Introduction. Porphyria cutanea tarda (PCT) is the most common type of porphyria. The strong association between PCT and hepatitis C virus (HCV) infection is well established. Although antiviral treatment of chronic hepatitis C may improve PCT in some cases, de novo onset of PCT has been observed in patients undergoing peginterferon/ribavirin treatment. We present a rare case of a genotype 3 HCV-positive liver transplant recipient who developed PCT during antiviral treatment and discuss its probable etiopathogenesis.
Case presentation. A genotype 3 HCV-positive liver transplant recipient, a 42-year-old man, was treated with peginterferon alfa-2a (180 μg/week) combined with ribavirin (1,200 mg/day) for recurrence of HCV infection after liver transplantation. He presented with hyperferritinemia but tested negative for genetic hemochromatosis (C282Y and H63D mutations). During antiviral therapy, he developed skin lesions on his hands characterized by vesicles and erosions consistent with PCT. PCT was confirmed by skin biopsy and elevated urinary uroporphyrin levels (1,469 mg/24 h). He was treated with chloroquine (200 mg) twice weekly, resulting in gradual regression of the skin lesions. Antiviral treatment was stopped after 48 weeks, and the patient achieved a sustained virological response. In conclusion, we report an extremely rare case of PCT in a genotype 3 HCV-positive liver transplant patient treated with antiviral therapy. We believe that the combination of HCV genotype 3 infection; hemolysis due to ribavirin treatment; and increased plasma levels of cytokines, such as IL-6 and TNFα, could have altered the patient's iron metabolism and thus caused PCT.
Porphyria cutanea tarda (PCT) is the most common type of porphyria and results from reduced activity of uroporphyrinogen decarboxylase (UROD), an enzyme implicated in the heme biosynthetic pathway. The strong association between PCT and hepatitis C virus (HCV) infection is well established. The percentage of PCT patients found to be antibody-positive for HCV ranges from approximately 50-90%.1 However, the percentage of HCV antibody-positive patients who develop PCT is small, which argues against HCV infection as a direct cause of PCT.2 Although antiviral treatment of chronic hepatitis C may improve PCT in some cases,3-6de novo onset of PCT has been observed in patients undergoing peginterferon/ribavirin treatment.7-9 Other factors that could be implicated in PCT are hemo-chromatosis, alcohol consumption, and drug use. Only one case of sporadic PCT in a liver transplant recipient has been reported in the literature. Herein, we describe the case of an HCV-positive liver transplant recipient who developed PCT during antiviral treatment and discuss its probable etiopathogenesis.
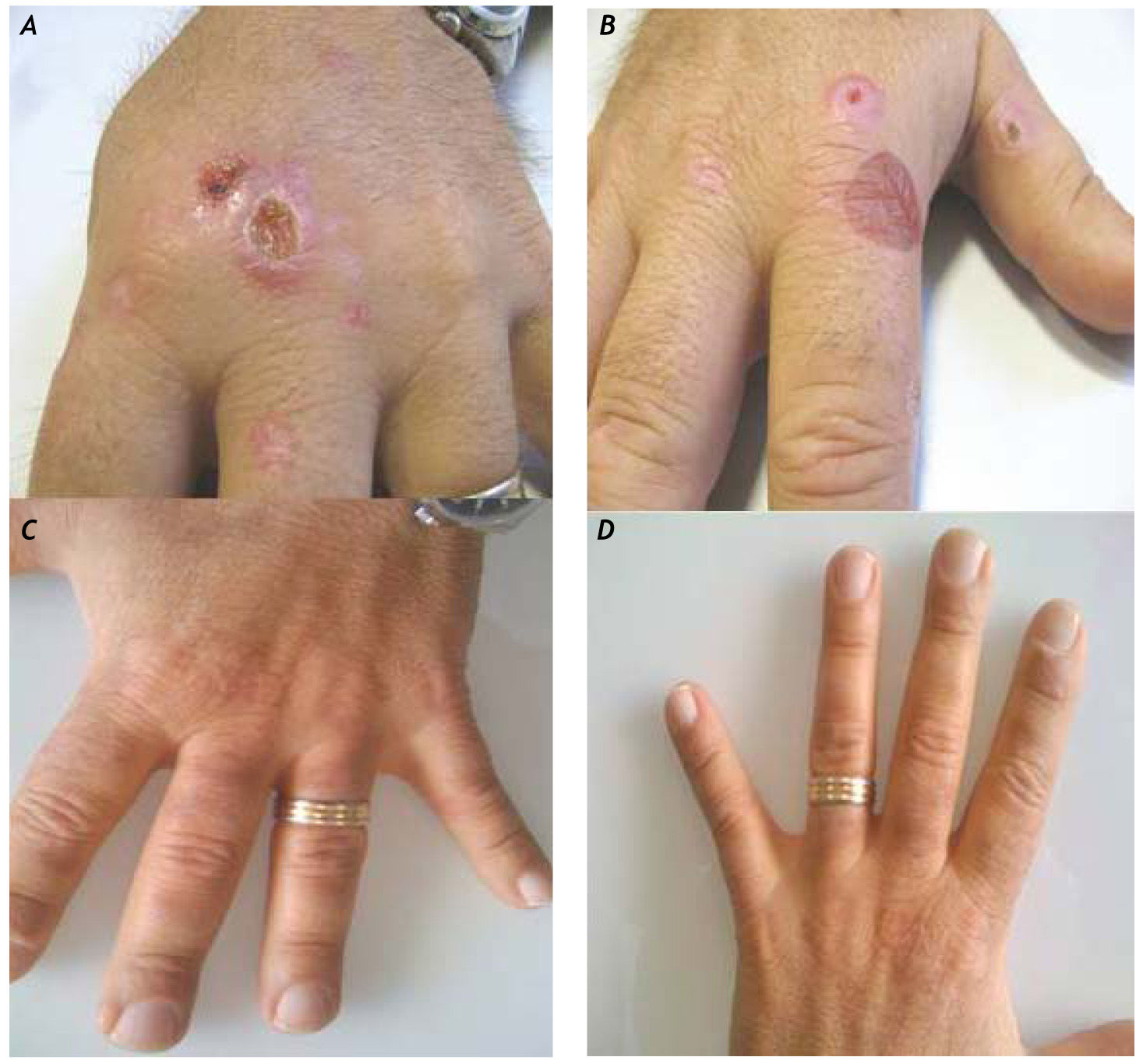
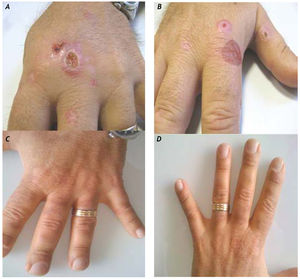
Case ReportA 42-year-old man with a history of drug addiction had been diagnosed with chronic hepatitis C (genotype 3) in 1994. His medical history was not significant except for alcohol intake. Diabetes mellitus was diagnosed in 2002, and the patient was started on insulin therapy. A liver biopsy examination in 2002 revealed liver cirrhosis (Ishak grade (G) 14, stage (S) 6). In the same year, he was treated with peginterferon alfa-2b (100 μg/week) and ribavirin (800 mg/day) for 6 months, but his HCV infection relapsed 2 months after suspension of antiviral treatment. At that time, his ferritin level was found to be 950 ng/mL, but tests for hemochromatosis (C282Y and H63D mutations) were negative. The patient was started on a new, 10-month course of treatment with peginterferon alfa-2a (180 μg/week) and ribavirin (800 mg/day) in 2004; however, as on the previous occasion, HCV relapsed 2 months after the end of treatment. The patient developed ascites and experienced rapid deterioration of liver function (MELD 18) in January 2006, and received a liver transplant in July 2006. His immunosuppressive treatment consisted of tacrolimus in combination with mycophenolate. Due to persistent hypertransa-minasemia, a liver biopsy was performed in June 2008, and active chronic moderate hepatitis C (Ishak G8 S3) with grade II liver steatosis was revealed. Positive Prussian blue iron staining was observed in the Kupffer cells and scattered in the hepatocytes. There were no signs of liver rejection. The patient's HCV RNA level was 2,841,000 IU/mL (TaqMan, Roche), and his ferritin level was 1,100 ng/mL. The patient was treated with peginterferon alfa-2a (180 μg/week) (Hoffman-La Roche Ltd.-Basel) and ribavirin (1,000 mg/day) for 44 weeks but again relapsed 2 months after the end of treatment. A new course of treatment with peginterferon alfa-2a (180 mg/week) and a higher dosage of ribavirin (1,200 mg/day) (Hoffman la Roche, Basel) was initiated in March 2010. In April 2010, the patient started on epoetin alfa (40,000 IU/week) for ribavirin-induced anemia (Hb 10.5 g/dL). In July 2010, the patient developed skin lesions on his hands characterized by vesicles and erosions consistent with PCT (Figures 1A-1B). PCT was confirmed by skin biopsy and elevated urinary uroporphyrin levels (1,469 mg/24 h). At the time of the PCT diagnosis, the patient's hemoglobin level was 11.0 g/dL and his ferritin level was 1,150 ng/mL. The plasma levels of the cytokines IL-6 and TNFα were > 10 pg/mL and 21.2 pg/mL, respectively (Endogen Inc., Massachusetts) (normal value range for (IL)-6 is 3 ± 0.6 pg/ mL; normal value range for TNFα is 3.1 ± 0.2 pg/mL). As the patient's chronic ribavirin-induced anemia did not permit phlebotomy, the PCT was treated with chloroquine (200 mg) twice weekly, which allowed gradual regression of the skin lesions (Figures 1C-1D). Antiviral treatment was stopped after 48 weeks, and the patient achieved a sustained virological response.
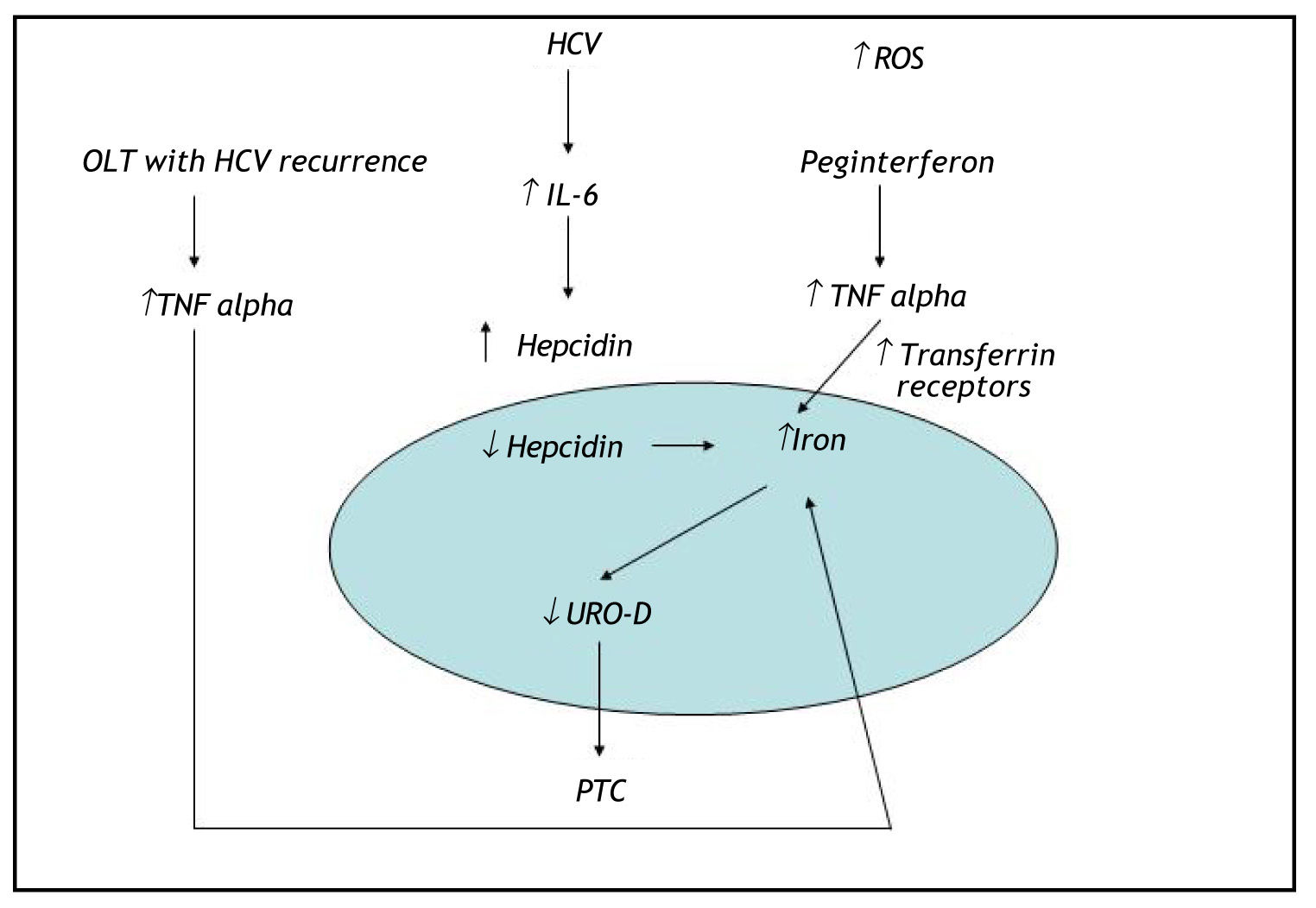
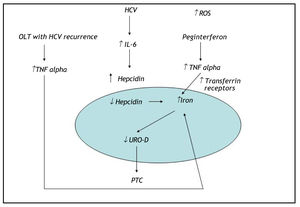
DiscussionHerein, we describe the case of an HCV-positive liver transplant patient who developed de novo PCT during antiviral therapy. To our knowledge, this is the first case of PCT reported in an HCV-positive liver transplant patient treated with antiviral therapy. Huang, et al. have described another case of post-liver transplant PCT in an HCV-positive patient with iron overload but not receiving antiviral therapy. In that report, the patient developed an alcohol abuse habit after the transplant, which could have contributed to increased hepatic iron deposits and thus to the development of PCT. Our patient had a high plasma ferritin level despite the absence of genetic hemochromatosis. PCT is actually identified as the most frequent extrahepatic manifestation of HCV infection in countries where the prevalence of this viral infection is high, and HFE gene mutations may be present in up to 73% of patients with PCT. High ferritin levels are frequently reported in chronic hepatitis C patients lacking an HFE mutation. Sebastiani, et al. demonstrated that hepatic iron deposits and liver steatosis in well-compensated chronic hepatitis C infection were strongly associated with HCV genotype 3.10 Several studies have established that there are increased serum prohepcidin or hepcidin levels in chronic hepatitis C patients with high plasma ferritin levels.11,12 This could be due to inflammatory stimuli induced by the release of a cytokine such as interleukin (IL)-6, which can induce production of these 2 substances.13 At high levels, hepcidin inhibits iron efflux from hepatocytes, macrophages, and enterocytes by binding to ferroportin, which subsequently increases hepatic iron deposits.13 Tumor necrosis factor alpha (TNFα) is a proinflammatory cytokine that contributes to the regulation of iron metabolism through several mechanisms. TNFα has been reported to stimulate the synthesis of ferritin and inhibit iron release from macrophages. A transient increase in TNFα gene expression during anti-HCV treatment has been demonstrated; this may be connected with the patient's response to interferon a/ribavirin therapy.14 Our patient presented with high plasma levels of TNFα and (IL)-6, which could have further contributed to increased liver iron deposits. In addition, the high dosage of ribavirin prescribed to our patient could have increased hepatic iron levels via hemolysis and thus contributed to the de novo onset of PCT. Five cases of porphyria cutanea tarda during interferon and ribavirin therapy for chronic HCV infection have been described in the literature.15 In all 5 cases, the patients had clinical evidence of systemic or hepatic iron overload. The excess of iron in the cytosol of hepatocytes can damage UROD, reducing the action of this enzyme and causing accumulation of its precursor. In our case, we believe that HCV infection was indirectly involved in the pathogenesis of PCT and that altered iron metabolism appears to play the key role in triggering PCT. We hypothesize that HCV genotype 3 infection, hemolysis due to ribavirin treatment, and increased plasma levels of cytokines such as (IL)-6 and TNFα could have triggered the onset of PCT by altering the patient's iron metabolism (Figure 2).
Hemolysis due to ribavirin treatment, genotype 3 HCV infection, and increased plasmas levels of the cytokines IL-6 and TNFα could have contributed to the development of PCT by altering intracellular iron metabolism. PCT: porphyria cutanea tarda. OLT: orthotopic liver transplant. UROD: uroporphyrinogen decarboxylase enzyme. TNF alpha: tumor necrosisfactor alpha. ↑: increase. ↓: decrease.