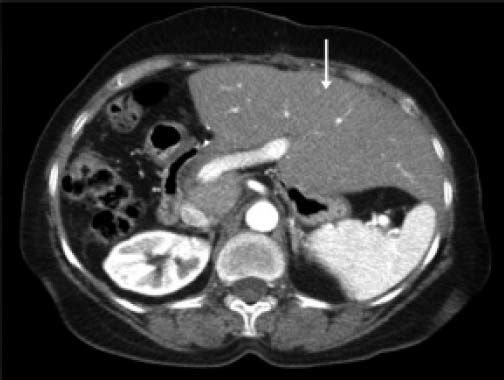
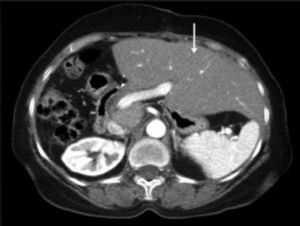
CT scan of the abdomen showing the hypertrophied left lobe (arrow) three months after resection of the lesion (right hepatectomy) in figure 4.
The clinical presentation of BCTs varies considerably. The age at diagnosis may be highly variable, but the incidence of BCA peaks in the fourth or early fifth decade of life and the incidence of BCAC peaks another decade or two later.4 In our series, a wide variation was observed in the age at presentation (30-70 years). Some patients may be asymptomatic and detected incidentally due to the increasingly liberal use of imaging. The majority of patients present with right upper quadrant or epigastric pain, discomfort or distension. One of these symptoms or a combination of them was present in all our patients. Since the symptoms are insidious in onset and evolve over a long period of time (due to the slow growing nature of the tumors), most of the tumors are large at diagnosis. A small proportion of patients may have an obstructed biliary tract and consequently present with jaundice or signs of colangitis.5 Most often these episodes are transient and tend to resolve spontaneously, which is compatible with the migration of mucus material or tumor embolus from the cyst into the bile duct.6 One patient in our series presented with pancreatitis as a consequence of the biliary communication.
CT and ultrasonography (USG) are the most helpful diagnostic modalities for diagnosis of the BCTs. They are seen as well-demarcated cystic lesions, usuallywith internal septations, on CT; calcification of the wall is rare and the presence of papillary projections or wall excrescences should raise a suspicion of cystadenocarcinoma.7 A sharply demarcated anechoic mass with echogenic internal septations is the usual finding on USG. BCAC is more likely to contain intracystic debris, mural or septal nodules and polypoid protrusions.8 MRI is another useful tool and is typically seen as a fluid-containing multilocular cyst with varying signal intensities depending on the content of the fluid within (mucinousisosignal; serous-hyposignal; hemorrhagic-hyperintense signal).9
BCAs are multiloculated cysts lined by biliary type cuboidal or non-ciliated columnar epithelial cells. In approximately 85-90% of BCAs, the cysts are surrounded by a stroma that mimics ovarian stroma.10 BCACs can be easily distinguished from BCAs in the presence of certain typical findings, namely, a loss of nuclear stratification in the epithelial lining, a tubulopapillary architecture, and mild nuclear pleomorphism. The presence of invasion confirms the diagnosis of a cystadenocarcinoma. Biliary intraductal papillary mucinous neoplasms (or IPMN-B) have been identified by some authors to be a separate group of BCTs associated with biliary tree communication and absence of ovarian stroma.11 Although management is similar, preoperative identification of IPMN-B can forewarn the surgeon to its typical pattern of superficial spreading tumor growth.
The risk of a BCA transforming into a BCAC has been reported to be up to 20%.12 Therefore, appropriate management is of utmost importance and there is a high likelihood of recurrence with operations other than total ablation. Over the years BCAs have been approached and treated in several ways- marsupialisation, internal Rouxen-Y drainage, aspiration, sclerosis, deroofing or partial resection. This leads to near universal recurrence and progression to malignancy. Formal surgical resection with negative margins is recommended for BCA. Liver resection or enucleation, either open or laparoscopic, are acceptable surgical therapies, anddepends on the patient’s characteristics, anatomical location of the cyst and the experience of the surgeon in tackling these tumors. Although peripherally located BCTs should be treated with formal resection, centrally located lesions can be enucleated as they may involve central biliary or vascular structures. It is advisable to perform a wide resection rather than an enucleation for BCAC as tumor extension cannot be reliably diagnosed on preoperative imaging. Many authors recommend routine intra-operative cholangiography for these tumors as some of them may communicate with the biliary tree. Besides documenting a potential biliary communication, it also helps to exclude the presence of mucus or tumor material in the bile duct.13
Based on published series for BCAs, recurrence rates seem to be very low (5-10%) following appropriate surgical treatment. In a large series of patients with benign BCA and a follow up period of 18 years, overall survival was >90%.14 Most of the recurrences associated with BCAC are in the liver itself and are probably a reflection of inadequate initial local management. Recurrence of disease in an extra-hepatic location is comparatively uncommon. The 5-year survival associated with BCAC is much better when compared to other hepatic malignancies such as hepatocellular carcinoma and cholangiocarcinoma (57%vs. 40% vs. 22%, respectively).15 The presence of ovarian-like stroma in a patient with BCAC confers a considerably higher long-term survival as compared to patients with BCAC lacking the ovarian-like mesenchymal stroma. Although the duration of follow-up is not as long as in the studies mentioned above, all the 4 patients (including one with BCAC) are asymptomatic and disease free at present.
In summary, BCTs should be suspected in a patient, especially a woman, whose radiological imaging shows a well-encapsulated, multilocular, cystic hepatic lesion. Radiological modalities like USG, CT and MRI yield important information but cannot reliably distinguish BCA from BCAC. Suspected BCTs should be extirpated surgically with complete removal of the lesion when feasible. Long term outcomes after surgery for BCA are good, whereas patients who have undergone for BCAC have a comparatively worse long-term prognosis.
AcknowledgementThe authors would like to thank senior pathologist, Dr. Anita S. Bhaduri, for her help with the pathology slides.
Abbreviations- •
BCA: biliary cystadenoma.
- •
BCAC: biliary cystadenocarcinoma.
- •
BCT: biliary cystic tumor.
- •
CT: computed tomography.
- •
IPMN-B: intraductal papillary mucinous neoplasm (biliary).
- •
MRI: magnetic resonance imaging.
- •
USG: ultrasonography.
The authors declare that they have no conflict of interest.
Funding SourcesNone.
Author’s ContributionAll the authors have contributed to the manuscript in one or more of the following areas- conception and design; analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article.
Informed consent was taken from the patients for the procedures and for publication.