Introduction. Viral hepatitis in children is a major public health problem worldwide.
Aim. To evaluate the prevalence of serological markers for hepatitis A, B and C infections in Mexican children diagnosed with hepatitis during a five-year period.
Material and methods. A total of 31,818 children admitted to a tertiary level hospital in Mexico from 2005 to 2009 were evaluated for hepatitis.
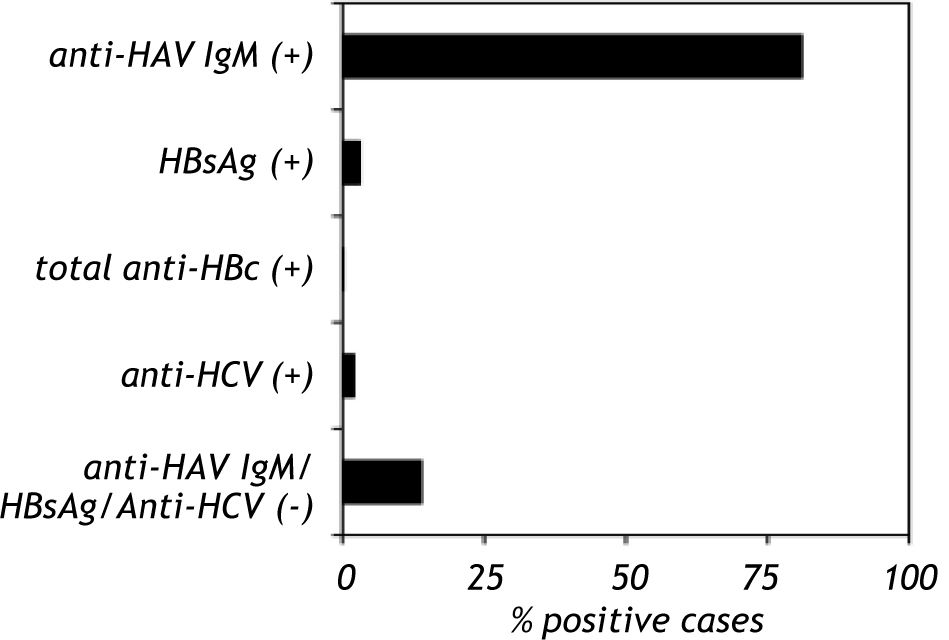
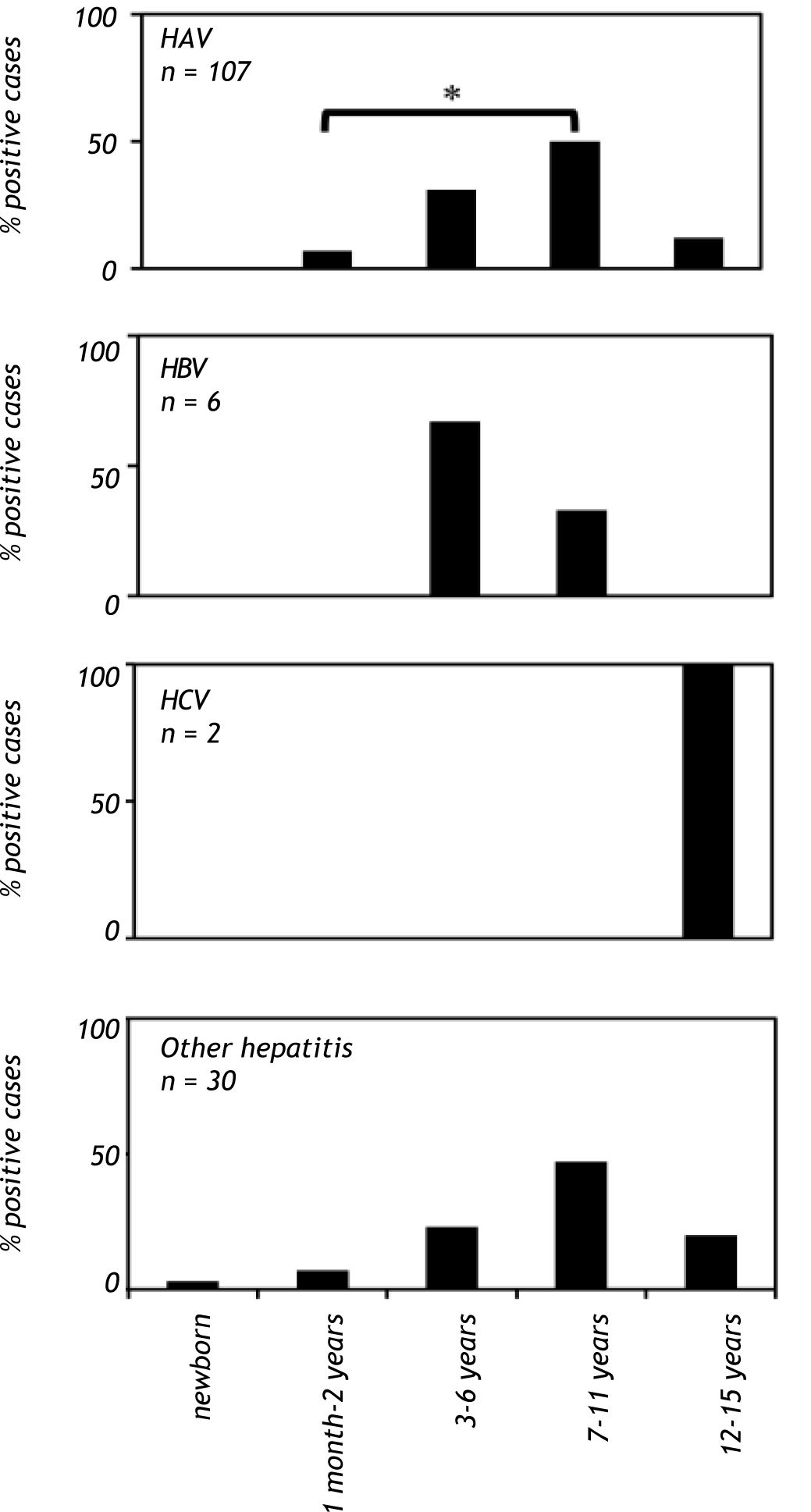
Results. Hepatitis was found in 215 (0.7%) of the children. Serum samples from hepatitis-positive children were screened for anti-HAV IgM, HBsAg, total anti-HBc and anti-HCV. HAV was the leading cause of viral hepatitis (81%), followed by HBV and HCV (3.1 and 2%, respectively), whereas no serological marker was observed in 13.9% of the analyzed samples. Furthermore, when children were categorized by age, a significant increase in anti-HAV detection was observed in school-aged children (7-11 years old) (p < 0.001) and a reduction in adolescents (12-15 years old).
Conclusion. In conclusion, hepatitis A is the most prevalent viral hepatitis infection detected in children, followed by HBV and HCV. In addition, the high percentage of hepatitis infections without a known etiological agent and the serological test limitations require the detection of occult HBV, HCV and hepatitis E infections. The age-dependent vulnerability of groups with HAV infections emphasizes the importance of HAV vaccination in young children in Mexico.
Viral hepatitis imposes an important burden on children’s health worldwide. In preschoolers, hepatitis A virus (HAV) infection is recognized as the most frequent cause of hepatitis, whereas hepatitis B virus (HBV) and hepatitis C virus (HCV) are important causes of chronic liver disease in children and adolescents.1-4 It is widely known that HAV spreads primarily through contaminated food or water.5 In contrast, childhood HCV and HBV infections mainly occur through mother-to-infant transmission.6,7
In recent years, the rates of liver diseases have increased in Mexico. Nearly 193,000 viral hepatitis cases in the general population were reported between 2000 and 2007; HAV, HBV and HCV were the main etiologies. HAV is the leading cause of viral hepatitis (79%) in the general population, followed by HCV and HBV (6 and 3.3%, respectively).8 The most common risk factors for viral hepatitis in the general Mexican population are lack of sanitation, blood transfusions and history of surgery.9,10 In addition, the prevalence of HBsAg (0.93-2.52%) and anti-HCV (2%) reported in pregnant women in Mexico9,11 suggests that young children are exposed to these viruses. However, the data relevant to the prevalence of serological markers for hepatitis A, B and C infections in children have not yielded conclusive results to date.12–24 Furthermore, we do not have data regarding HAV, HBV and HCV prevalence in hepatitis-diagnosed children, and to date, the impact of age on viral hepatitis prevalence has not been analyzed in Mexico. The aim of the present study was to evaluate the prevalence of hepatitis A, B and C viral infections in Mexican children diagnosed with hepatitis during a five-year period.
Material and MethodsPatient selectionThe pediatric patients (< 15 years old) evaluated in this study were admitted to the Pediatric Infectious Disease Section of the Civil Hospital of Guadalajara Fray Antonio Alcalde from 2005 to 2009. Hepatitis was defined as hepatomegaly, fever (> 38°C) and/or jaundice with elevated levels of serum aspartate ami-notransferase (AST) > 38 UI/L and alanine amino-transferase (ALT) > 35 UI/L. In addition, direct bilirubin > 0.3 mg/dL, albumin levels and other clinical features including: age, sex, time of onset of clinical symptoms (months), nausea, vomiting, abdominal pain, choluria, acholia and acute liver failure were investigated. Patients with hepatic-based coagulopathy (prothrombin time ≥ 20 s or international normalized ratio ≥ 2.0) that was not corrected by parenteral vitamin K and/or hepatic encephalopathy with unknown evidence of chronic liver disease were considered to have acute liver failure.25 Patients with liver disease who were undergoing treatment with a hepatotoxic drug, patients with chronic hepatitis non-related to viral hepatitis or patients with autoimmune hepatitis were excluded from the study.
Serum samplesA total of 215 serum samples were collected from children with clinical hepatitis. Only one blood sample was obtained from each participant in the study. After the serum fraction was isolated, the samples were stored at-80°C. Immunological tests were performed as described below.
SerologyAll serum samples from the children with clinical hepatitis were screened to detect anti-HAV IgM (im-munoglobulin M), HBsAg, total anti-HBc and anti-HCV. The anti-HAV IgM, HBsAg and anti-HCV were analyzed by using a third-generation micropar-ticle immunoenzymatic assay (AxSYM HAVAB-M 2.0, AxSYM HBsAg (V2) and AxSYM HCV 3.0, Abbott Laboratories, Chicago IL) on the AxSYM analyzer. Total anti-HBc (total immunoglobulin M and immunoglobulin G) was tested with an immu-noenzymatic assay (Monolisa Anti-HBc PLUS, BIO-RAD Laboratories, Chicago, IL) on a PR 3100 TSC analyzer. Patients with positive anti-HAV IgM, HB-sAg or anti-HCV samples were considered as HAV-, HBV-or HCV-infected patients, respectively. Exposure to HBV infection was defined as a total anti-HBc positive result with an HBsAg negative result.26–28
Clinical history and demographical dataAll cases were referred to a trained pediatrician who conducted a confidential interview using a structured questionnaire to investigate clinical history and demographical data including age, gender, hepatitis A and B vaccination and the main risk factors related to HAV, HBV and HCV infections. As previously described, the following risk factors known for HBV and HCV infection were investigated: history of hospitalizations, promiscuity, history of major surgery, drug use (IV or inhaled), blood transfusion, tattoos, history of sex with a sex worker, contact with contaminated body fluids, accidental needle-stick, acupuncture, injections with contaminated needles and hemodialysis.10,29 Other risk factors, including history of hepatitis without a known etiological agent, hepatitis B infection, hepatitis C infection, cirrhosis and hepatocellular carcinoma in the children’s parents were investigated. All data were obtained from the children and the children’s parents. Sanitation was defined as housing sanitary conditions including sewage disposal, cesspool, piped water, artesian well and public waste collection system12 and was investigated using questionnaires completed by the children’s parents. The children’s parents provided written, informed consent at the time of evaluation, and this study was approved by the Institution Ethics Committee.
Statistical analysesContinuous variables were reported as mean, standard deviation (SD) and median. The demographic and risk factors and clinical data are reported as simple frequencies and proportions. The serological markers anti-HAV, HBsAg and anti-HCV were analyzed by comparing the children’s age groups:
- •
Newborn, < 1 month old.
- •
Infant, 1 month to 2 years old.
- •
Pre-school age, 3 to 6 years old.
- •
School age, 7 to 11 years old, and
- •
Adolescents, 12 to 15 years old.
The age groups were categorized as previously described.30 Statistical differences were evaluated by applying a Chi-square test and Fisher’s exact test. P values less than 0.05 were considered significant.
ResultsDemographics and clinical feature dataFrom a total of 31,818 children, 215 (0.7%) were diagnosed with hepatitis. Of these patients, 110 were males and 105 were females (age range, 0-15 years; mean, 7.2 SD 3.8 years).
- •
The mean transaminase levels were ALT 1276 UI/L, SD (2407); range 156-25,894 UI/L; median 861 UI/L and AST 830 UI/L, SD (1040); range 84-6,600 UI/L; median 453 UI/L.
- •
The mean direct bilirubin level was 3.7 mg/dL, SD (4.1); range 0.5-39 mg/dL; median 3.1 mg/dL.
- •
The mean albumin level was 4.5 mg/dL, SD (1.1); range 1.8-7.6 mg/dL; median 4.6 mg/dL.
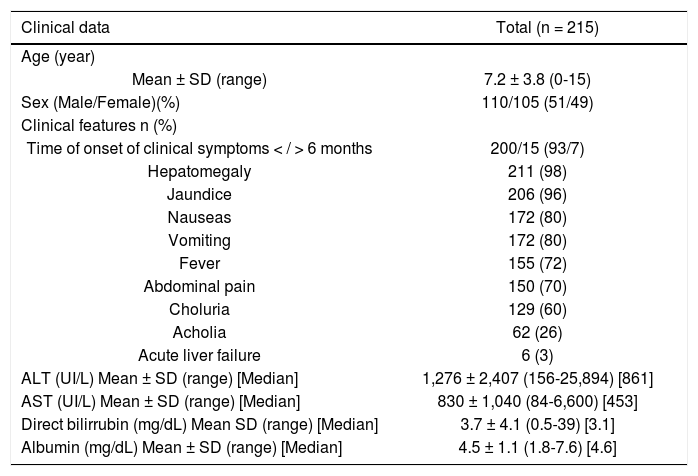
The time of onset of clinical symptoms was < 6 months in 93% (200/215) of the cases. In the 215 total patients, the following occurred (Table 1):
Clinical characteristics of Mexican children with viral hepatitis.
| Clinical data | Total (n = 215) |
|---|---|
| Age (year) | |
| Mean ± SD (range) | 7.2 ± 3.8 (0-15) |
| Sex (Male/Female)(%) | 110/105 (51/49) |
| Clinical features n (%) | |
| Time of onset of clinical symptoms < / > 6 months | 200/15 (93/7) |
| Hepatomegaly | 211 (98) |
| Jaundice | 206 (96) |
| Nauseas | 172 (80) |
| Vomiting | 172 (80) |
| Fever | 155 (72) |
| Abdominal pain | 150 (70) |
| Choluria | 129 (60) |
| Acholia | 62 (26) |
| Acute liver failure | 6 (3) |
| ALT (UI/L) Mean ± SD (range) [Median] | 1,276 ± 2,407 (156-25,894) [861] |
| AST (UI/L) Mean ± SD (range) [Median] | 830 ± 1,040 (84-6,600) [453] |
| Direct bilirrubin (mg/dL) Mean SD (range) [Median] | 3.7 ± 4.1 (0.5-39) [3.1] |
| Albumin (mg/dL) Mean ± SD (range) [Median] | 4.5 ± 1.1 (1.8-7.6) [4.6] |
- •
Hepatomegaly in 98% (211/215).
- •
Jaundice in 96% (206/215).
- •
Nausea in 80% (172/215).
- •
Vomiting in 80% (172/215).
- •
Fever in 72% (155/215).
- •
Abdominal pain in 70% (150/215).
- •
Choluria in 60% (129/215).
- •
Acholia in 26% (62/215), and
- •
Acute liver failure in 3% (6/215).
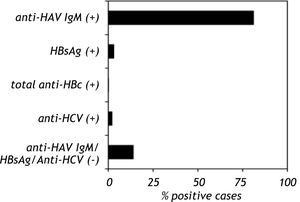
Anti-HAV IgM was positive in 81% (107/132) of the samples, HBsAg was positive in 3.1% (6/184) of the samples and total anti-HBc was positive in 1% (1/113) of the samples. Anti-HCV was detected in 2% (2/129) of the study group. Co-infection of HAV with HBV was found in three cases (1.4%), whereas co-infection of HAV with HCV was found in two cases (1%). Anti-HAV, HBsAg, total anti-HBc and anti-HCV were not found in 13.9% of the samples (hepatitis without a known etiological agent) (Figure 1). A total of six patients presented acute liver failure; two were HAV-infected, one was HAV-HCV co-infected and three patients lacked positive serological marker. Only one child presented with chronic hepatitis B infection.
Seroprevalence of anti-HAV, HBsAg, total anti-HBc and anti-HCV in Mexican children with viral hepatitis. The serum samples from children with hepatitis were screened to detect anti-HAV IgM, HBsAg, total anti-HBc and anti-HCV. The serological markers were detected by an immunoenzymatic assay according to the manufacturer’s instructions (Abbot Laboratories, Chicago IL and BIO-RAD Laboratories, Chicago IL). The data are presented as percentages of positive and negative serological markers.
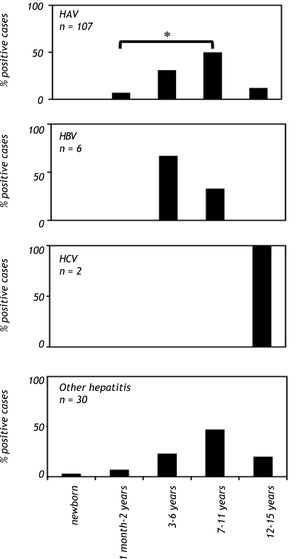
When patients were analyzed on the basis of age, a significant increase (p < 0.001) in anti-HAV IgM prevalence was observed in the school-aged group (age 7 to 11 years old), and there was a reduction in anti-HAV IgM prevalence in adolescents (12 to 15 years old). In contrast, anti-HAV IgM antibodies were not found in children younger than one month (Figure 2). The highest prevalence of hepatitis B cases was observed in patients between the ages of 3 to 6 years, whereas anti-HCV antibodies were found only in patients between the ages of 12 and 15 years (Figure 2). Hepatitis cases diagnosed without a known etiological agent were mainly observed in patients between the ages of 7 to 11 years (Figure 2).
Viral hepatitis by age group in Mexican children with hepatitis (2005-2009). The number of patients positive for HAV, HBV, HCV infections and other hepatitis cases in children of each age group. The serological markers were detec¬ted as described infigure 1. A P value < 0.01 was deemed significant. HAV: hepatitis A virus. HBV: hepatitis B virus. HCV: hepatitis C virus.
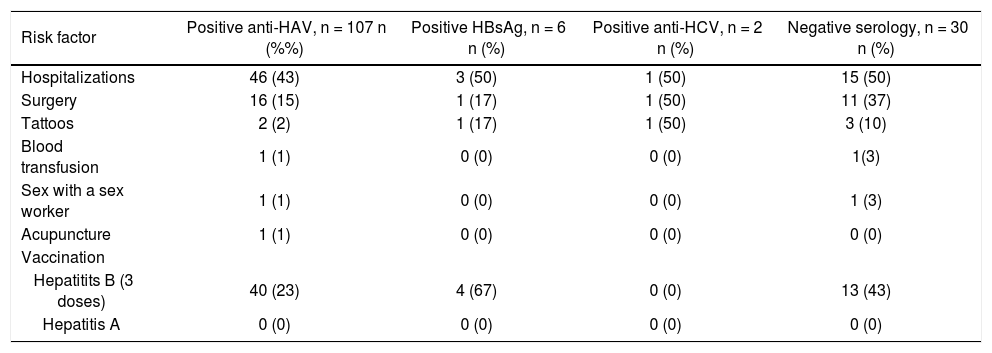
The most frequent risk factors found in the anti-HAV-positive children were history of hospitalization (43%, 46/107) and surgery (15%, 16/107). Other risk factors found in the anti-HAV-positive children were:
- •
Tattoos (2%, 2/107).
- •
Blood transfusion (1%, 1/107).
- •
History of sex with a sex worker (1%, 1/107), and
- •
Acupuncture (1%, 1/107).
Of the HBsAg positive patients, 50% (3/6), 17% (1/6) and 17% (1/6) of the patients had histories of hospitalizations, surgery and tattoo, respectively. Fifty percent of the anti-HCV positive children (1/2) had a history of hospitalization, surgery and tattoo.
Of the hepatitis without a known etiological agent patients:
- •
50% (15/30) had a history of hospitalizations.
- •
37% (11/30) had undergone surgery.
- •
10% (3/30) had a tattoos.
- •
3% (1/30) had a blood transfusion, and
- •
3% had history of sex with a sex worker (1/30).
Hepatitis B vaccination was found in 23% (40/107) of the anti-HAV-positive children, 67% (4/6) of the HBsAg positive patients and 43% (13/30) of the patients with hepatitis without a known etiological agent. None of the anti-HCV positive patients had been vaccinated for hepatitis B, and no child had received hepatitis A vaccination (Table 2).
Frequency of viral hepatitis risk factors and hepatitis A and B vaccination in children diagnosed with hepatitis.
| Risk factor | Positive anti-HAV, n = 107 n (%%) | Positive HBsAg, n = 6 n (%) | Positive anti-HCV, n = 2 n (%) | Negative serology, n = 30 n (%) |
|---|---|---|---|---|
| Hospitalizations | 46 (43) | 3 (50) | 1 (50) | 15 (50) |
| Surgery | 16 (15) | 1 (17) | 1 (50) | 11 (37) |
| Tattoos | 2 (2) | 1 (17) | 1 (50) | 3 (10) |
| Blood transfusion | 1 (1) | 0 (0) | 0 (0) | 1(3) |
| Sex with a sex worker | 1 (1) | 0 (0) | 0 (0) | 1 (3) |
| Acupuncture | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Vaccination | ||||
| Hepatitits B (3 doses) | 40 (23) | 4 (67) | 0 (0) | 13 (43) |
| Hepatitis A | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
The most common risk factors found in parents of children with HAV and HBV infections and hepatitis without an etiological agent were history of hospitalization: 78% (83/107), 100% (6/6) and 70% (21/30), respectively; and history of previous surgery: 86% (92/107), 67% (4/6) and 63% (19/30), respectively. Hospitalizations, drug use and tattoos were the most frequent risk factors found in the parents of the HCV positive children (100%, 2/2). Promiscuity, drug use, blood transfusion, tattoos and history of sex with a sex worker were additional risk factors found in the parents of HBV and HCV positive children. A history of hepatitis was found in the parents of three HBV positive children. From those parents, one presented hepatitis without a known etiological agent (father), one presented hepatitis B infection (mother) and one was previously diagnosed with cirrhosis (mother). A previous diagnosis of hepatocellular carcinoma was found in two parents of HCV positive children (one mother and one father). A history of hepatitis was found in 35 parents of HAV positive children. Of those parents, 22 were infected with hepatitis without a known etiological agent, two had previous diagnoses of hepatitis B infection, two had previous diagnoses of hepatitis C infection, six had previous diagnoses of cirrhosis and three had previous diagnoses of hepatocellular carcinoma. Finally, a total of 16 parents of children with hepatitis without a known etiological agent had a history of hepatitis. Of these parents, six did not present an etiological agent for hepatitis, five were diagnosed previously with hepatitis B infection, two were diagnosed previously with hepatitis C infection, two were diagnosed previously with cirrhosis and one was diagnosed previously with hepatocellular carcinoma (Table 3).
Frequency of viral hepatitis risk factors in parents of children with hepatitis.
| Risk factor | Positive anti-HAV, n = 107 n (%) | Positive HBsAg, n = 6 n (%) | Positive anti-HCV, n = 2 n (%) | Negative serology, n = 30 n (%) |
|---|---|---|---|---|
| Hospitalizations | 83 (78) | 6 (100) | 2(100) | 21 (70) |
| Surgery | 92 (86) | 4 (67) | 0 (0) | 19 (63) |
| Promiscuity | 33 (31) | 2 (33) | 1 (50) | 10 (33) |
| Drugs use | 27 (25) | 3 (50) | 2 (100) | 11 (37) |
| Blood transfusion | 22 (21) | 2 (33) | 0 (0) | 8 (27) |
| Tattoos | 25 (23) | 2 (33) | 2 (100) | 5 (17) |
| Sex with a sex worker | 3 (3) | 1 (17) | 1 (50) | 3 (10) |
| Contact with contaminated body fluids | 6 (6) | 0 (0) | 0 (0) | 2 (7) |
| Accidental needle-stick | 7(6) | 0 (0) | 0 (0) | 2 (7) |
| Acupuncture | 2 (2) | 1 (17) | 0 (0) | 2 (7) |
| Injections with contaminated needles | 1 (1) | 1 (17) | 0 (0) | 2 (7) |
| Hemodialysis | 2 (2) | 0 (0) | 0 (0) | 1 (3) |
| History of hepatitis: | ||||
| Hepatitis without a known etiological diagnosis | 22 (21) | 1 (17) | 0 (0) | 6 (20) |
| Hepatitis B infection | 2 (2) | 1 (17) | 0 (0) | 5 (17) |
| Hepatitis C infection | 2 (2) | 0 (0) | 0 (0) | 2 (7) |
| Cirrhosis | 6 (6) | 1 (17) | 0 (0) | 2 (7) |
| Hepatocellular carcinoma | 3 (3) | 0 (0) | 2 (100) | 1 (3) |
| Sanitary conditions housing | ||||
| Sewage disposal | 85 (86) | 4 (67) | 2 (100) | 19 (63) |
| Cesspool | 12 (12) | 2 (33) | 0 (0) | 0 (0) |
| None sewage disposal | 2 (2) | 0 (0) | 0 (0) | 2 (7) |
| Piped water | 86 (87) | 4 (67) | 2 (100) | 18 (60) |
| Artesian well | 13 (13) | 2 (33) | 0 (0) | 2 (7) |
| Public waste collection system | 83 (84) | 4 (67) | 2 (100) | 19 (63) |
Lack of sanitation was found in 2% of the analyzed cases. Of the anti-HAV IgM-positive patients:
- •
86% (85/107) had sewage disposal.
- •
12% (12/107) cesspool.
- •
2% (2/107) no sewage disposal.
- •
87% (86/107) pipe water.
- •
13% (13/107) artesian well, and
- •
84% (83/107) public waste collection.
The frequencies of the risk factors are shown in table 3.
DiscussionViral hepatitis is a major public health problem in children worldwide, resulting in school absence and negative economic effects.31,32 In Mexico, the frequency of liver disease has increased and viral hepatitis represents the main cause of liver damage in the country. However, we do not have data regarding the prevalence of serological markers for hepatitis A, B and C infections in children with clinical hepatitis.
We investigated the prevalence of anti-HAV IgM, HBsAg, total anti-HBc and anti-HCV serological markers in Mexican children diagnosed with hepatitis during a five-year period. The low incidence of HBV and HCV infections in Mexico9,10 resulted in a small number of samples testing positive for serological markers for these viruses. An additional limitation of our study is the fact that serological tests (HBsAg and anti-HBc) are based on genotypes A and D of the HBV prevalent in Europe. This is important because genotypes H and F, which are predominant in Latin American countries, including Mexico,33 are different than those reported around the world (the HBV-H genotype is almost exclusively found in Mexico). Thus, a plausible underestimation of HBV prevalence in Mexico could have been made. Another fact is that occult HBV and HCV infections, defined as the absence of serological markers in the presence of low viral load DNA or RNA, may not be determined using serological probes.28,34
In our study, the anti-HAV antibody was the most prevalent serological marker found in children diagnosed with hepatitis, followed by HBsAg and anti-HCV. This result is consistent with the finding that the prevalence of HAV infections is higher than that of HBV or HCV.8–10 However, the prevalence of Hepatitis A reported in this study did not correlate with the reduction in HAV infection that has been documented over the last several years.12–18 Our data revealed a significant age-dependent prevalence for anti-HAV, namely, an age-dependent increase in this marker was observed after infancy. The highest anti-HAV prevalence was found in school age children (7 to 11 years old), with a reduction in adolescents (12 to 15 years old). These data are consistent with the fact that Mexico is a country with an intermediate endemic of HAV infection, as previously reported.35 Notably, although no statistical significance was found, deficient sanitation was not the main risk factor for HAV infection (Table 3). These issues, together with the high prevalence of HAV observed in children of school age, suggest the impact of unidentified risk factors associated with hepatitis infection being the quality of the water an issue to be analyzed. Furthermore, education campaigns must be reinforced in order to prevent HAV dissemination. Moreover, although efficacious vaccines containing formalin-inactivated HAV have been licensed in multiple countries,31 HAV vaccination is not included in public health programs in Mexico.36 Thus, children from homes of low-income status as the included in the present study, which represent the most susceptible group of children, are not vaccinated against HAV. Therefore, we recommend vaccination for all children over the age of one year in Mexico to avoid dissemination of the HAV infection and its complications.
Interestingly, in our study hepatitis cases without a known etiological agent showed the same age-dependent profile as that of children with hepatitis A (Figure 2); thus, it is potentially plausible that these patients were infected with hepatitis E virus, as they were negative for hepatitis A serological markers. Further studies must be conducted to corroborate this possibility. In our study, a distinctive age pattern was observed in hepatitis B and C. The highest prevalence of hepatitis B was observed in preschool-aged children, whereas anti-HCV antibodies were exclusively found in adolescents (Figure 2). The presence of anti-HCV antibodies may be due to high-risk behavior in adolescents, such as unprotected sexual activity and drug use. However, the low number of samples that tested positive for hepatitis B and C did not allow us to perform statistical analyses.
For children infected with HBV and HCV, the risk factors in their parents were more frequent than those found in the children. This finding is important because the main transmission route in children is vertical.1,3,4 In our study, the vertical transmission of HBV and HCV infections were not evaluated due to the lack of serological markers in the children’s parents. The most frequent risk factors in the parents in all study groups were history of hospitalization and previous surgery. These risk factors may indicate that proper handling of contaminated body fluid and surgical equipment in hospitals may reduce their hepatitis transmission. Therefore, large-scale studies related to risk factors related to viral hepatitis and the vertical transmission are required in Latin American countries, including Mexico.
In summary, the large percentage of hepatitis without serological markers for HAV, HBV or HCV as well as the high anti-HAV prevalence in children support the necessity to improve prevention, control and treatment strategies in Mexico. The analytical limitations of serological testing reveal that molecular techniques are required to rule out HBV and HCV infections in children. Finally, to reduce the prevalence of hepatitis, HAV vaccination should be mandatory, especially in the vulnerable groups identified in this study.
ConclusionWe found that hepatitis A is the most prevalent viral hepatitis in children; thus, vaccination campaigns should be reinforced. Moreover, the high percentage of hepatitis cases without serological markers for HAV, HBV and HCV may suggest a role of other viruses, including hepatitis E virus, occult HBV or HCV infections, in the development of hepatitis in children. Thus, the data presented in this study together with the fact that serological studies may have analytic limitations support the mandatory detection of viral nucleic acid content in these patients.
Abbreviations- •
HAV: Hepatitis A virus.
- •
HBV: Hepatitis B virus.
- •
HCV: Hepatitis C virus.
- •
AST: Aspartate aminotransferase.
- •
ALT: Alanine aminotransferase.
- •
Anti-HAV: Antibodies against HAV.
- •
HBsAg: Hepatitis B surface antigen.
- •
Total anti-HBc: Total antibodies against Hepatitis B core antigen.
- •
Anti-HCV: Antibodies against HCV.
The authors thank Drs. Bertha Garcia-Armenta and Roberto Hinojosa for their medical assistance, and Rogelio Troyo for his statistical assistance.
DisclosureNo author has a financial conflict of interest.