Non-alcoholic fatty liver disease (NAFLD) is a common and serious disease. Literature reports the central role of the gut-liver axis in the pathogenesis of NAFLD, and recently suggests that the microbiota is a casual factor of the disease, involved in the interactions between intestinal lumen and liver. Probiotic are commensal bacteria, able to modulate the microbiota with benefits for the health of humans. Several data suggest a range of potentially beneficial medicinal uses for probiotics, in particular in NAFLD patients. However, the species with higher efficacy in this disease are not identified. The present review focuses on the role of gut-liver axis in the pathogenesis, and on the potential therapeutic role of probiotics in the managing of NAFLD.
There is a natural link between the intestinal flora and the gut, and many species of bacteria have adapted to survive and live in the intestine.1 The human gastrointestinal tract is a complex ecosystem integrated by up to 1,014 total bacteria, called gutmicrobiota that plays main functions in human: provide a barrier for colonization of pathogens; it exerts important metabolic functions such as fermentation of non-digestible fibers, salvage of energy as short chain fatty acids and synthesis of vitamin K; moreover, it stimulates the development of the immune system.2,3 Lifestyle, dietary habits, age, host genotype and exposure to antibiotics may affect the composition of the intestinal microflora.4 Some data reported that gut bacteria contribute to differences in body weight, insulin sensitivity, glucose metabolism and other cardiometabolic risk factors among individuals.5,6 In fact, the derangement of the microbiota, in particular small intestinal bacterial overgrowth (SIBO), occurs in a large percentage (20-75%) of patients with chronic liver disease, and in particular in non-alcoholic fatty liver disease (NAFLD) patients, and has been associated with the severity of steatosis.7
Probiotics have been defined in many ways over the years. The most widely accepted definition recognized by the World Health Organization and the Food and Agriculture Organization, states that they are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”.8 Probiotics are bacteria that help to maintain the balance of microbiota in the gut. Literature suggests a range of potentially beneficial medicinal uses for probiotics. The present review focuses on the role of gut-liver axis in the pathogenesis of fatty liver and on the potential therapeutic role of probiotics in the managing of NAFLD.
The Gut-Liver Axis: The Nafld CaseThe idea that increased intestinal permeability and gut-microbiota might contribute to the development of several diseases, was first suggested by Llewellyn Jones (Theory of auto-intoxication from gut bacteria-since 1890). The presence of a link between the gut and the liver mediated by the microbiota is an hypothesis that could explain the hepatobiliary changes associated with several inflammatory and infectious intestinal diseases.9
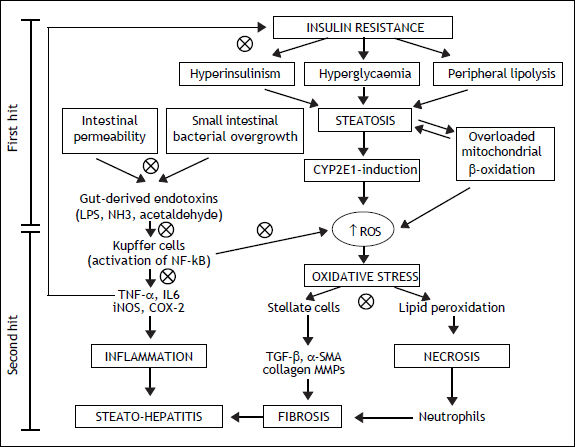
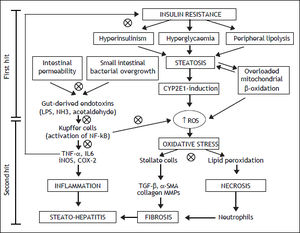
NAFLD is the most common cause of liver disease in Western Countries, that usually develops in the setting of insulin resistance and obesity.10 This is an umbrella term that refers to a broad spectrum of lesions that range from isolated steatosis, to active lesions with inflammation [non-alcoholic steatohepatitis (NASH)], to cirrhosis and hepatocellular carcinoma. Obesity has been associated with changes in the gut microflora and increased intestinal permeability.11,12 The two-hit hypothesis is a widely accepted theory of the pathogenesis of NAFLD.13 The first hit presents the insulin resistance leading to the fat accumulation, resulting in hepatic steatosis. The second hit is an oxidative stress, caused by different liver pathogens, determining lipid peroxidation and increasing cytokine production, at the base of the liver inflammation and necrosis. The first barrier of the gut in preventing antigens encountering the immune system is the single line of the intestinal epithelium. The intestinal epithelium is a very dynamic structure that limits, but does not exclude, antigens from contact with the tissues.14
Intestinal microflora is the primary source of bacterial endotoxins [lipopolysaccharide (LPS)], produced by Gram-negative bacteria, normally crosses the mucosa only in trace amounts, enters the portal blood, and becomes cleared in the liver.15 Several mechanisms have been identified in this process that relies on a balance between the barrier functions of the gut and the ability of the liver to detoxifying.16 Literature suggests that the intestinal microflora is directly implicated in the induction and progression of chronic liver diseases, including not only NAFLD, but also hepatitis C infection, alcoholic liver disease, cirrhosis and cholestasis of pregnancy (Figure 1).17,18 In patients with NAFLD, intestinal permeability and the prevalence of SIBO are increased.19 The increased permeability appears to be caused by disruption of intercellular tight junctions in the intestine, and it may play an important role in the pathogenesis of hepatic fat deposition.
Mechanisms involved in the pathogenesis of NAFLD and the possible therapeutic action (cross circles) of probiotics. CYP2E1: cytochrome P450 2E1. NH3: ammonia. ROS: reactive oxygen species. IL6: interleukin6. iNOS: inducible nitric oxide synthase. COX-2: cyclooxygenase-2. TGF-β:transforming growth factor beta. MMPs: matrix metallopro-teinases.
In addition, the high-fat diet has been reported not only to increase intestinal translocation of endotoxin, but also to reduce enteric concentration of Bifidobacteria, a group of bacteria that have been shown to lower intestinal LPS levels and to improve mucosal barrier function.20
The progression of NAFLD, from steatosis to steatohepatitis, is attributed to the effect of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), oxidative stress, and endotoxins.21 LPS can stimulate innate immune system-mediated production of pro-inflammatory cytokines from adipose tissues and also can lead to mitochondrial dysfunction in Kupffer cells.22 The Kupffer cells are the major immune effector cells in the pathogenesis of NAFLD. These activated cells express CD14, which is a receptor for a complex consisting of LPS and LPS-binding protein, on the surface of macrophages. CD14 plays an important role in endotoxin signaling, and can mediate the effects of endotoxin as well as other receptors.23 CD14, a LPS receptor on monocytes, macrophages, and neutrophils, has no intracellular domain but enhances signalling through another LPS receptor, toll-like receptor-4 (TLR4). Ligand engagement of TLR4 induces downstream signaling via recruitment of two molecular ways.24 The myeloid differentiation protein 88 (MyD88)-de-pendent pathway signals interleukin 1 receptor associated kinase 1/4, goes on to phosphorylate TNF receptor-associated factor 6, and stimulates nuclear factor kappa-B (NF-kB) and activator protein 1, which initiates the production of pro-inflammatory cytokines. On the other hand, the MyD88 independent pathway signals through TIR-domain-containing adapter-inducing interferon-β/translocation associated membrane protein, stimulates interferon regulatory factor 3 phosphorylation, and ultimately results in the stimulation of interferon (IFN)-inducible genes and the production of type I IFNs.25 On the contrary, the TLR4 deficiency improves hepatic steatosis induced by high-fat diets.26 These data globally support the hypothesis that the LPS/CD14 system has a role in the induction of the low-grade inflammation of metabolic disease, setting the threshold of insulin sensitivity and the onset of diabetes, obesity and cardiovascular complications.
The Role of Probiotics in Nafld: Which and WhenOn this topic there are several questions that are open in the research domain and in the clinical practice. The first is whether or which preparation of probiotics to prescribe? Another question is when to prescribe? Today, a large range of probiotic preparations is available in supermarkets and health food shops, which can also be available in internet. The global probiotic market generated US $15.9 billion in 2008 and is expected to be worth US$ 32.6 billion by 2014 with a compound annual growth rate of 12.6% from 2009 to 2014.27 There are drinks, yoghurts, powders and capsules. Powders and capsules have high numbers of bacteria but may lose a variable amount of these during the storage. Milk derivatives often present a lower number of bacteria when they are refrigerated. The use of certain types of bacteria in the probiotic formulations may depend on the historical use in foods by that society, even when clinical evidence is provided to support it. Such preparations differ greatly for bacterial number according to species and brand, which is generally > 106-108 CFU/g, or 108-1010 CFU/day.28,29Saccha-romyces cerevisiae boulardii and Escherichia coli nissle are widely used in European consumers. North America populations, frequently request Acidophilus when referring to probiotics with Lactobacillus. On the other hand, Asian cultures provide other bacteria, and for example in India there are products based on microbes such as Bacillus coagulans and in Japan they have used preparations containing Clostridium butyricum. In China and South Korea a combination of Enterococcus faecium R0026 and Bacillus subtilis R0179 has gained wide acceptance.30 The regulatory mechanism for probiotics differs from country to country and also even within a country.31 However international guidelines on probiotics in food broadly specify the kind of tests that may be required to determine the safety and to assess the effects on health.32
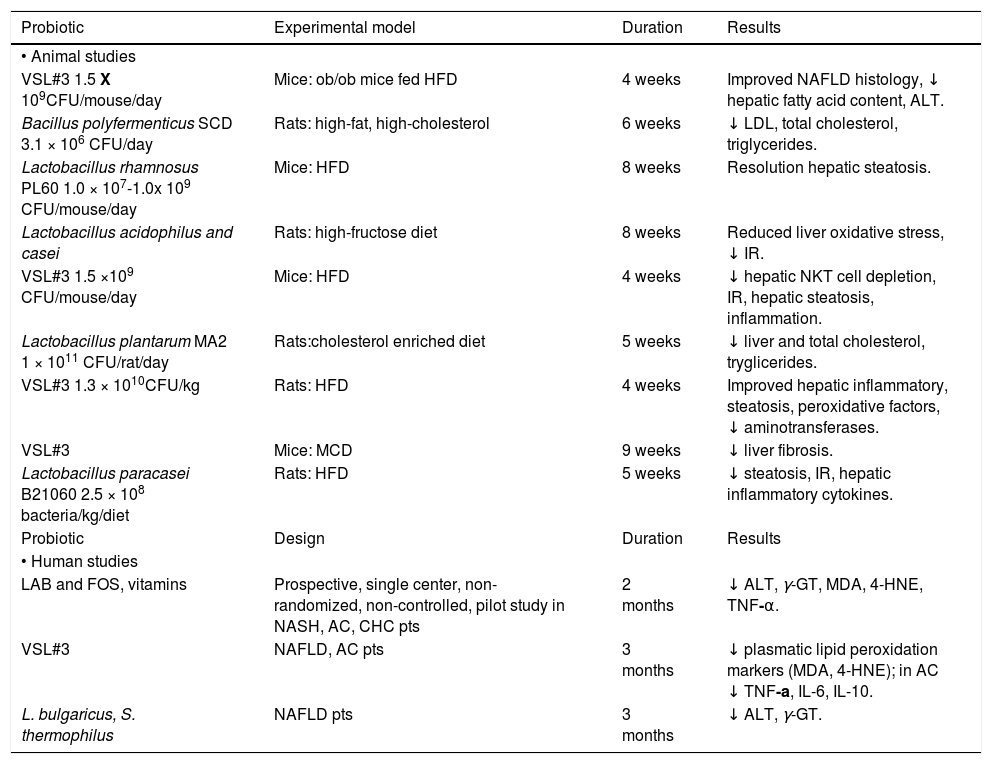
It is difficult to assess the real effect of probiotics in NAFLD prevention and treatment since evidences from literature arise from different animal models using different bacterial strains (Table 1). Among the probiotics available, several strains of lactobacillus have shown protective properties on NAFLD damage evolution.33 Eight-week oral administration of Lactobacillus rhamnosus PL60 has anti-obesity effect and leads to liver steatosis reduction in diet-induced obese mice, as histologically confirmed on liver specimens.34Lactobacillus acidophilus and Lactobacillus casei-treated mice fed with a high-fructose diet, a dietary model of type 2 diabetes associated with insulin-resistance (IR), hyper-insulinemia and hyper-triglyceridemia, showed a delayed onset of glucose intolerance, reduced insulinemia and liver glycogen, and improved steatosis, reducing malonyldialdehyde and increasing gluthathione content.35 A Chinese study has demonstrated that the administration of Lactobacillus plantarum MA2 in rats fed with an enriched cholesterol diet, beyond the hypolipidemic effect, reduces both liver cholesterol and triglycerides, and increases the number of fecal lactobacilli and bifidobacteria, as previously observed with Bacillus polyfermenticus.36,37
Probiotic strains used in animal and human studies.
| Probiotic | Experimental model | Duration | Results |
|---|---|---|---|
| • Animal studies | |||
| VSL#3 1.5 X 109CFU/mouse/day | Mice: ob/ob mice fed HFD | 4 weeks | Improved NAFLD histology, ↓ hepatic fatty acid content, ALT. |
| Bacillus polyfermenticus SCD 3.1 × 106 CFU/day | Rats: high-fat, high-cholesterol | 6 weeks | ↓ LDL, total cholesterol, triglycerides. |
| Lactobacillus rhamnosus PL60 1.0 × 107-1.0x 109 CFU/mouse/day | Mice: HFD | 8 weeks | Resolution hepatic steatosis. |
| Lactobacillus acidophilus and casei | Rats: high-fructose diet | 8 weeks | Reduced liver oxidative stress, ↓ IR. |
| VSL#3 1.5 ×109 CFU/mouse/day | Mice: HFD | 4 weeks | ↓ hepatic NKT cell depletion, IR, hepatic steatosis, inflammation. |
| Lactobacillus plantarum MA2 1 × 1011 CFU/rat/day | Rats:cholesterol enriched diet | 5 weeks | ↓ liver and total cholesterol, tryglicerides. |
| VSL#3 1.3 × 1010CFU/kg | Rats: HFD | 4 weeks | Improved hepatic inflammatory, steatosis, peroxidative factors, ↓ aminotransferases. |
| VSL#3 | Mice: MCD | 9 weeks | ↓ liver fibrosis. |
| Lactobacillus paracasei B21060 2.5 × 108 bacteria/kg/diet | Rats: HFD | 5 weeks | ↓ steatosis, IR, hepatic inflammatory cytokines. |
| Probiotic | Design | Duration | Results |
| • Human studies | |||
| LAB and FOS, vitamins | Prospective, single center, non-randomized, non-controlled, pilot study in NASH, AC, CHC pts | 2 months | ↓ ALT, γ-GT, MDA, 4-HNE, TNF-α. |
| VSL#3 | NAFLD, AC pts | 3 months | ↓ plasmatic lipid peroxidation markers (MDA, 4-HNE); in AC ↓ TNF-a, IL-6, IL-10. |
| L. bulgaricus, S. thermophilus | NAFLD pts | 3 months | ↓ ALT, γ-GT. |
IR: insulin resistance. MCD: methionine-choline deficient. CHC: chronic hepatitis. MDA: malondialdehyde. 4-HNE: 4-hydroxylnonenal. AC: alcoholic cirrhosis.
VSL#3 is a mixture of different strains (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei and Lactobacillus bulgaricus). Administration of VSL#3 for four weeks to ob/ob mice under high-fat diet, improves liver fat deposition analyzed histologically, reduces total fatty acids content and decreases amino-transferase plasma levels by down-regulation of c-Jun N-terminal kinase and NF-kB synthetic pathways (Figure 1).38 Moreover VSL#3 is able to improve insulin resistance and hepatic steatosis in the same mouse model.39 In fact, these probiotic strains are able to prevent natural killer T cells depletion induced by high fat diet. Moreover other studies have shown the anti-inflammatory, anti-oxidant and anti-fibro-genetic effects of these probiotics in mice.40
There are only a few pilot human studies on the efficacy of probiotics in NAFLD/NASH treatment and/or prevention. NASH biopsy proven patients have been treated with a symbiotic Lactobacillus acidophilus, bifidus rhamnosus, plantarum, salivarius, bulgaricus, lactis, casei, breve and fructo-oligosac-charides together with vitamins for sixty days.41 Subsequently, the same research group treated NAFLD with VSL#3 for three months.42 Both studies confirmed the same effects of probiotics, as shown in animal models with lowered aminotransferases, y-glutamyl transpeptidase, TNF-a levels, and improvement of oxidative stress markers, such as malondilaldehyde and 4-hydroxinonenal (Table 1). These data are perhaps confirmed from a recent meta-analysis by the Cochrane Library, assessing the absence of randomized placebo controlled trials in literature and the impossibility to give clear recommendations in probiotics use in the clinical treatment of NAFLD/NASH.43 To date the only placebo-randomized but not controlled clinical trial available in literature on the efficacy of probiotics in treatment of biopsy-proven steatosis, was conducted in NALFD patients randomized to one tablet per day with 500 million of Lactobacillus bulgaricus and Streptococcus thermophilus or to one placebo tablet (120 mg of starch). The first group of patients showed a significant decrease of both alanine- and aspartate-aminotransferases and gamma-glutamine transferases compared to placebo.44
ConclusionNAFLD is a common and serious disease. Literature reports the central role of the gut-liver axis in the pathogenesis of NAFLD and recently, suggests that the microbiota is a casual factor of the disease, involved in the interactions between lumen and liver. In fact, the change in the equilibrium of the intestinal microflora, associated with increased intestinal permeability, can experimentally induce progression of NAFLD, mediated by several bacterial products, such as LPS. Probiotic are commensal bacteria, able to modulate intestinal microbiota with benefits for the health of humans. There is no substitute for eating the right foods and getting regular exercise for preventing fat accumulation in the liver. Adding probiotics to lifestyle standards may increase help efforts towards a lean liver. However, today a species with higher efficacy in this disease has not been identified. The administration of Lactobacillus species is very promising. It is our opinion that, larger, long-term, biopsy-controlled, double-blind prospective clinical trials are needed to build up recommendations in probiotics use and to define a new, safe, well-tolerated and natural treatment of NAFLD. So far any recommendation about which and when is not yet possible for their use both in treatment and prevention of liver steatosis.
Abbreviations- •
IFN: interferon.
- •
IR: insulin-resistance.
- •
LPS: lipopolysaccharide.
- •
MyD88: myeloid differentiation protein 88.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
NF-kB: nuclear factor kappa-B.
- •
SIBO: small intestinal bacterial overgrowth.
- •
TLR4: toll-like receptor-4.
- •
TNF-a: tumor necrosis factor-a.
There are no conflicts of interest associated with this work.