Cirrhosis is characterised by a prolonged asymptomatic period in which the inflammation persists, increasing as the disease progresses. Characteristic of this is the increase in pro-inflammatory cytokines and pro-oxidant molecules which are determining factors in the development of multiple organ dysfunction. In the early development of cirrhosis, splanchnic arterial vasodilation, activation of vasoconstrictor systems (renin-angiotensin-aldosterone) and the sympathetic nervous system (noradrenaline) bring about bacterial translocation and systemic dissemination via portal circulation of bacterial products, and molecular patterns associated with damage, which exacerbate the systemic inflammation present in the patient with cirrhosis. Albumin is a molecule that undergoes structural and functional changes as liver damage progresses, affecting its antioxidant, immunomodulatory, oncotic and endothelial stabilising properties. Our knowledge of the properties of albumin reveals a molecule with multiple treatment options in patients with cirrhosis, from the compensated then decompensated phases to multiple organ dysfunction. Its recognised uses in spontaneous bacterial peritonitis, post-paracentesis circulatory dysfunction, acute kidney injury and hepatorenal syndrome are fully validated, and a treatment option has opened up in decompensated cirrhosis and in acute-on-chronic liver disease.
Liver cirrhosis (LC) is a relatively irreversible disease characterised by a prolonged period of inflammation which leads to remodelling of the extracellular matrix and accumulation of collagen (fibrosis) in liver tissue. As liver cirrhosis develops, there is a decrease in serum albumin levels associated with an increased risk of death in patients with cirrhosis and infections. The non-oncotic properties of the albumin molecule, such as antioxidant, anti-inflammatory and immunomodulatory properties, play a fundamental role here [1].
Albumin infusion in patients with cirrhosis dates back more than 70 years, with proven efficacy in uses such as large-volume paracentesis, acute kidney injury and hepatorenal syndrome, and spontaneous bacterial peritonitis. However, there is increasing debate surrounding other uses of albumin, some emerging, such as its long-term use in decompensated patients (MACHT, ANSWER and pilot-PRECIOSA studies), patients hospitalised due to decompensation (ATTIRE study), post-liver transplantation, intrinsic or post-renal acute kidney injury, infections other than spontaneous bacterial peritonitis, hyponatraemia and hepatic encephalopathy (HE). The aim of this review was to create a scientific position on the uses of albumin in liver cirrhosis by authors from Mexico (Asociación Mexicana de Hepatología [Mexican Hepatology Association]).
2Pathophysiology of cirrhosis and properties of albumin2.1Natural history of liver cirrhosisIn 2016, there were more than 40,000 deaths from LC complications in the United States, and worldwide it causes 800,000 deaths annually [2]. LC is one of the leading causes of death in the world, the twelfth in the United States, and the fifth in Mexico [2,3] with projections estimating an increase in the coming years due to the fatty liver disease pandemic. It is estimated that only one third of patients with chronic liver disease are aware of their diagnosis, with an increase in the prevalence in the non-Hispanic black population and in Mexican-Americans [4].
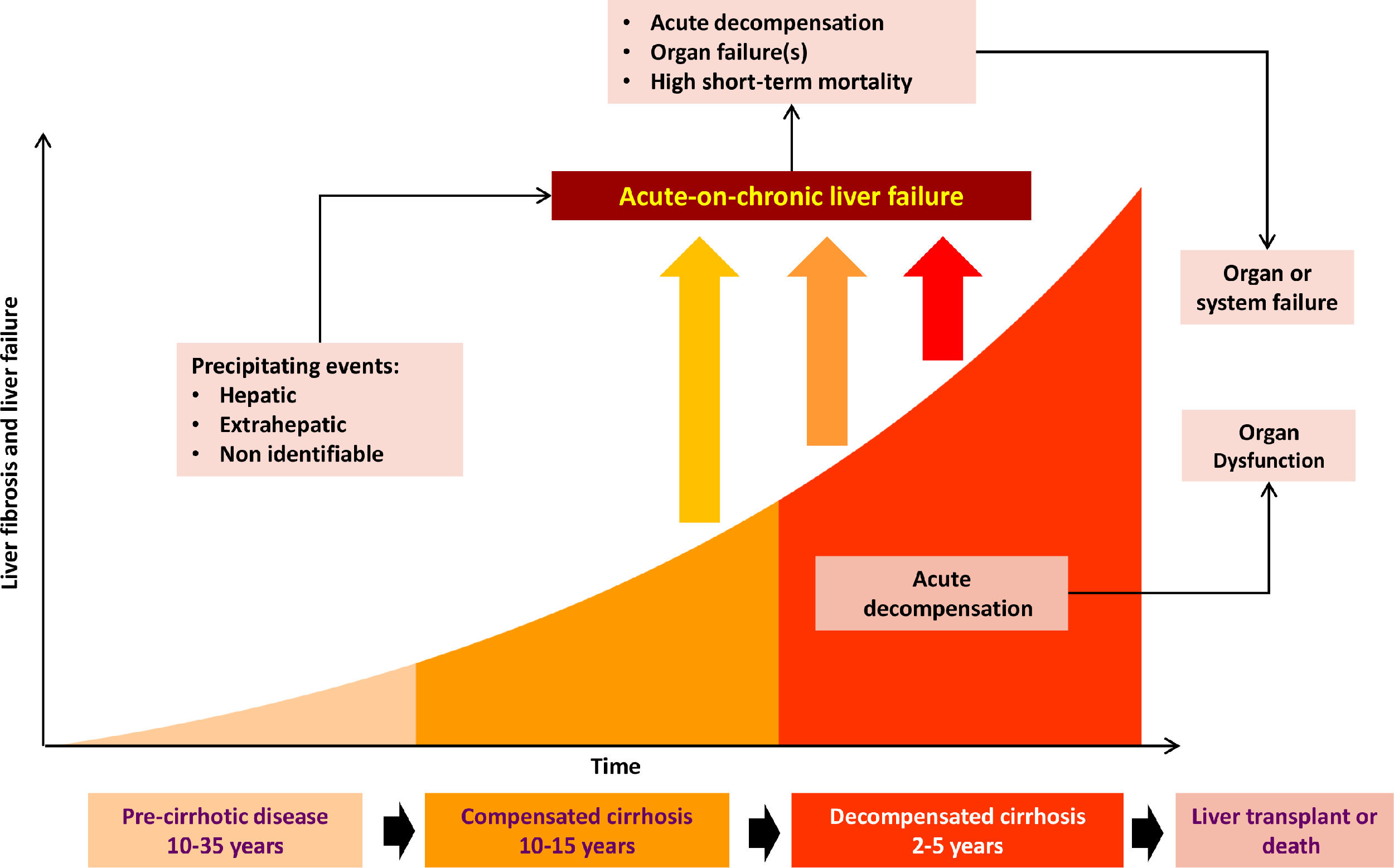
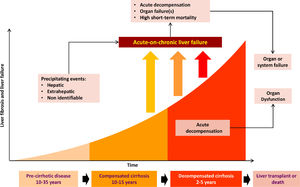
LC complications are due to increased portal pressure [5], with normal pressure being <5 mmHg. Non-significant portal hypertension is defined as values of 6-9 mmHg, while complications of cirrhosis occur when there is a clinically significant increase (≥10 mmHg) [4]. The disease stage preceding the onset of complications is known as compensated cirrhosis, and it becomes decompensated cirrhosis when these complications develop in the form of ascites, variceal haemorrhage, HE, coagulopathy, jaundice, bacterial infections and acute kidney injury (AKI). Patients with compensated cirrhosis have a better prognosis than decompensated patients, with average survival of 12 years and 2 years respectively [6] (Fig. 1).
The progression from compensated to decompensated liver damage occurs in 5-7% of patients per year [6], and usually occurs in the presence of clinically significant portal hypertension [7].
2.2Impact of the decrease in serum albumin in patients with liver cirrhosisThe decrease in serum albumin is characteristic of cirrhosis progression. It has been associated with an increase in mortality, with 5-year survival being markedly lower in patients with albumin levels <4 g/dl [3].
Patients with low albumin levels who have a triggering event (such as excessive consumption of alcohol, medications, infections and bleeding) are known to progress to acute-on-chronic liver failure (ACLF), a clinical syndrome characterised by an acute decompensation of liver function in the presence of underlying chronic liver disease, and which is associated with multiple organ failure and high mortality at 28 days [5,8].
2.3Peripheral arterial vasodilationArterial circulation dysfunction in LC is secondary to a reduction in systemic vascular resistance due to vasodilation, primarily of the splanchnic arterial circulation. The vasodilation is the result of an increase in the production of glucagon, nitric oxide, endogenous cannabinoids and carbon monoxide, associated with abnormal bacterial translocation from the intestine secondary to portal hypertension, as well as dysfunctional contractile pathways. In decompensated cirrhosis, peripheral arterial vasodilation increases, and blood pressure must be maintained through compensatory activation of systemic vasoconstrictor systems, such as the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system (SNS), and in the more advanced stages, the non-osmotic secretion of antidiuretic hormone [9,10].
2.4Pathophysiological bases of decompensated cirrhosisMany of the complications of decompensated cirrhosis are due to the decreased effective circulating volume secondary to peripheral arterial vasodilation. It is also known that in decompensated cirrhosis there is a pro-inflammatory and pro-oxidant state which plays a primary role in the development of complications.
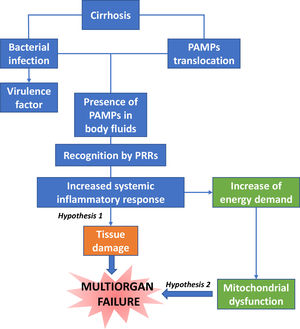
The decompensated patient has a greater predisposition to infections, resulting from the systemic spread of bacteria or bacterial products associated with abnormal translocation from the intestine, as well as molecular patterns associated with damage from the diseased liver, which trigger the release of pro-inflammatory mediators, such as tumour necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-1-β, granulocyte colony stimulating factor (G-CSF) and vascular endothelial growth factor (VEGF), by activating immune system cells [4].
Persistent systemic inflammation and the pro-oxidant state lead to structural and functional changes in the albumin molecule, affecting its antioxidant, immunomodulatory, capillary permeability, oncotic pressure maintenance, and endothelial protection properties [5].
The decrease in albumin in decompensated patients is a consequence of this chronic inflammatory state, causing qualitative changes in the effective circulating albumin leading to an increase in infections, renal dysfunction, refractory ascites, hospitalisations and higher mortality rates [11].
2.5Factors to consider in the development of acute-on-chronic liver failureRepeated episodes of acute decompensation characterise the clinical course of decompensated cirrhosis, which may or may not be associated with ACLF. The PREDICT study showed that there are three different clinical courses in the pathophysiology and prognosis of patients with cirrhosis hospitalised for exacerbation. All three types coincide with specific changes in the degree of systemic inflammation and have been characterised as: pre-ACLF patients, i.e. patients who develop ACLF within the first 90 days of damage; unstable decompensated patients, those with at least one hospital readmission without ACLF within the 90 days after the acute injury; and stable decompensated patients, who are those without ACLF or hospital readmissions within a 90-day period [12].
Systemic inflammation is pronounced in these episodes (higher levels of leucocyte counts, C-reactive protein, pro-inflammatory cytokines, chemokines, and levels of irreversibly oxidised albumin [human non-mercaptoalbumin: HNA2]) and is further increased in patients with ACLF [13]. Although the mechanisms which cause the inflammation are still not fully understood, it is known that bacterial infections are involved in 33% of cases [14].
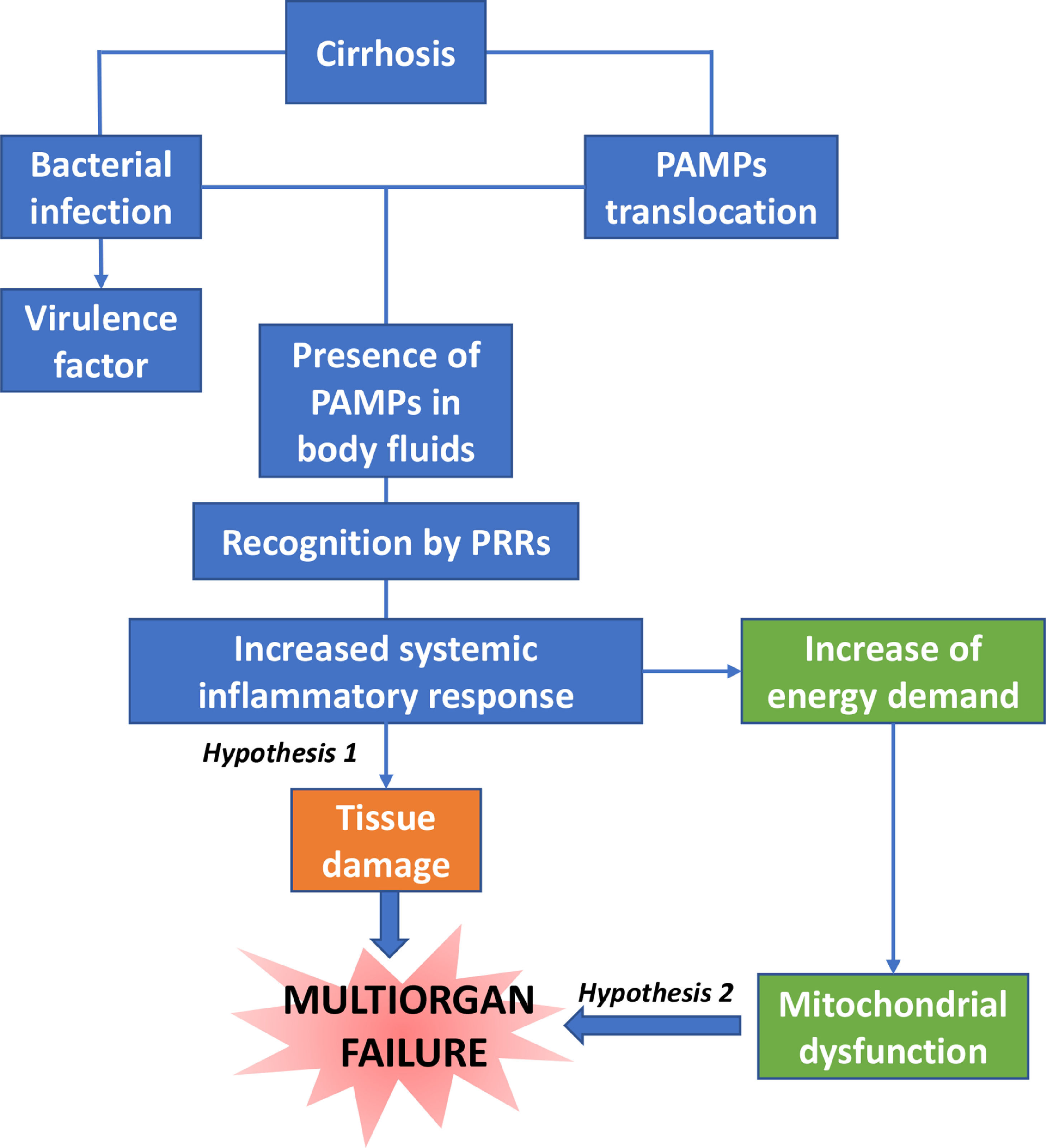
Dysfunction in the elimination of bacteria by neutrophils is associated with the risk of infection and death [15]. There is a close correlation between the severity of systemic inflammation and the number of organ failures, and among them, renal dysfunction is the most common in patients with moderate systemic inflammation [16]. One suggested hypothesis is that innate immune cell activation causes the release of cytokines and proteases within vital organs causing collateral tissue damage, in a process known as immunopathology [17].
Another hypothesis supports that the increase in energy demand by immune cells causes the diversion of nutrient resources for these cells at the expense of cells of vital tissues not belonging to the immune system (Fig. 2).
2.6Human albuminHuman albumin is a protein, which comprises 50% of plasma proteins in healthy individuals. It is synthesised in the liver at a rate of 10-15 g/day, and has a concentration of 35-50 g/l [17]. Its half-life in healthy individuals is 20 days, but it may be longer in patients with cirrhosis, suggesting that hypoalbuminaemia in these patients is mediated by multiple factors [19].
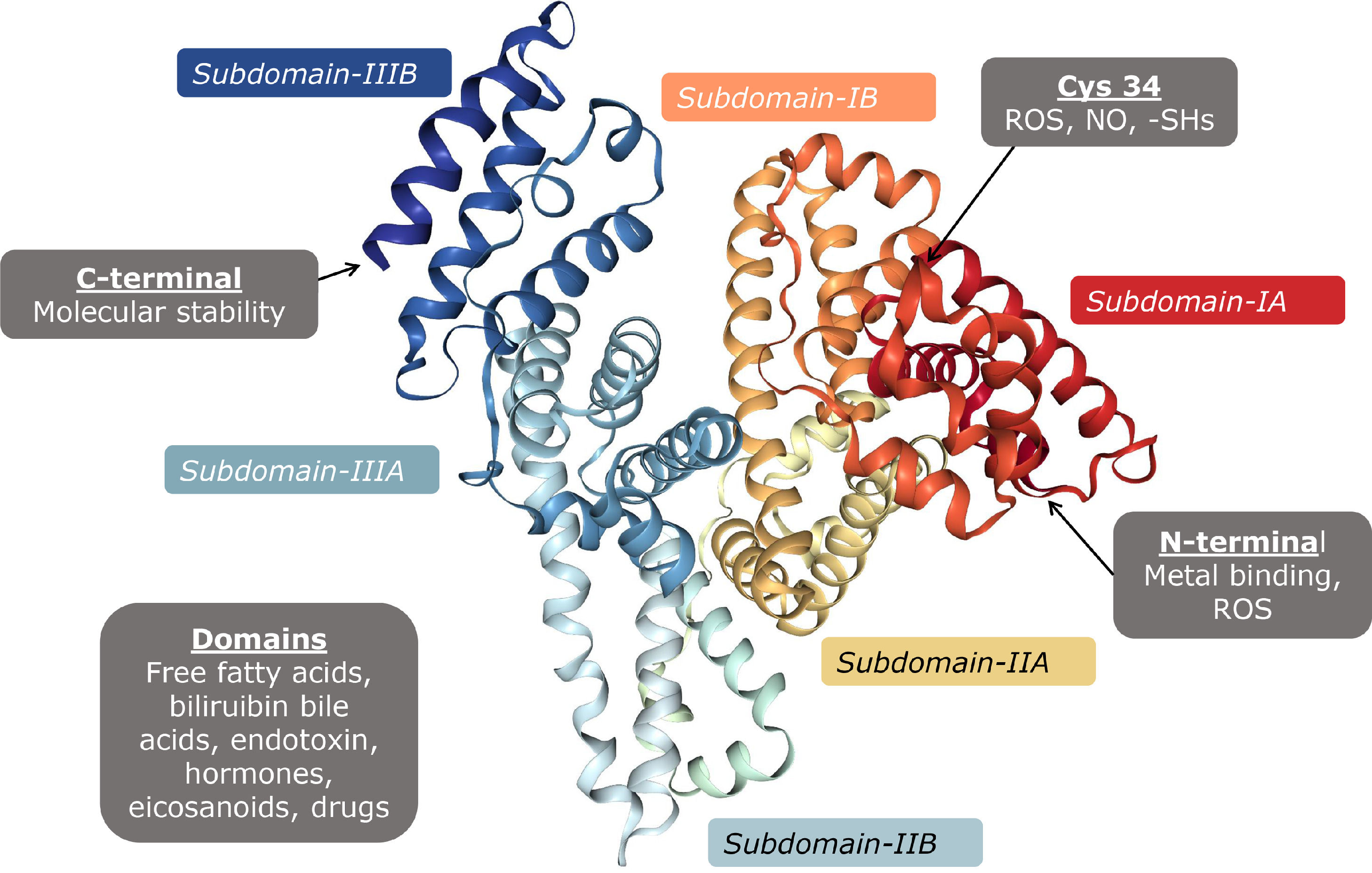
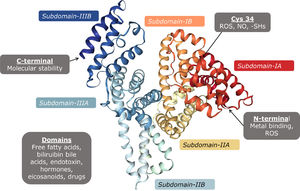
Albumin is a protein encoded in chromosome 4, small in size (67 kDa), with a globular configuration made up of 609 amino acids, predominantly acidic pH, which provides it with a negative charge at pH 7. It is formed by several α helices which together form two subdomains A and B, and three identical pairs of them unite to form the albumin molecule (Fig. 3). The properties of albumin depend on its structure, and it is formed by post-translational modifications which add 18 tyrosine residues, six methionine residues, one tryptophan, 59 lysine, 17 disulphides, and one cysteine (Cys34) [20]
Disulphide bonds contribute to the formation of its tertiary structure, giving it flexibility to be able to bind to different substances in circulation. Its antioxidant properties are due to the free cysteine residues which inactivate reactive oxygen species [21].
Recent evidence demonstrated that periodic administration of albumin in ascites resulted in lowering the incidence of infections, spontaneous bacterial peritonitis (SBP), HE, hepatorenal syndrome (HRS), hyponatraemia, hospitalisations, refractory ascites, the need for paracentesis, as well as the costs derived from complications, primarily when concentrations above 4 g/l were achieved [22]
The best-known function of albumin is that of maintaining the oncotic pressure of the plasma,[5,23–25] but it has other effects such as: facilitating the metabolism, transport and solubilisation of molecules; antioxidant and immunomodulatory effects; stabilising capillary permeability and the vascular endothelium; and fulfilling other functions which promote homeostasis (Table 1) [19,21,24,26–33]
Albumin functions and their biological basis
Hypoalbuminaemia is characteristic of cirrhosis. Albumin levels can be reduced by up to 60-80% by haemodilution, water and sodium retention, and sequestration of albumin in the extracellular space and ascites fluid [9].
Splanchnic vasodilation produces an increase in blood volume through the splanchnic circulation, thus allowing the formation of ascites. Despite the increase in blood volume, there is a low effective circulating volume, with the subsequent activation of compensatory systems, which stimulate the renal reabsorption of sodium and water, which contributes to the formation of ascites [23,24].
2.8Effects of albumin on circulatory dysfunctionPersistent systemic inflammation causes tissue and cell damage, which increases circulating inflammatory cytokines. Circulatory dysfunction and inflammation occur simultaneously in the decompensated patient with multiple organ failure. In this situation, in addition to expanding plasma volume, increasing preload and cardiac output, albumin increases peripheral vascular resistance and reduces the inflammatory state in the patient with cirrhosis as a result of its antioxidant and immunomodulatory properties [21,28–32].
3Proven uses of albumin in patients with cirrhosis: scientific position3.1Albumin in large-volume paracentesisAscites is the most common cause of decompensation, with an annual incidence of 5-10% [34]. There are three grades: Grade 1, only detectable by ultrasound; grade 2 detectable on clinical examination and manifested by distension; grade 3 or tense, with compromise of breathing and/or eating [9].
Abdominal paracentesis is performed for either diagnostic or therapeutic purposes. A single 5-litre paracentesis can be performed safely in the patient with diuretic-resistant tense ascites [35], but large-volume paracentesis (i.e. more than 5 litres) increases the risk of developing post-paracentesis circulatory dysfunction (PPCD), defined as a 50% increase in plasma renin activity, with a final value greater than 4 ng/ml, on day five or six post-paracentesis [36]. PPCD is characterised by a reduction in effective circulating volume and is associated with death, and with the development of AKI and hyponatraemia [37,38]. The incidence of PPCD after large-volume paracentesis is 70%, but the risk may be reduced when fluid replacement therapy is given [39]. In one study, patients with tension ascites were randomised to receive albumin versus standard treatment after therapeutic paracentesis. The group which did not receive albumin developed more hyponatraemia, and RAAS activity and creatinine were increased to a greater extent [40]. A meta-analysis of randomised trials showed that albumin is superior to any other expander or vasoconstrictor not only in preventing PPCD, but also its consequences. Furthermore, albumin infusion after large-volume paracentesis appears to be more cost-effective than a plasma volume expander and less expensive, due to fewer liver-related complications and hospital costs over a 30-day period [41]. Albumin should be administered for paracentesis of more than 5 litres to prevent PPCD [9–35]. Regarding the optimal dose, studies have tested from 4-10 grams per litre drained, but the doses with the best evidence are 6-8 grams per litre [42–44].
Position:
In order to avoid PPCD in large-volume paracentesis, 6-8 grams of albumin should be administered for each litre drained.
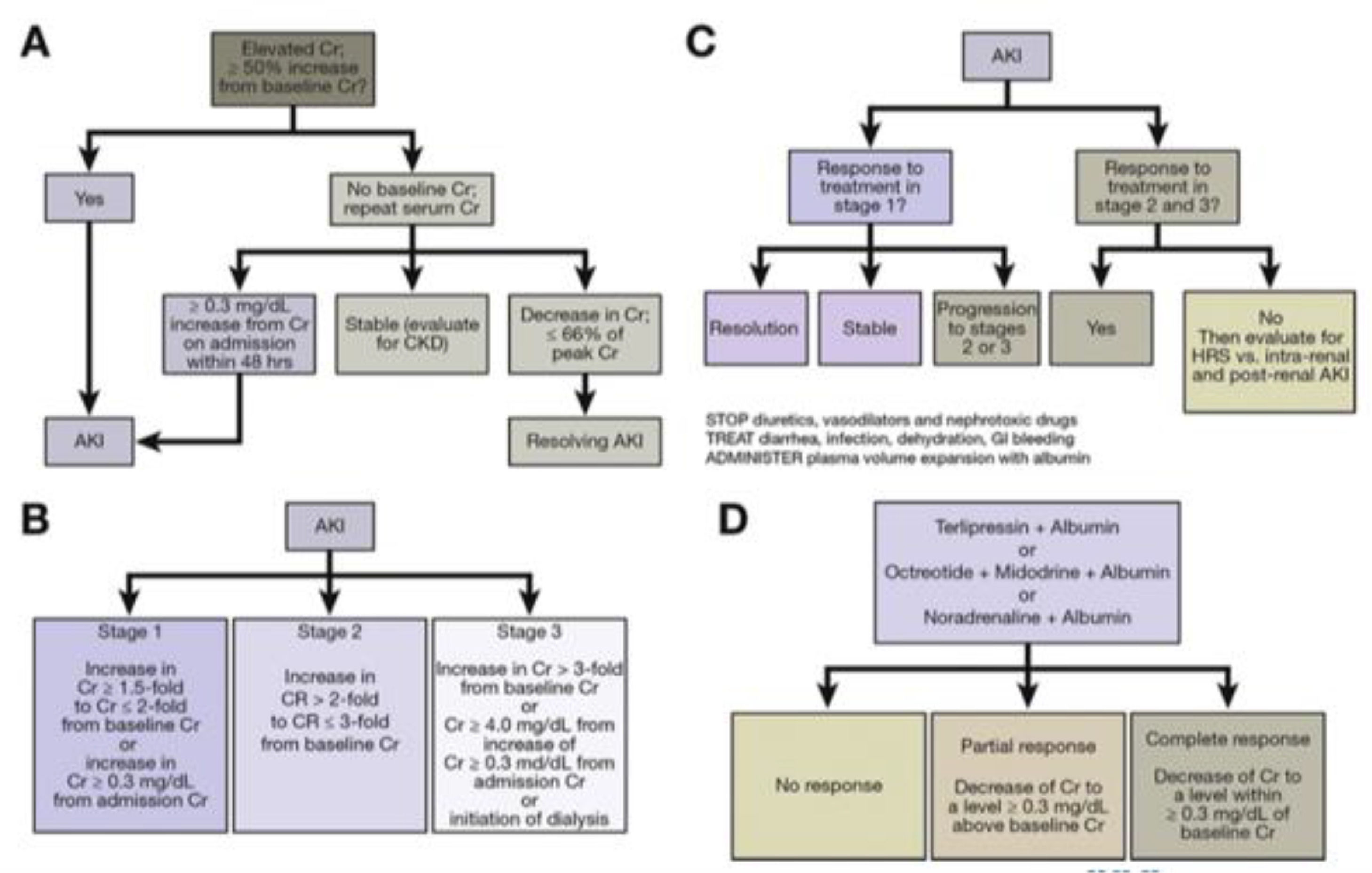
3.2Albumin in patients with acute kidney injury and hepatorenal syndromeHRS is the complication with the worst prognosis in patients with cirrhosis, with median survival of 2 weeks to 2 months [45]. The first definition of this syndrome was proposed in 1978 [46] and subsequently redefined by the International Club of Ascites as the increase in serum creatinine of 50% or ≥0.3 mg/dl after ruling out other causes of kidney injury. Historically, it had been classified as HRS type 1 when there was a significant alteration in renal function within a period of two weeks, and HRS type 2 when it occurred over a longer period of time [47]. It is currently classified as HRS Acute Kidney Injury (HRS-AKI) when there is an elevation of creatinine ≥0.3 mg/dl or ≥50% with respect to baseline in a period of 48 h or a urinary output ≤0.5 ml/kg for ≥6 h, or as HRS Non-Acute Kidney Injury (HRS-NAKI).
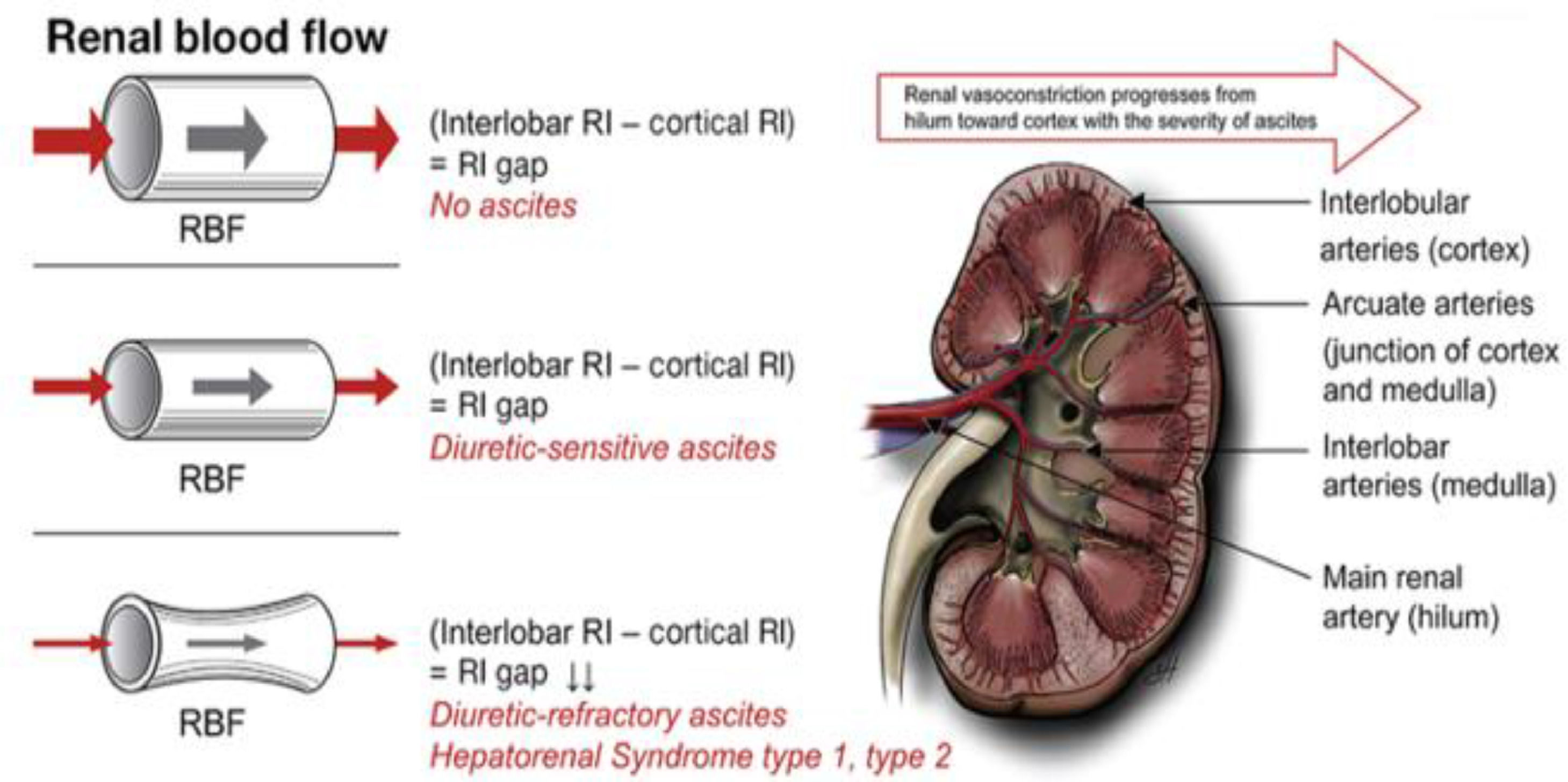
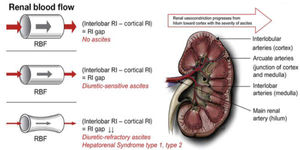
The pathophysiology involves renal vasoconstriction, which begins in the hilum and extends to the cortex, and is necessary to maintain equilibrium in response to splanchnic arterial vasodilation (Fig. 4) [48]. Treatment is based on expanding the effective volume (generally with albumin) and the use of vasoactive medications such as midodrine, octreotide, terlipressin and norepinephrine.
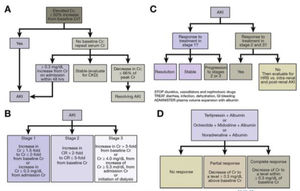
The guidelines of the European Association for the Study of the Liver recommend that resuscitation in HRS-AKI be consistent with the cause and severity of the fluid loss [9]. In the event of no obvious cause or in the context of HRS-AKI 1A (i.e. creatinine <1.5 mg/dl) or lesion associated with infection, an albumin dose of 1 g/kg of body weight is recommended (maximum 100 g daily) for two consecutive days. In patients with HRS-AKI in a stage beyond 1A, vasoconstrictors are recommended, preferably terlipressin, plus albumin. Albumin should be at 20-40 g daily, ideally titrated with central venous pressure or other measures of blood volume, to reduce the risk of fluid overload, pulmonary oedema and respiratory failure [49]. Should HRS recur once the treatment is finished, it can be repeated. Fig. 5 shows the algorithms for the diagnosis and treatment of HRS-AKI [48].
There are few studies which have evaluated the role of albumin alone in the management of HRS. In a randomised, open-label, uncontrolled study, Neri et al [50] assigned patients to terlipressin/albumin vs albumin (first day 1 g/kg and subsequently 20-40 g/day) and found that the combined treatment showed improvement in both kidney function and survival. Boyer et al compared the use of terlipressin/albumin (20-40 g/day) vs placebo/albumin in patients with HRS1, and although they found no difference in the rate of reversal of HRS1, the decrease in creatinine was greater in the combined-treatment group [51]. In a study of 16 patients with cirrhosis, Guevara et al [52] showed that the simultaneous administration of albumin and ornipressin boosted the reversal of HRS. Ortega et al [53] showed that the simultaneous administration of terlipressin/albumin (20-40 g/day) normalised creatinine in 50% of the patients, and was more effective than monotherapy with terlipressin. Similarly, an analysis of the two longest randomised, placebo-controlled studies in patients with HRS1 showed that terlipressin/albumin is more effective than placebo/albumin [54].
Position:
- a)
Fluid resuscitation in patients with AKI should be linked to the cause and severity of fluid loss. If there is no obvious cause, or if there is HRS-AKI or lesion associated with infection, albumin is recommended at 1 g/kg of weight for 48 h and then reassess.
- b)
The use of vasoconstrictors and albumin reduces mortality rates and improves renal function in HRS-AKI and is recommended in all patients with stage >1a.
- c)
Albumin should be given at a daily dose of 20-40 g during treatment with vasoconstrictors after the initial treatment for 48 h of albumin at 1 g/kg.
SBP is the most common bacterial infection in patients with ascites, with a prevalence of 10% and an incidence of 7-30% among those who are hospitalised [9,55]. The causal agents are generally enteric, and presumed to originate from the gastrointestinal tract by bacterial translocation, but it appears that the ascites fluid is inoculated by the haematogenous route. Diagnosis is established with a neutrophil count >250/µl in the absence of any source of intra-abdominal infection [9,34]. A puncture is recommended to rule out SBP in all patients with cirrhosis and de novo ascites, in all patients with ascites admitted to hospital, and in all patients with HE, impaired liver function, AKI, fever, abdominal pain, or shock. One third of patients will develop AKI despite the resolution of the infection, which is associated with increased mortality [56]; the aim of the use of albumin in SBP is to prevent AKI.
Sort et al studied 126 patients with SBP and compared two groups: one of patients who received albumin 1.5 g/kg at diagnosis and 1 g/kg on day 3 in infusion + cefotaxime; and another of patients who only received cefotaxime. AKI was more common in the group without albumin (33% vs 10%, p = 0.002). In-hospital mortality (29% vs 10%, p = 0.01) and at 90 days (41% vs 22%, p = 0.03) showed that the use of albumin in patients with SBP reduces both AKI and mortality rates [57]. With regard to whether or not to use other expanders, a pilot study comparing albumin and hydroxyethyl starch 200/0.5 in patients with SBP found haemodynamic improvement only in patients who received albumin [58].
There is a debate as to whether or not all patients with cirrhosis and SBP benefit from albumin, as the incidence of HRS is low in patients with creatinine <1 mg/dl and bilirubin <4 mg/dl [59].
Position:
- a)
The combined use of albumin and antibiotics in patients with SBP reduces mortality and AKI, particularly in high-risk groups (bilirubin >4 mg/dl, blood urea nitrogen >30 mg/dl and/or creatinine >1 mg/dl).
- b)
The recommended dose of albumin is 1.5 g/kg on day one, and 1 g/kg on day 3, but it must be individualised and monitored to avoid fluid overload. Summarises the indications for albumin.
Long-term use of albumin in decompensated patients has been investigated in at least three studies: Pilot-PRECIOSA, ANSWER and MACHT.
The Pilot-PRECIOSA study looked at the effect of administering albumin for 12 weeks secondary to hypoalbuminaemia, circulatory dysfunction, portal hypertension and markers of systemic inflammation in decompensated patients. Two doses were compared: 1.5 g/kg weekly versus 1 g/kg every two weeks. High doses of albumin were found to be associated with normalisation of albumin levels, left ventricular circulatory stability, and reduced levels of inflammatory cytokines, but not with any significant changes in portal pressure [15].
The ANSWER study, which randomised 440 patients with cirrhosis and uncomplicated ascites to standard therapy versus albumin at 40 g twice a week for the first two weeks, and subsequently 40 g per week, showed improvement in survival and control of ascites [21], fewer decompensation events and hospitalisation, and better quality of life. The administration of albumin was associated with an increase in its serum levels, and a post-hoc analysis found that an albumin value of 40 g/l or above 30 days after starting the infusions was associated with a notable benefit in survival. It was also found that even patients who did not reach this threshold benefited from the administration of albumin [22,60]. This benefit in mortality rates was corroborated in a non-randomised clinical trial in which 70 patients with refractory ascites received albumin 20 g twice a week [61].
In contrast, the MACHT study, a multicentre, randomised, double-blind, placebo-controlled study in 196 decompensated patients on the waiting list for liver transplantation, which assessed the role of long-term administration of midodrine and albumin 40 g every 15 days, showed no reduction in complications (AKI, hyponatraemia, infections, HE, or gastrointestinal bleeding), or mortality at one year [62,63].
The differences in the results of the ANSWER and MACHT studies can be explained by different factors. Patients in ANSWER had less liver dysfunction (model for end-stage liver disease [MELD] 12 vs 17), suggesting that albumin administration should be started in early stages. Furthermore, the doses used were different, and this was reflected in the increase in serum levels in ANSWER, not in MACHT, suggesting that an insufficient dose of albumin may result in not obtaining the expected benefit. Lastly, the duration of the intervention in ANSWER was 17.6 months versus 63 days in MACHT, suggesting that the response is not only dose-dependent, but also time-dependent.
Position:
- a)
The long-term use of albumin on an outpatient basis in decompensated patients is very promising, both in reducing mortality and decompensation, and in facilitating the management of ascites. However, its use cannot yet be routinely recommended, as there is a lack of evidence to define the most appropriate group of patients to receive this intervention, how to calculate the dose, how to monitor it, and of studies assessing the cost of this strategy.
- b)
The use of albumin in decompensated patients with portal hypertension for the sole purpose of reducing portal pressure is not recommended.
The ATTIRE study, which included 777 patients hospitalised for decompensation, assessed the use of repeated infusions of albumin to bring their levels up to 30 g/l or more, compared to standard care. The study was negative as albumin did not reduce AKI events, infections or death, and instead increased the number of serious adverse effects, including fluid overload and pulmonary oedema [49].
Position:
The use of repeated infusions of albumin in decompensated hospitalised patients to prevent AKI, infection or death is not recommended.
4.3Post-liver transplantIn the immediate post-transplant context, a retrospective study compared 15 patients who had received continuous infusion of albumin (100 g/day) for seven days with 15 controls [64]. Patients who received albumin had a lower score on the Sequential Organ Failure Assessment (SOFA) scale, in cardiovascular SOFA and higher albumin concentration, colloid osmotic pressure and total proteins, but there were no differences in fluid balance, length of stay, in-hospital deaths, ICU admission or mortality at one year [64]. Another retrospective study assessed whether maintaining albumin levels >30 g/l during the first week after transplant was associated with a lower SOFA score. For this, 60 recipients were analysed: 30 always maintained albumin levels >30 g/l; and 30 had at least one day with levels <30 g/l. In this study, levels >30 g/l were not associated with lower SOFA, but there were lower cardiovascular SOFA scores and less need for vasopressors [65]. In one randomised clinical trial, 40 living donor recipients received either albumin infusion to maintain levels >30 g/l or standard management. The administration of albumin did not lead to differences in terms of organ failure, postoperative course or complications [66].
Position:
The use of albumin after liver transplant in patients with hypoalbuminaemia can be considered as part of the management of fluid balance, but there is no evidence that its use improves clinical outcomes.
4.4Intrinsic or post-renal acute kidney injuryThe most common type of AKI in hospitalised patients with decompensated cirrhosis is pre-renal, reported in 68% of cases [67]. Intrinsic AKI is primarily acute tubular necrosis (ATN) and usually results from persistent hypovolaemia leading to ischaemia or from using nephrotoxins. As regards post-renal AKI, it is not common in decompensated patients, and if it does occur, the obstruction must be corrected. In patients with ATN-AKI there is no evidence that favours the use of albumin over other solutions [9,68–70].
Position:
The use of albumin is not justified in patients with intrinsic or post-renal AKI.
4.5Muscle crampsThese occur more frequently in patients with cirrhosis. The presence of ascites, low mean blood pressure, and advanced disease have been reported as risk factors (Child Pugh C). A crossover design study in which 12 patients with cirrhosis and cramps were administered 100 ml of 25% albumin weekly or placebo found a significant reduction in the frequency of cramps [71]. The European guidelines for patients with decompensated cirrhosis recommend weekly infusion of albumin in patients with muscle cramps [9]. A systematic review reported a reduction in cramps in 75% of the patients who received albumin, probably due to the improvement in circulatory function [72].
Position:
A weekly infusion of albumin may be considered in patients with decompensated cirrhosis with muscle cramps which affect quality of life and who have not responded to other forms of treatment.
4.6Infections other than SBPIn a randomised study of 110 hospitalised decompensated patients with non-SBP infections, the use of albumin at doses of 1.5 g/kg on day 1 and 1 g/kg on day 3 resulted in improvement in renal function and circulatory function, and tended to reduce the rate of type 1 HRS, but did not show improvement in survival, which was the primary endpoint. In the per protocol multivariate analysis adjusted to factors with prognostic value (i.e. age, bilirubin, albumin, blood urea nitrogen and nosocomial infection), treatment with albumin was associated with lower mortality rates at three months; the same analysis, but by intention to treat, was not significant [73]. In another multicentre study, 193 decompensated patients (i.e. Child Pugh >8) with sepsis not associated with SBP, were randomised to receive albumin at doses for SBP/antibiotics versus antibiotics only; the infusion of albumin delayed the onset of AKI, but there were no differences in three-month mortality or the AKI rate [74]. In a meta-analysis of 407 patients included in three studies, the use of albumin was not associated with lower mortality at 30 days (RR 1.62, 95% CI: 0.92-2.84, p = 0.09, I2 = 0%) or at 90 days (RR = 1.27, 95% CI: 0.89-1.83, p = 0.19, I2 = 0%), nor with lesser renal dysfunction (RR 0.55, 95% CI 0.25-1-19, p = 0.13, I2 = 0%), thus concluding that there is insufficient evidence to indicate albumin in all infected patients with cirrhosis [75]. In an open-label multicentre study of 118 patients with non-SBP infections, in which one group was administered albumin/antibiotics and the other antibiotics only, no differences were found in hospital mortality rates, but albumin patients improved their renal and circulatory function within the first seven days, inflammation markers (leucocytes, C-reactive protein, IL-6) showed a tendency to improve, and they had fewer nosocomial infections and a higher resolution rate of ACLF [76].
Position:
There is insufficient evidence to recommend the use of albumin in patients with decompensation and infections other than SBP.
4.7HyponatraemiaHyponatraemia in patients with cirrhosis is defined as sodium below 130 mEq/l, and is usually hypervolaemic and hypo-osmolar, the result of excess free fluid [77]. There is limited evidence supporting the use of albumin to correct hyponatraemia [78].
Position:
The use of albumin may be considered as adjuvant in the treatment of hyponatraemia, especially for short-term and transitory correction, as there is no evidence that its use controls chronic hyponatraemia.
4.8Hepatic encephalopathyMost studies which have found a benefit in the use of albumin in HE focus on patients with ascites, where a reduction in HE has been found as a secondary outcome [61]. There are few studies aimed at HE, and their results are inconsistent. Sharma et al compared the use of albumin 1.5 g/kg/day/lactulose vs lactulose alone in the treatment of HE in 120 patients with cirrhosis and found that the combination was more effective in reversing HE (75% vs 53%, p = 0.03); furthermore, the use of albumin was associated with lower mortality rates (18.3% vs 31.6%, P < 0.05) [79]. In contrast, Simón-Talero et al compared albumin at SBP doses vs isotonic saline, in conjunction with standards of treatment (i.e. laxatives, rifaximin 1200 mg daily) in patients with acute high-grade HE and found no differences in the resolution of HE (57.7 % vs. 53.3%, p > 0.05), but did see an improvement in mortality at three months (69.2% vs. 40.0%, p = 0.02) [80], which suggests that HE could help identify a subset of patients who would benefit from albumin administration. The above is in line with the decrease in mortality which has been evident in studies of long-term use of albumin in decompensated patients [22,61]. In a meta-analysis that included the studies by Sharma and Simón-Talero, the use of albumin was associated with a reversal of HE symptoms (OR 2.4, p = 0.04), as well as a tendency towards reduction in the risk of developing HE (OR 1.63, p = 0.07) [81].
Position:
The use of albumin as preventive or treatment strategy for HE is promising, although further studies are required to support this evidence.
5USE of albumin in ACLF, immunomodulation and future options: scientific position5.1Use in ACLFThe decompensated patient scenario is characterised by a pro-inflammatory and pro-oxidant environment, accentuated in patients with acute decompensation (AD) and even more so in ACLF. These patients have increased intestinal permeability, which causes the translocation of bacteria and pathogen-associated molecular patterns (PAMP), which together with damage-associated molecular patterns (DAMP), contributes to a pro-inflammatory state that can lead to ACLF. Another mechanism influencing multiple organ failure is the development of mitochondrial oxidative phosphorylation dysfunction, which causes a significant reduction in energy production [5].
Considering the pathophysiological mechanisms in the development of ACLF, albumin is emerging as a good candidate for prevention or treatment due to its properties as an immunomodulator, antioxidant, transporter, and protector of the endothelium. Although the ACLF scenario was not specifically analysed in ANSWER [22], it did find a reduction in the episodes of bacterial infections, which are usually the main trigger for ACLF. In this regard, in the INFECIR-2 study [76], which assessed albumin in patients with cirrhosis with non-SBP infections, a higher rate of ACLF resolution was found in the group receiving albumin, with no benefit in terms of mortality [76]. Based on that, and the results of the Pilot-PRECIOSA study, which determined the dose of albumin that exerts an immunomodulatory effect, the administration of albumin could have a use in the context of ACLF [15]. In the development of future trials, the use of albumin in immunomodulating doses should be considered, seeking an “effective concentration of albumin” to guide its dosage.
Position:
Early administration of albumin in patients with cirrhosis and AD may reduce the risk of developing ACLF, but further studies are required.
When using albumin in ACLF, potential clinically useful predictors of response have been reported. In the study by Piano et al, [82] which analysed four different cohorts of European patients with HRS who were treated with albumin/terlipressin, only 53% responded adequately to treatment. When analysing the subgroups according to CLIF-C score, 60%, 48%, and 29% of the patients with ACLF grades 1, 2, and 3 responded to treatment respectively (p < 0.001). Factors associated with mortality were the degree of ACLF, the presence of HRS type 1, age, leucocytes, lack of response to treatment, and late start of treatment. Assessing the patient with ACLF by CLIF-C criteria should therefore be standard, as it helps provide predictors of mortality, useful for management in intensive care, and of response to albumin infusion [83–85].
Position:
The administration of albumin may be useful as a complementary treatment in ACLF, but studies focused on this scenario are needed. In the particular case of patients with HRS-AKI, the grade of ACLF assessed by CLIF-C is a predictor of the response to treatment with albumin/terlipressin, so it is essential to identify this condition early to start treatment in a timely manner.
5.2Use in immunomodulation and systemic inflammationThe long-term dose of albumin required to normalise the serum albumin concentration is much higher than that used in most studies [22,86]. The effect of treatment with albumin on its concentration is related to two factors: the administered dose and the baseline albumin concentration. In the Pilot-PRECIOSA study, in patients with hypoalbuminaemia, of the seven patients who received low doses (1 g/kg every 2 weeks) only one had an increase to ≥34 g/l in all measurements, while in the six patients who received high doses (1.5 g/kg every week) their albumin concentration returned to normal [15]. There is an inverse correlation between baseline albumin concentration and the increase obtained during treatment; the lower the albumin concentration, the greater the increase achieved during treatment [15].
Position:
Studies are necessary to assess the albumin dose required in long-term treatment to increase and normalise the albumin concentration.
In the Pilot-PRECIOSA study, a greater reduction in inflammatory cytokine concentrations was found in the group receiving high doses of albumin, confirming that the immunomodulatory effect requires adequate dosing. In INFECIR-2, treatment with albumin and antibiotics was associated with a reduction in circulating cytokines, but not in the group which received antibiotics only [15].
Position:
Short- and long-term treatment with albumin at high doses has been shown to have immunomodulatory effects in decompensated cirrhosis, and its use is promising, but more evidence is required.
Left ventricular function plays an important role in circulatory dysfunction in patients with cirrhosis [87], thus the cardiac index progressively falls from compensated to decompensated cirrhosis and HRS [88]. Fernández et al compared the systemic haemodynamic changes in patients with SBP before and after the administration of albumin or hydroxyethyl starch, and found that the markers of circulatory dysfunction (left ventricular stroke work, cardiac output, peripheral vascular resistance and mean arterial pressure) improved only in the group receiving albumin [58,89]. The data in the Pilot-PRECIOSA study shows that normalisation of albumin concentration in decompensated uninfected patients who received high doses did not induce significant changes in central blood volume and portal pressure, but did so in left ventricular function. This observation is important as it is known that systemic inflammation has a harmful effect on cardiac function, and the beneficial effect found in the PRECIOSA study could be mediated, at least in part, by the immunomodulatory effect of albumin [15].
Position:
Treatment with albumin improves circulatory dysfunction and decreases markers of systemic inflammation in decompensated patients, but studies are needed to define the group of patients who can benefit the most from this strategy with regard to clinical outcomes, and the required dose and periodicity of administration.
6PharmacoeconomicsWithin the framework of the Answer Study, a cost-utility analysis was carried out from the perspective of the Italian health service using a time horizon of one year [22]. The analysis considered the incremental benefit in terms of quality-adjusted life-years (QALY) calculated from the results of the EQ-5D questionnaire, observing a gain of 0.117 QALY after 12 months of follow-up for patients receiving albumin treatment compared to those who received standard therapy only.
The incremental cost considered hospitalisations, paracentesis, albumin used as an intervention in the clinical trial and follow-up visits for its administration, and albumin used in routine clinical practice. The incremental cost per patient/year was found to be €2,488, which implied a higher cost per QALY of €21,265, much lower than the cost-effectiveness criterion of €35,000 established for the country. The authors assessed the robustness of the analysis of the incremental cost-utility ratio (ICUR) using a sensitivity analysis, which showed that it had a 92.5% probability of being cost-effective considering a willingness-to-pay threshold of €35,000 [22].
In Spain, a study by Fernández J et al estimated the cost per patient with cirrhosis and uncomplicated ascites receiving standard treatment and compared it to the cost per patient treated with weekly intravenous albumin infusion scheme and standard treatment. This comparison considered the ratios of the different complications observed in the ANSWER study, but applied the costs from the perspective of the Spanish healthcare system. Preliminary results showed a net reduction of €1,377 per patient/year when patients were treated with long-term albumin infusion and standard treatment compared to standard treatment alone [90].
Lastly, in Mexico, within the framework of preparing this position paper, a cost-of-illness analysis was performed in order to compare the cost per patient with cirrhosis and uncomplicated ascites receiving standard treatment and that for a patient treated with the scheme of long-term weekly intravenous albumin infusion and standard treatment based on the ANSWER protocol. The economic analysis followed the perspective of the Mexican healthcare system and showed an estimated net reduction of Mex$33,417 per patient per year [91].
Position:
Data from Spain and Italy, when adapted to the Mexican healthcare system, seem to indicate a favourable direct impact on the pharmaco-economics of albumin use in patients with liver cirrhosis and its complications.
7ConclusionsOur understanding of the biology of cirrhosis and its complications has increased considerably over the last 20 years. This knowledge has been accompanied by a clearer understanding of the therapeutic application of albumin based on the non-oncotic properties of this molecule. As a result, albumin now has indications well established by international guidelines, such as in large volume paracentesis, SBP and HRS. However, a higher level of evidence is needed for other indications.
From the positions on controversial or emerging uses of albumin established in this review, we can conclude that, in decompensated patients, the routine use of albumin on an outpatient basis is very promising, although evidence is lacking to recommend its routine use, as well as in decompensated patients and infections other than SBP. The use of albumin for the sole purpose of reducing portal pressure, or repeated infusions of albumin in decompensated hospitalised patients with the purpose of preventing AKI, infection or death is also not recommended. The lack of evidence also affects the use of albumin in patients with intrinsic or post-renal AKI, for improving clinical outcomes after liver transplantation, and for the control of chronic hyponatraemia.
Promising uses of albumin, but which require additional studies to support the evidence, include: preventive or treatment strategy for HE; decreasing risk of developing ACLF in cirrhotic patients with AD; adjuvant treatment in ACLF; immunomodulation in decompensated cirrhosis; improving circulatory dysfunction; and reducing markers of systemic inflammation in decompensated patients.
Authors' contributionsAll authors contributed equally to the intellectual content of this position paper. GCN, CMV and AT wrote the manuscript. AT is the author who is responsible for the integrity of the work as a whole.
Conflicts of interestAll authors have received support from Grifols for the present manuscript regarding provision of study materials, medical writing assistance, and with article processing charges. Graciela Castro-Narro received honoraria for lectures from Grifols. Rene Male-Velázquez received honoraria for lectures from Gilead. Francisco Javier Bosques received honoraria for lectures from Grifols, AbbVie, and Janssen. José Antonio Velarde Ruiz-Velasco received honoraria for lectures from Abbot. Javier Manuel Meza-Cardona received honoraria from Gilead for an educational event. Norberto Chávez-Tapia received honoraria for lectures from Merck. Aldo Torre received honoraria for lectures from: Seria, Viatri, Medix, Grünenthal, Alfa Wasserman, and Cell Pharma. Carlos Moctezuma-Velázquez, Rafael Trejo-Estrada, Rosalba Moreno-Alcántar, Heriberto Rodríguez-Hernández, Aleida Bautista-Santos, Carlos Córtez-Hernández, Eira Cerda-Reyes, Juanita Pérez-Escobar, Jonathan Aguirre-Valadez, Raúl Contreras-Omaña, Godolfino Miranda-Zazueta, Monica del Rocío Reyes-Bastidas, Edgar Santino García-Jiménez, Nicolas Joaquín Fernández-Pérez, and Juan Manuel Aldana-Ledesma declare no other conflict of interest.
Cristina Fuster PhD and Elisabet Viayna PhD (Grifols) are acknowledged for their support in the literature and manuscript review. Jordi Bozzo PhD, CMPP (Grifols), is acknowledged for editorial support in the preparation of this manuscript. Grifols is a manufacturer of plasma-derived albumin products.