Introduction and aim. There is scarce information about primary prophylaxis in cirrhotic patients. The aim was to assess the efficacy of ciprofloxacin for primary prophylaxis for bacterial infections in patients with cirrhosis of the liver and ascites.
Material and methods. A randomized, double-blind placebo-controlled clinical trial was conduced. Patients were randomized to receive oral ciprofloxacin 500 mg/day or placebo for one month. A basal evaluation and repeated assessments at 4, 6, 12, 18, and 24 weeks afterwards, or whenever a primary endpoint occurred were done. Statistical analysis: probability curves were constructed with the Kaplan-Meier method and compared by the log-rank test.
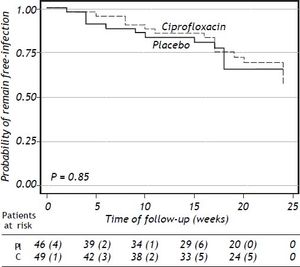
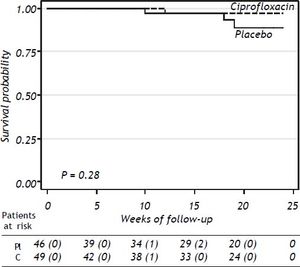
Results. 95 patients were randomized to ciprofloxacin group (n = 49; 51.6%) and placebo group (n = 46; 48.4%). Six-teen (32.6%) patients in the ciprofloxacin group developed bacterial infections and thirteen (28.2%) patients developed bacterial infections in the placebo group (p = NS). The probability to remain free of bacterial infections did not reach statistical significance (p = 0.38). Probability of survival at 24 weeks was 91% in placebo group and 98% in the ciprofloxacin group (p = 0.28). The absolute risk reduction was 5%, the relative risk reduction was 6% and the NNT was 20 patients.
Conclusion. Primary prophylaxis with ciprofloxacin for one month in cirrhotic patients with ascites who do not have a currently accepted indication, did not show a preventive effect on the development of bacterial infections at one month follow-up. Moreover in women could increases the odds for UTI. The administration of ciprofloxacin seemed to decrease the risk of mortality.
Bacterial infections account for 30 to 50% hospital admissions among patients living with cirrhosis of the liver, and a high risk of mortality (50%) despite resolution of the infection.1,2 Important progress regarding prophylaxis and treatment of spontaneous bacterial peritonitis (SBP) has been made in previous years.3–9 Up now, secondary prophylaxis of SBP has been strongly recommended for patients with previous SBP because they are at high risk of SBP recurrence and death (32-70%) at one year.3,10–12 Data from a recent meta-analysis indicate that short-term antibiotic prophylaxis decreases the infection rate and improve survival in patients with gastrointestinal hemorrhage.13 However, there is scarce information about primary prophylaxis, since the role of antibiotics is controversial and the only risk-factor considered for development SBP is poor protein concentration in the ascitic fluid.4,7,14,15
There is growing evidence about different bacterial infections in addition to SBP play an important role in morbidity and mortality in cirrhotic patients.2,16,17 In a systematic review,2 the mortality without infection was 13.6% (18 cohorts, 2,317 patients) vs. the 40% mortality in patients with infection. The pooled odds ratio for mortality with infection was 3.75 (95%CI, 2.12-4.23). Regardless of these data, up to now all studies have focused on the evaluation of prophylaxis specifically for SBP. The main mechanism involved in the development of bacterial infections in cirrhotic patients with ascites is bacterial translocation. Considering ascites as a marker of decompensated cirrhosis and bacterial translocation as the main factor associated with infection, it is possible that patients with ascites without any other complication could benefit with the use of primary prophylaxis.
The primary aim of the present study was to assess the efficacy of oral administration of ciprofloxacin for primary prophylaxis for bacterial infections in patients with cirrhosis of the liver and ascites, without any indication for SBP prophylaxis currently accepted. Secondly, basal concentrations of proinflammatory and anti-inflammatory cytokines were determined and evaluated after administration of ciprofloxacin.
Material and MethodsThis was a randomized, double-blind placebo-controlled clinical trial. The Institutional Human Biomedical Research Committee approved the study protocol. We included patients aged from 19 to 79 years, who were able to give written informed consent.
PatientsOne hundred and seventy seven patients with cirrhosis of the liver and ascites were screened from April 2008 to November 2009. Diagnosis of cirrhosis was supported by means of clinical (jaundice, ascites, hepatic encephalopathy, evidence of portal hypertension, variceal hemorrhage), laboratory (abnormal liver function test as decreased serum albumin, elevated serum globulins, prolonged prothromin time, elevated serum bilirubin, elevated serum aminotransferases), ultrasound (hyperechoic hepatic parenchyma, heterogeneous liver, nodularity of the liver surface, and selective enlargment of the caudate lobe) and/or histologic data (diffuse involvement of the liver with progressive fibrosis with nodule formation and distortion of the hepatic architecture). Patients were excluded if cirrhosis was due to autoinmmune disease, history of SBP, active gastrointestinal bleeding, total protein in ascitic fluid < 1.5 g/dL, use of antibiotics within the last 30 days, pregnancy, encephalopathy ≥ grade 2, immune-related comorbidities, immunesuppressive therapy, hepatocarcinoma or other malignancies, allergy to fluoroquinolones, and bacterial infection at the time of enrollment.
Eligible patients were randomized to receive oral ciprofloxacin 500 mg/day (Ciproflox, Laboratorios Senosiain, S.A. de C.V., México) or 500 mg/day of an equally appearing placebo for one month, both ciprofloxacin and placebo tablets had the same appearance, and were dispensed in undistinguishable containers. A random allocation sequence was generated and kept in a sealed envelope until occurrence of a severe adverse event or end of trial. Treatment containers were numbered with consecutive numbers and assigned to patients on a first-come-firstserve basis. Upon enrollment, physical examination and laboratory tests (liver and renal function tests, red and white cell counts, platelet count, and prothrombin time) were performed. The same assessment was repeated 4, 6, 12, 18, and 24 weeks afterwards, or whenever a primary endpoint occurred. Enrolled patients continued with their regular baseline medications during the six-month follow-up of the trial. Compliance with the study medication was assessed by tablet counts at the end of the four-week treatment. Patients taking the study medication for less than two weeks were considered as non-compliers and were withdrawn from the per-protocol analysis. Study medication was discontinued when a primary endpoint occurred. Patients with encephalopathy secondary to dietary transgression, constipation, or diuretic use continued in the study under treatment adjustments.
Infection, gastrointestinal (GI) bleeding, hepatic encephalopathy, severe adverse event and death were the trial outcomes of interest. An infection was suspected when fever, abdominal pain, urinary or respiratory symptoms were present. SBP was diagnosed if ≥ 250 PMN/mm3 ascites were detected.18,19 Urinary tract infection (UTI) was diagnosed if dysuria, frequency, and/or urgency, flank pain and/or fever, and confirmed by culture of urine.20,21 Lower respiratory-tract infection was diagnosed if breath sound, localized rales, and/or acute infiltrate on a chest X-ray were accompanied at least two of the following: fever or hypothermia, rigors, sweats, new cough with or without sputum, chest discomfort or dyspnea.22 If localized rales and/or acute infiltrate on a chest X-ray were absent, then the disease was considered as upper respiratory-tract infection. Spontaneous bacteremia was diagnosed on the basis of systemic inflammatory response and a positive blood culture in absence of a recognized primary infection.23 An infection was considered as severe if hospitalization was required. Occurrence of a primary endpoint was taken as a study failure, implicating interruption of the study medication and initiation of the proper standard care required by the patient.
Lipopolysaccharide and cytokine assaysPeripheral venous blood (20 mL) was collected with heparinized sterile pyrogen-free disposable syringes (Becton Dickinson, Mississauga, Ontario, Ca).
Plasmatic lipopolysaccharideThe plasma endotoxin was determined using the quantitative chromogenic assay of limulus amoebocyte lysate (LAL) QCL-1000, (Biowhitakker, Inc Walkersville, MD, USA) according to the manufacturer’s instructions: endotoxin inhibitors were removed by diluting the plasma 1:10 with pyrogen-free water, and heating the samples to 70 °C for 5 min. The pH of the samples was adjusted in a range of 7-8 through the use of sodium hydroxide solution and hydrochloric acid 0.1N 0.1N. In a sterile microplate was placed standards (7 standards prepared a stock solution of Escherichia coli endotoxin provided by Biowhitakker), blank, and duplicate samples. LAL will be added and incubated the plate for 10 min. Chromogenic substrate was added (preheated to 37 ± 1.0 °C) and incubated the plate for 6 min. Finally, glacial acetic acid was added 25% to stop the reaction. The reaction was carried out at 37 ± 1.0°C. Optical density was read in microplate reader at a wavelength of 405-410 nm.
CytokinesConcentrations of TNF, IL-1, IL-6, IL-12 and IL-10 were determined by enzyme-linked immunosorbent assay (ELISA) (OptEIA™, BD Pharmingen, San Diego, CA, USA) according to the manufacturer,s instructions. Detection limits for each assay were 4 pg/mL for TNF, IL-1β, IL-6, and IL-10, and 15 pg/mL for IL-12. In each patient, every test was done in duplicate. The polystyrene microwell plate was covered by a specific monoclonal antibody against each of these cytokines. Culture medium was added or cytokine standard solutions and incubated for 3 h (approximately). Adding a second specific polyclonal antibody. Finally, a chromogenic solution was added. The absorbance was measured at 450 nm and concentrations (pg/mL) of cytokines were obtained based on the standard curve.
Statistical analysis and sample sizeSample size was estimated assuming a 25% difference in the infection incidence between the ciprofloxacin and placebo groups.4,6–8 To detect this difference at a 5% significance level (one sided) with an 80% power, 48 patients per group needed to be assessed considering a 20% of missing data. Results were summarized as means ± standard deviations (SD), medians (minimum and maximum values), or absolute frequencies (%), as appropriate. Probability curves were constructed with the Kaplan-Meier method and compared by the log-rank test. Differences between ciprofloxacin and placebo groups were analyzed by means of the Student’s t, MannWhitney U, chi-square, Fisher’s exact or Mantel-Haenszel tests. Intra-group repeated observations were analyzed by means of the Friedman test. All analyses were carried on both intention-to-treat and per-protocol basis. A P < 0.05 was considered as statistically significant’ and a Bonferroni adjustment was used in multiple pairwise comparisons. All statistical procedures were conducted with The Stata/Mac 10.0 software.
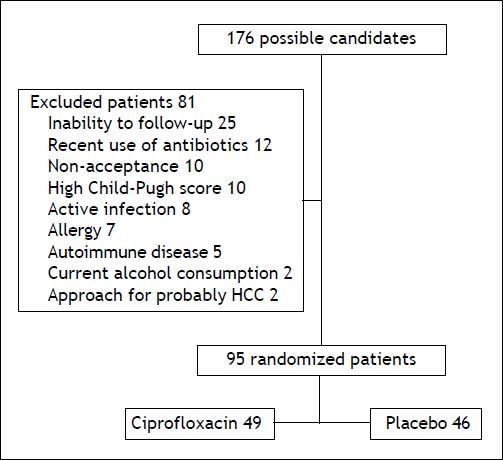
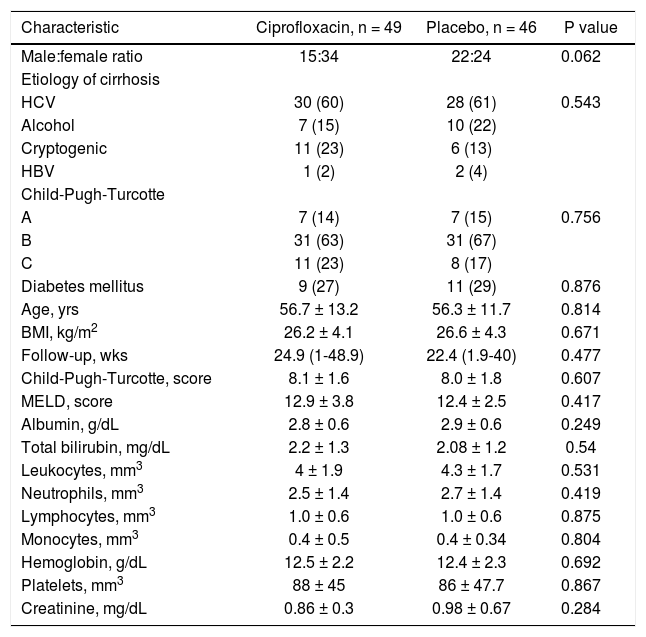
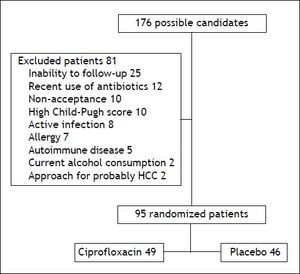
ResultsA total of 176 patients were evaluated, eighty-one were excluded (Figure 1) and the remaining 95 were randomized to ciprofloxacin group (n = 49; 51.6%) and placebo group (n = 46; 48.4%). Table 1 shows the clinical and laboratory data at baseline. The mean follow-up in the ciprofloxacin and placebo groups was 18.5 (1-24) weeks and 18 (1-24) weeks, respectively (p = NS). In table 2 are shown the clinical and laboratory characteristics at follow-up.
Baseline clinical and biochemical characteristics by treatment group.
| Characteristic | Ciprofloxacin, n = 49 | Placebo, n = 46 | P value |
|---|---|---|---|
| Male:female ratio | 15:34 | 22:24 | 0.062 |
| Etiology of cirrhosis | |||
| HCV | 30 (60) | 28 (61) | 0.543 |
| Alcohol | 7 (15) | 10 (22) | |
| Cryptogenic | 11 (23) | 6 (13) | |
| HBV | 1 (2) | 2 (4) | |
| Child-Pugh-Turcotte | |||
| A | 7 (14) | 7 (15) | 0.756 |
| B | 31 (63) | 31 (67) | |
| C | 11 (23) | 8 (17) | |
| Diabetes mellitus | 9 (27) | 11 (29) | 0.876 |
| Age, yrs | 56.7 ± 13.2 | 56.3 ± 11.7 | 0.814 |
| BMI, kg/m2 | 26.2 ± 4.1 | 26.6 ± 4.3 | 0.671 |
| Follow-up, wks | 24.9 (1-48.9) | 22.4 (1.9-40) | 0.477 |
| Child-Pugh-Turcotte, score | 8.1 ± 1.6 | 8.0 ± 1.8 | 0.607 |
| MELD, score | 12.9 ± 3.8 | 12.4 ± 2.5 | 0.417 |
| Albumin, g/dL | 2.8 ± 0.6 | 2.9 ± 0.6 | 0.249 |
| Total bilirubin, mg/dL | 2.2 ± 1.3 | 2.08 ± 1.2 | 0.54 |
| Leukocytes, mm3 | 4 ± 1.9 | 4.3 ± 1.7 | 0.531 |
| Neutrophils, mm3 | 2.5 ± 1.4 | 2.7 ± 1.4 | 0.419 |
| Lymphocytes, mm3 | 1.0 ± 0.6 | 1.0 ± 0.6 | 0.875 |
| Monocytes, mm3 | 0.4 ± 0.5 | 0.4 ± 0.34 | 0.804 |
| Hemoglobin, g/dL | 12.5 ± 2.2 | 12.4 ± 2.3 | 0.692 |
| Platelets, mm3 | 88 ± 45 | 86 ± 47.7 | 0.867 |
| Creatinine, mg/dL | 0.86 ± 0.3 | 0.98 ± 0.67 | 0.284 |
Categorical variables are expressed as n (%) and continuous numerical as (x ± s) or median (range). HCV: hepatitis C virus. HBV: hepatitis B virus. BMI: body mass index. MELD: Model for End-Stage Liver Disease.
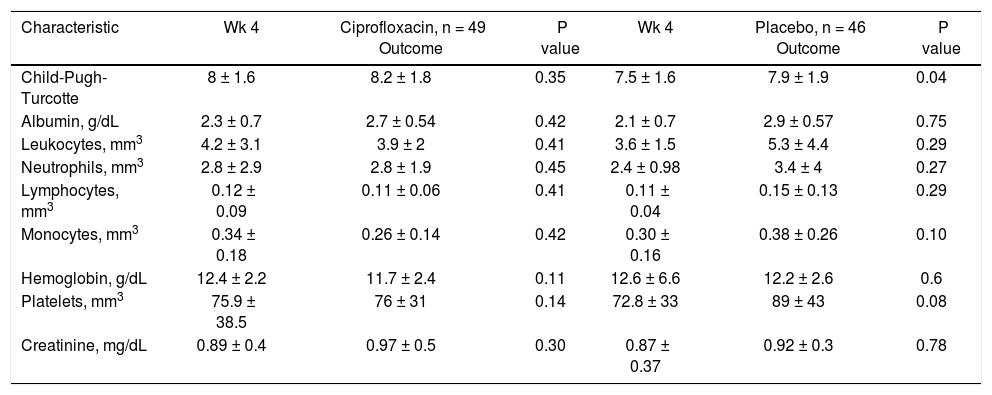
Clinical and biochemical characteristics by treatment group at follow-up.
| Characteristic | Wk 4 | Ciprofloxacin, n = 49 Outcome | P value | Wk 4 | Placebo, n = 46 Outcome | P value |
|---|---|---|---|---|---|---|
| Child-Pugh-Turcotte | 8 ± 1.6 | 8.2 ± 1.8 | 0.35 | 7.5 ± 1.6 | 7.9 ± 1.9 | 0.04 |
| Albumin, g/dL | 2.3 ± 0.7 | 2.7 ± 0.54 | 0.42 | 2.1 ± 0.7 | 2.9 ± 0.57 | 0.75 |
| Leukocytes, mm3 | 4.2 ± 3.1 | 3.9 ± 2 | 0.41 | 3.6 ± 1.5 | 5.3 ± 4.4 | 0.29 |
| Neutrophils, mm3 | 2.8 ± 2.9 | 2.8 ± 1.9 | 0.45 | 2.4 ± 0.98 | 3.4 ± 4 | 0.27 |
| Lymphocytes, mm3 | 0.12 ± 0.09 | 0.11 ± 0.06 | 0.41 | 0.11 ± 0.04 | 0.15 ± 0.13 | 0.29 |
| Monocytes, mm3 | 0.34 ± 0.18 | 0.26 ± 0.14 | 0.42 | 0.30 ± 0.16 | 0.38 ± 0.26 | 0.10 |
| Hemoglobin, g/dL | 12.4 ± 2.2 | 11.7 ± 2.4 | 0.11 | 12.6 ± 6.6 | 12.2 ± 2.6 | 0.6 |
| Platelets, mm3 | 75.9 ± 38.5 | 76 ± 31 | 0.14 | 72.8 ± 33 | 89 ± 43 | 0.08 |
| Creatinine, mg/dL | 0.89 ± 0.4 | 0.97 ± 0.5 | 0.30 | 0.87 ± 0.37 | 0.92 ± 0.3 | 0.78 |
Continuous numerical variables are expressed as (x ± s). Wk: week.
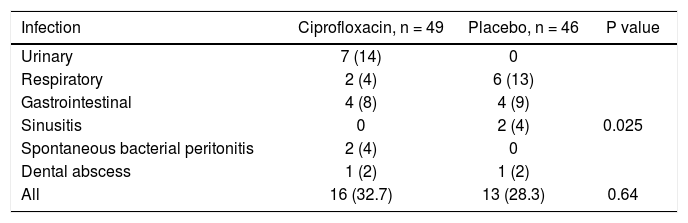
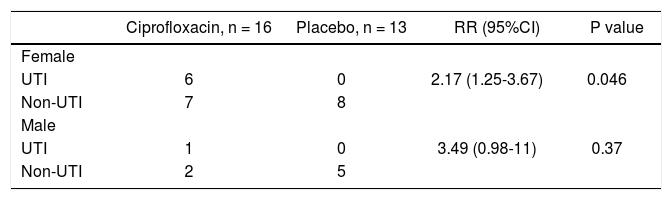
Sixteen (32.7%) patients in the ciprofloxacin group developed bacterial infections and thirteen (28.3%) in the placebo group (p = NS). Table 3 shows the episodes of infection in each group. Interestingly, UTI were more frequent in patients in the ciprofloxacin group with a statistical significance in relationship to women (P = 0.04) (Tables 3 and 4).
Incidence and type of infection in each group, n (%).
| Infection | Ciprofloxacin, n = 49 | Placebo, n = 46 | P value |
|---|---|---|---|
| Urinary | 7 (14) | 0 | |
| Respiratory | 2 (4) | 6 (13) | |
| Gastrointestinal | 4 (8) | 4 (9) | |
| Sinusitis | 0 | 2 (4) | 0.025 |
| Spontaneous bacterial peritonitis | 2 (4) | 0 | |
| Dental abscess | 1 (2) | 1 (2) | |
| All | 16 (32.7) | 13 (28.3) | 0.64 |
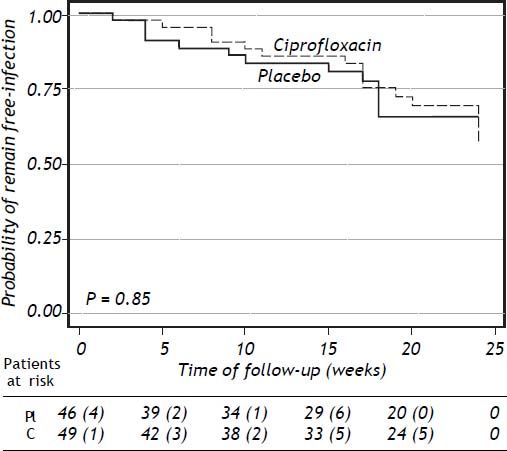
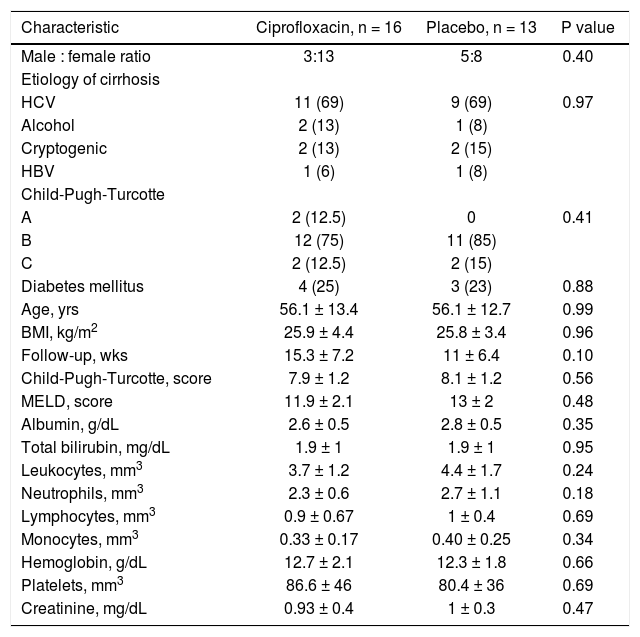
Clinical and laboratory data at baseline of patients who developed a bacterial infection are shown in table 5 (supplementary data). The probability to remain free of infections did not reach statistical significance (p = 0.85) (Figure 2).
Baseline clinical and biochemical characteristics of patients who developed an infectious event according to treatment.
| Characteristic | Ciprofloxacin, n = 16 | Placebo, n = 13 | P value |
|---|---|---|---|
| Male : female ratio | 3:13 | 5:8 | 0.40 |
| Etiology of cirrhosis | |||
| HCV | 11 (69) | 9 (69) | 0.97 |
| Alcohol | 2 (13) | 1 (8) | |
| Cryptogenic | 2 (13) | 2 (15) | |
| HBV | 1 (6) | 1 (8) | |
| Child-Pugh-Turcotte | |||
| A | 2 (12.5) | 0 | 0.41 |
| B | 12 (75) | 11 (85) | |
| C | 2 (12.5) | 2 (15) | |
| Diabetes mellitus | 4 (25) | 3 (23) | 0.88 |
| Age, yrs | 56.1 ± 13.4 | 56.1 ± 12.7 | 0.99 |
| BMI, kg/m2 | 25.9 ± 4.4 | 25.8 ± 3.4 | 0.96 |
| Follow-up, wks | 15.3 ± 7.2 | 11 ± 6.4 | 0.10 |
| Child-Pugh-Turcotte, score | 7.9 ± 1.2 | 8.1 ± 1.2 | 0.56 |
| MELD, score | 11.9 ± 2.1 | 13 ± 2 | 0.48 |
| Albumin, g/dL | 2.6 ± 0.5 | 2.8 ± 0.5 | 0.35 |
| Total bilirubin, mg/dL | 1.9 ± 1 | 1.9 ± 1 | 0.95 |
| Leukocytes, mm3 | 3.7 ± 1.2 | 4.4 ± 1.7 | 0.24 |
| Neutrophils, mm3 | 2.3 ± 0.6 | 2.7 ± 1.1 | 0.18 |
| Lymphocytes, mm3 | 0.9 ± 0.67 | 1 ± 0.4 | 0.69 |
| Monocytes, mm3 | 0.33 ± 0.17 | 0.40 ± 0.25 | 0.34 |
| Hemoglobin, g/dL | 12.7 ± 2.1 | 12.3 ± 1.8 | 0.66 |
| Platelets, mm3 | 86.6 ± 46 | 80.4 ± 36 | 0.69 |
| Creatinine, mg/dL | 0.93 ± 0.4 | 1 ± 0.3 | 0.47 |
Categorical variables are expressed as n (%) and continuous numerical as (x ± s). HCV: hepatitis C virus. HBV: hepatitis B virus. BMI: body mass index. MELD: Model for End-Stage Liver Disease.
A positive culture was found in 10 patients: 7 urine (E. coli), 2 in ascites (E. coli), and 1 in sputum (Staphylococcus aureus). All patients with positive cultures, except one (S. aureus), belonged to the ciprofloxacin group. E. coli resistant to ciprofloxacin was found in 6/7 patients with UTI, all of them required IV ceftriaxone and cured.
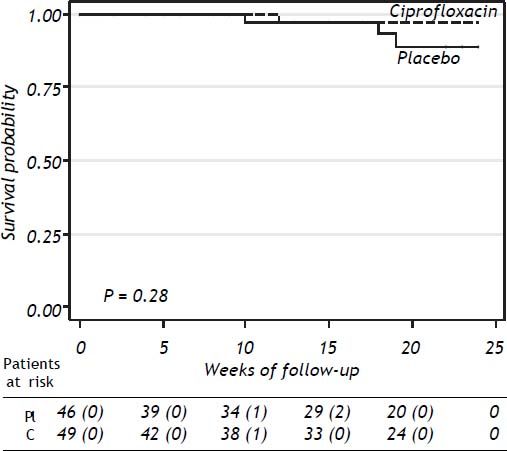
SurvivalThe probability of survival at 24 weeks was lower in the placebo group compared to patients receiving ciprofloxacin, without statistical significance (P = 0.28) (Figure 3). Three patients in the placebo group and one in the ciprofloxacin group died during the study period, all of them because of variceal bleeding. The patients in the placebo group died at weeks 10, 18, and 19 of follow-up’ respectively. The patient in the ciprofloxacin group died at week 12. Probability of survival at 24 weeks was 91% in placebo group and 98% in the ciprofloxacin group.
Compliance and side effectsFour patients in the ciprofloxacin group and seven patients in the placebo group were lost during follow-up. Five patients in the ciprofloxacin group had nausea transiently but none of them required to stop medication. There were no complications directly related to the use of ciprofloxacin or placebo. The significance of rate of infections (P = 0.83) and survival probability (P = 0.26) were not modified by per-protocol analysis.
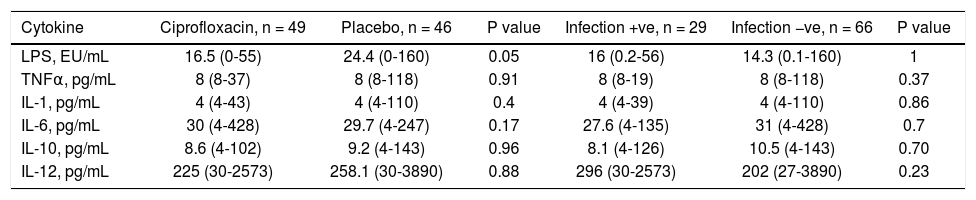
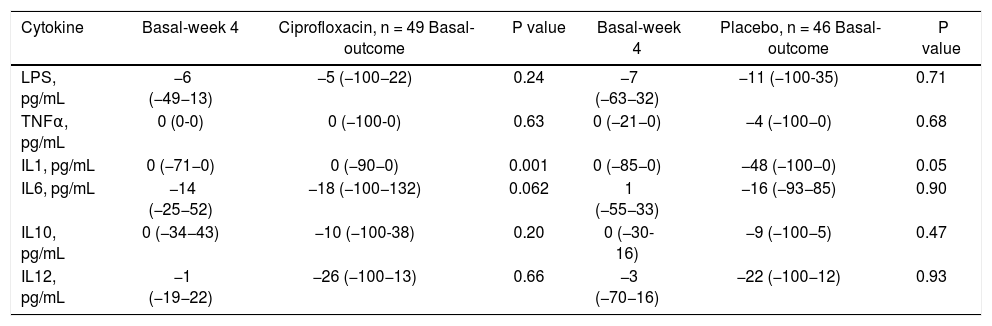
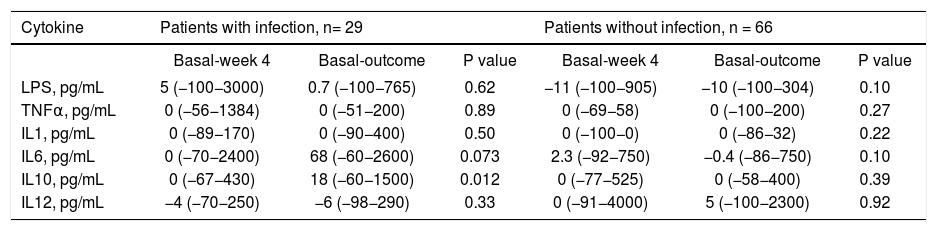
Lipopolysaccharides and cytokinesThere were no differences in serum levels between ciprofloxacin group vs. placebo group (Table 6) or patients who did not develop an infection compared with patients whose developed an infection during follow up (Table 6). We found significant differences in the time of mean values for IL-1 (both groups; ciprofloxacin vs placebo; data shown in table 7). In the case of IL-10, patients who developed infections had higher levels at follow-up, and a tendency in the case of IL-6 (Table 8).
Baseline serum LPS and cytokine levels by treatment group and infectious event.
| Cytokine | Ciprofloxacin, n = 49 | Placebo, n = 46 | P value | Infection +ve, n = 29 | Infection −ve, n = 66 | P value |
|---|---|---|---|---|---|---|
| LPS, EU/mL | 16.5 (0-55) | 24.4 (0-160) | 0.05 | 16 (0.2-56) | 14.3 (0.1-160) | 1 |
| TNFα, pg/mL | 8 (8-37) | 8 (8-118) | 0.91 | 8 (8-19) | 8 (8-118) | 0.37 |
| IL-1, pg/mL | 4 (4-43) | 4 (4-110) | 0.4 | 4 (4-39) | 4 (4-110) | 0.86 |
| IL-6, pg/mL | 30 (4-428) | 29.7 (4-247) | 0.17 | 27.6 (4-135) | 31 (4-428) | 0.7 |
| IL-10, pg/mL | 8.6 (4-102) | 9.2 (4-143) | 0.96 | 8.1 (4-126) | 10.5 (4-143) | 0.70 |
| IL-12, pg/mL | 225 (30-2573) | 258.1 (30-3890) | 0.88 | 296 (30-2573) | 202 (27-3890) | 0.23 |
Values are expressed as median (range). LPS: lipopolysaccharide. TNFa: tumor necrosis factor alpha. IL-1: interleukin 1. IL-6: interleukin 6. IL-10: interleukin 10. IL-12: interleukin 12.
Differences in serum levels of LPS and cytokines at week 4 and the outcome compared to baseline for patients classified according to the received maneuver (expressed in percentage change).
| Cytokine | Basal-week 4 | Ciprofloxacin, n = 49 Basal-outcome | P value | Basal-week 4 | Placebo, n = 46 Basal-outcome | P value |
|---|---|---|---|---|---|---|
| LPS, pg/mL | −6 (−49−13) | −5 (−100−22) | 0.24 | −7 (−63−32) | −11 (−100-35) | 0.71 |
| TNFα, pg/mL | 0 (0-0) | 0 (−100-0) | 0.63 | 0 (−21−0) | −4 (−100−0) | 0.68 |
| IL1, pg/mL | 0 (−71−0) | 0 (−90−0) | 0.001 | 0 (−85−0) | −48 (−100−0) | 0.05 |
| IL6, pg/mL | −14 (−25−52) | −18 (−100−132) | 0.062 | 1 (−55−33) | −16 (−93−85) | 0.90 |
| IL10, pg/mL | 0 (−34−43) | −10 (−100-38) | 0.20 | 0 (−30-16) | −9 (−100−5) | 0.47 |
| IL12, pg/mL | −1 (−19−22) | −26 (−100−13) | 0.66 | −3 (−70−16) | −22 (−100−12) | 0.93 |
P25: percentile 25. P75: percentile 75. LPS: lipopolysaccharide. TNF: tumor necrosis factor alpha. IL-1: interleukin 1. IL-6: interleukin 6. IL-10: interleukin 10. IL-12: interleukin 12.
Differences between serum concentrations of LPS and cytokines at week 4 and the outcome compared to baseline for patients classified according to the development of infection (expressed in percentage change).
| Cytokine | Patients with infection, n= 29 | Patients without infection, n = 66 | ||||
|---|---|---|---|---|---|---|
| Basal-week 4 | Basal-outcome | P value | Basal-week 4 | Basal-outcome | P value | |
| LPS, pg/mL | 5 (−100−3000) | 0.7 (−100−765) | 0.62 | −11 (−100−905) | −10 (−100−304) | 0.10 |
| TNFα, pg/mL | 0 (−56−1384) | 0 (−51−200) | 0.89 | 0 (−69−58) | 0 (−100−200) | 0.27 |
| IL1, pg/mL | 0 (−89−170) | 0 (−90−400) | 0.50 | 0 (−100−0) | 0 (−86−32) | 0.22 |
| IL6, pg/mL | 0 (−70−2400) | 68 (−60−2600) | 0.073 | 2.3 (−92−750) | −0.4 (−86−750) | 0.10 |
| IL10, pg/mL | 0 (−67−430) | 18 (−60−1500) | 0.012 | 0 (−77−525) | 0 (−58−400) | 0.39 |
| IL12, pg/mL | −4 (−70−250) | −6 (−98−290) | 0.33 | 0 (−91−4000) | 5 (−100−2300) | 0.92 |
P25: percentile 25. P75: percentile 75. LPS: lipopolysaccharide. TNF: tumor necrosis factor alpha. IL-1: interleukin 1. IL-6: interleukin 6. IL-10: interleukin 10. IL-12: interleukin 12.
The results of this clinical trial do not support the efficacy of primary prophylaxis with oral ciprofloxacin against bacterial infections in patients with cirrhosis of the liver and ascites in absence of a formal indication currently accepted (low protein ascitic fluid concentration). Furthermore, it is possible that in female patients the administration of quinolones could be deleterious. The use of ciprofloxacin in this group of patients reduces the mortality rate, a major point that scarcely has been reported on primary prophylaxis.4 Patients receiving placebo presented three times more deaths than patients receiving ciprofloxacin. Finally, the use of ciprofloxacin does not warrant a significant influence over LPS or cytokines serum levels.
The use of antibiotics as prophylaxis in patients with cirrhosis of the liver has been studied previously and specific criteria required including a particular patient as candidate.3,4,7–9,13,24,25 Regarding primary prophylaxis, these studies except by one,7 were designed to evaluate the use of antibiotics to prevent SPB exclusively; however there is evidence regarding that practically any bacterial infection worsens morbidity and mortality in patients with cirohsis of the liver.2,26 The clinical trial was designed to evaluate the prevention of bacterial infections as a whole group. Because patients with cirrhosis and ascites regularly develop some immunologic deficiencies18,19,27–30 that predispose them to acquire bacterial infections with a higher risk of morbidity and mortality in compsrison with the general population,2 we evaluated the efficacy of primary prophylaxis with ciprofloxacin in patients without any currently accepted indication for primary prophylaxis. We found no significant difference in the incidence of bacterial infections in patients who received ciprofloxacin compared to those receiving placebo (p = 0.64; table 3). An interesting finding was that female patients under ciprofloxacin had UTI more often those female patients in placebo group; this phenomenon was not observed in male patients (Table 4). Although, UTI in patients in the ciprofloxacin group were not more severe than in patients in the placebo group, it is well known that UTI are more frequently seen in women, thereby the use of ciprofloxacin in this group patients could be an independent risk factor for UTI. The rate of infection in patients with cirrhosis of the liver in previous studies waries from 13 to 40% with primary prophylaxis and 24-58% in the placebo groups.4,6–8 Our results are, in general, consistent with these findings, since we did not find differences between groups.
The rate of mortality, altought not statistically significant, was three times higher in patients in the placebo group compared to the ciprofloxacin group (Figure 3, P = NS) although it is possible that this difference could be more evident with a longer follow-up. Mortality in this study is lower than previously reported4,6–8 among patients in similar conditions. In the study by Fernandez, et al.8 mortality after three months of follow-up was reported and it is similar with our results. We evaluated the administration of ciprofloxacin only during one month; hence it is possible that different lengths of ciprofloxacin administration could have better outcomes. The treatment groups included in previous studies were receiving antibiotics by longer time.4,6–8 Importantly, 6 out of 7 patients with positive urine culture in the ciprofloxacin group had documented E. coli resistant to ciprofloxacin; these patients were treated with ceftriaxone with good results. We did not observe differences between ciprofloxacin and placebo in relation with complications such as gastrointestinal bleeding, hepatic encephalopathy, or treatment compliance.
The administration of ciprofloxacin did not show any considerable effect over LPS or cytokines levels. Previous studies have shown variable LPS serum levels and this lack of consistence seems to be related with the short life of the molecule31 suggesting that this is not a reliable marker of bacterial translocation. Therefore, different surrogate markers of bacterial translocation have been searched, being LBP (lypopolysacharide-binding protein) serum determination the procedure with the best performance.23,31 IL-6 is a pro-inflammatory cytokine produced in response to persistent bacterial translocation present in cirrhotic patients with ascites23,31 and it is known that this substance is involved in cellular damage, hepatocytes death, cholestasis, and hepatic fibrosis.29,30 Regarding IL-10, as a response to persistent and consistent bacterial translocation, evidenced by the basal elevation of LPS levels and pro-inflammatory cytokines, a high secretion of IL-10 (anti-inflammatory cytokine) could be expected in patients who developed an infection on the follow-up. The variability in serum levels of cytokines may be related to different factors related to the characteristics of the patients included in previous studies. In a study of Albilos, et al. were included healthy controls, cirrhotic patients without ascites, and cirrhotic patients with ascites. Serum levels of TNF and IL-6 in the patients included in this study are similar to those previously reported in the group for cirrhotic patients with ascites and high serum levels of LBP. In the study of Berry et al were reported much higher values of TNF, IL-6, and IL-10 than those found in the work of Albilos, et al. and in our own study, this difference may be explained because they included patients with decompensated cirrhosis and acute complication at time of enrollment.30
Because translocation is associated with the migration of resident intraluminal bacteria to the bloodstream, intestinal decontamination with antibiotics may have an impact in serum levels of pro-inflammatory cytokines. Nevertheless, our results do not support this hypothesis in patients with “compensate” ascites. This could be related with life of these cytokines in the bloodstream; the presence of different stimuli for cytokine production, as well as a vigorous but permanent response to “previous” LPS exposure. Regarding IL-1 serum levels, the values obtained were unstable in both groups the explanation of this seems to be a instability of the cytokines, rather than the use of ciprofloxacin. In the case of IL-6 and IL-10 we observed significant differences before-after in patients who developed an infection (Table 8). This variability in serum concentrations were not observed related to ciprofloxacin administration (Table 7). An important found was that when patients with more severe infectious diseases (SBP, pneumonia) were analyzed as a group and compared with other groups, no differences were founded regarding LPS or cytokine profiles.
Some limitations of our study have to be considered:
- •
Patients with different etiologies were included.
- •
We did not include patients with higher ChildPugh scores (specifically patients with 14 and 15 points).
- •
Time of ciprofloxacin administration was relatively short.
Enrollment of patients with different etiologies of cirrhosis makes it possible to erroneously study different immunologic compromise. Although some patients (e.g. with HCV infection) have extra-hepatic manifestations mediated by immunologic phenomena, so far our knowledge, there is no existing data supporting the idea that this group is more prone to infections in comparison with cirrhotic patients with different etiologies. Patients with cirrhosis of the liver living with known immunologic alterations because of the etiology (AIH, PBC, overlap syndrome) or the treatments (immune suppressors) were not included. We decided to exclude patients with currently accepted indication for primary prophylaxis because significant evidence exists, and we considered more relevant to study different groups in which the effect of ciprofloxacin (or other antibiotics) as primary prophylaxis could be useful but until that moment unknown. Because of this uncertainty, and although previous studies have used prophylactic antibiotics for longer periods safely,3,4,6–8,25 we decided to use ciprofloxacin for a time that would minimize the likelihood of side effects, specifically secondary infections caused by resistant bacteria or fungi. It is possible that future clinical trials with different regimens of prophylaxis show a favorable effect on the development of infections and mortality in this group of patients. Our results are consistent with previous results27 to support the idea that patients with cirrhosis of the liver are at a basal state of hyper-stimulation of cytokine production and that at some point lack of reserves to allow greater responsiveness to stimuli of sharp.27 Further studies on the stimulation in vivo and in vitro of mononuclear cells of patients with cirrhosis may be useful to clarify this phenomenon.
In conclusion, primary prophylaxis with ciprofloxacin for one month in cirrhotic patients with ascites who do not have a currently accepted indication did not show a preventive effect on the development of bacterial infections at one month follow-up. Moreover in women could increases the odds for UTI. However, primary prophylaxis for longer period must be evaluated. Ciprofloxacin use as primary prophylaxis could diminish the mortality in cirrhotic patients.
Abreviations- •
GI: gastrointestinal.
- •
LAL: limulus amoebocyte lysate.
- •
LBP: lypopolysacharide-binding protein.
- •
SBP: spontaneous bacterial peritonitis.
- •
UTI: urinary tract infection.
All authors declare no financial disclosures.
Financial SupportThis study was supported by a grant from the Mexican National Council for Science and Technology CONACyT-193593 and a grant of Laboratorios Senosiain, S.A. de C.V.