Hepatic encephalopathy is a frequent complication of cirrhosis, when this event becomes persistent, treatment compliance should be verified and any precipitating factor need to be identified. Also the presence of portosystemic shunts, which are a rare cause of decompensation or persistence hepatic encephalopathy need to be ruled out. In this paper we report the case of a 57 year old man with persistent hepatic encephalopathy secondary to the presence of a porto-onfalo-femoral shunt successfully closed with the placement of an Amplatzer device.
Decompensation of hepatic encephalopathy by spontaneous portosystemic shunt is rare, the incidence is unknown in the presence of cirrhosis. Clinical symptoms occur with shunt diameters larger than 10 mm.1 The evidence obtained to determine its management is based on a review of published case series. Closure of the shunt, surgically or by interventional radiology has been proposed as a therapeutic option, however the main complication is aggravation of portal hypertension.1–3 Here we present the successful management of a long spontaneous shunt (porto onfalo femoral) with an Amplatzer Vascular Plug II device (AVP II, AGA Medical, Plymouth, MN USA).
Clinical DataA 57 years old, previously healthy male, started in 2006 with asthenia and adynamia. Liver function tests showed elevated transaminases (ALT 75U/L, AST 97U/L, AF 119U/L, GGT 195U/L). At the time of presentation a viral panel was negative, also he had a negative immunological profile (antinuclear antibodies, anti-mitochondrial and anti-smooth muscle).
Liver ultrasound suggested mild hepatic steatosis and impaired ecotexture with nodular surface. Endoscopy showed large esophageal varices. Liver biopsy was performed which showed liver cirrhosis. Hemochromatosis and Wilson’s disease were ruled out, so the diagnosis of cryptogenic cirrhosis was established.
On June 2011 he presented the first event of hepatic encephalopathy and lactulose therapy was started. During the next year the patient developed 15 episodes of hepatic encephalopathy that required hospitalization. L-ornithine-L-aspartate, rifaximin, zinc and magnesium were added to treatment. The serum ammonium was 169.9 mcg/dL in May 2012 and 140.2 mcg/dL in September 2012. The patient was listed for liver transplantation, with a B Child Pugh score and MELD of 18 points.
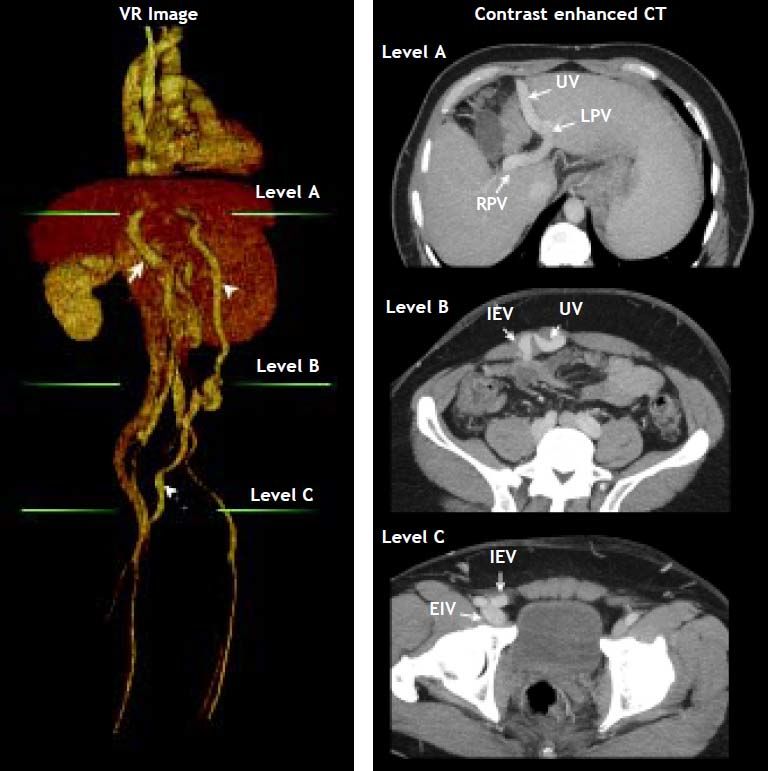
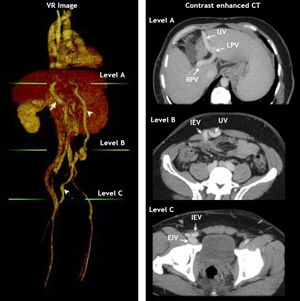
In October 2012 he developed a new hepatic encephalopathy episode, which was classified as persistent and severe (grade II-III). Treatment was adjusted, intravenous hydration and lactulose enemas were started, until the stabilization of the episode was achieved. The EEG and brain MRI ruled out other etiologies of neurological impairment. Infection and variceal bleeding were also ruled out. A thoracoabdominal CT was done which demonstrated the presence of a shunt with reperfusion of the umbilical vein into the right femoral vein, with a diameter of 12.34 (Figure 1). Since the patient referred adequate treatment adherence, we considered the porto-cava spontaneous shunt as the cause of the persistent encephalopathy. The case was evaluated with the Interventional Radiology department and decided to occlude the shunt with an Amplatzer Vascular Plug II device in the umbilical vein (Figure 2).
Volume rendering (VR) image with corresponding contrast enhanced CT images showing: Arrow: Main portal vein; arrow head: recanalized umbilical vein; pointed arrow: inferior epigastric vein. Green lines at VR image indicate axial CT level. At level A shows the recanalized umbilical vein (UV) from the left portal vein (LPV). RPV, rigth portal vein. Level B shows the place of anastomosis between umbilical vein (UV) and inferior epigastric vein (IEV). At level C the normal drainage of inferior epigastric vein (IEV) into external iliac vein (EIV) above the inguinal ligament is shown.
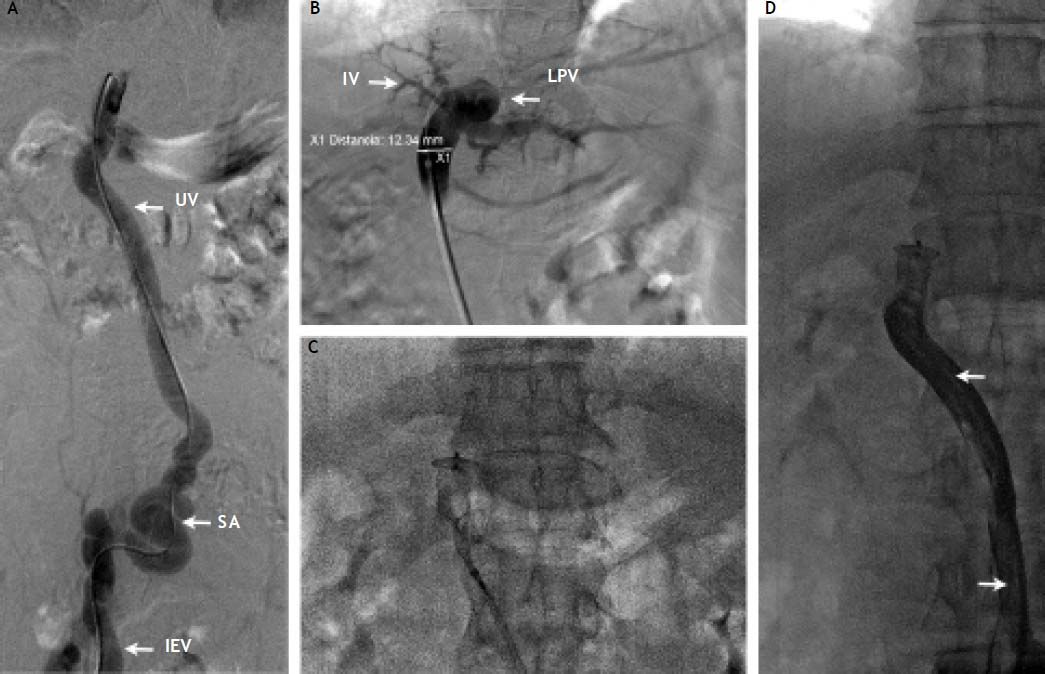
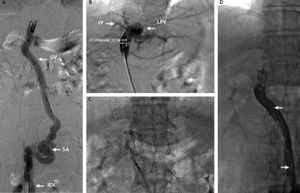
A. Contrast injection after introduction of a Cobra 2 (5 Fr; Cook, Bloomington, IN, USA) catheter through the IEV to the recanalized umbilical vein (UV). SA: Probable site of anastomosis between the UV and the IEV. B. Contrast injection through the Amplatzer II delivery system showing opacification of the left portal vein (LPV) and branches of segment IV. Measurement was done at the level of the umbilical vein showing a diameter of 12.3 mm. C. Deployment of the Amplatzer II vascular occlusion device at the level of the umbilical vein. D. Final contrast injection demonstrating occlusion of the umbilical vein and collapse of the umbilical vein. Note the long radio-lucid lines in the UV lumen suggesting contact between the walls of the umbilical vein (arrows).
A percutaneous access was performed at the epigastric vein and 8 Fr shaft was placed. After advancing a hydrolophilic guide wire trough the shunt into the portal vein, the vascular shaft was removed and the delivery shaft of the Amplatzer II was placed. An Amplazter II device of 18 mm of diameter was chosen. The oversizing of 50% (6 mm) was performed to adequately occlude the vessel and prevent device migration, final venography showed occlusion of the shunt. The procedure was carried out without complications. The patient was discharged three days later, with significant improvement of encephalopathy.
Follow UpTwo weeks after the procedure the patient was evaluated, on physical examination he remained without neurological alterations, only mild asterixis was detected and cataloged as encephalopathy grade I. The serum ammonium levels were reduced to 60 mcg/dL. No melena or hematemesis was referred and he continued treatment with lactulose and rifaximin.
DiscussionHepatic encephalopathy is a syndrome characterized by a wide range of neuropsychiatric symptoms, reversible and not attributable to neurological or metabolic causes different from liver disease.4–7 Every event is associated with high mortality, depending on the extent and severity of each episode. The appearance of hepatic encephalopathy varies according to different series from 42% in the first year to 23% after 3 years from the first event. It is a common complication of chronic liver disease, it is estimated that up to 28% of patients with cirrhosis have a clinically detectable event and 84% have subclinical neuropsychiatric disorders.4,8
The most accepted theory to explain the pathophysiology is related to deleterious effects of ammonium not metabolized by the liver, which causes abnormalities in neurotransmitters and astrocyte damage by oxidative stress.5,9–12
During patient assessment, it is important to accurately classify the type of encephalopathy. Hepatic encephalopathy (HE) can be classified according to their etiology into 4 types:13,14
- a)
Related to acute liver failure.
- b)
Related to the presence of portosystemic shunts without liver disease.
- c)
Related to cirrhosis.
- d)
Related to alterations in the urea cycle.
The type C, related to cirrhosis, can be subclassified according to Vienna Consensus (1988) in episodic, persistent and minimum. The episodic can be precipitated, spontaneous and recurrent.9,15 The persistent HE refers to the presence of neurological ab- normalities that fluctuate between level I (clinically detectable) and never down staging the 0 level. The hepatic encephalopathy is subclassified depending on the treatment on mild and severe. For this propose the West Haven scale is used.8,9,13,15
In relation to diet and medical treatment, overall care measures should start with preventing falls in confused patients and avoiding driving. Also infection prevention, blood volume control, and strict maintenance of glucose and electrolytes levels is of paramount importance. Any pH disturbance should be corrected.
Caloric intake should be between 35-40 kcal/kg per day with protein intake of 1.2 to 1.5 g/kg/ day. In this patients we can consider adding zinc and branched chain aminoacids (valine, leucine, isoleucine) and remove from the diet wheat and milk. Management should be given to the precipitating events, with emphasis to reduce ammonium levels.
There is more evidence to lactulose, although there is controversy about it, in a systematic review of Cochrane, Als-Nielsen et al., found no superiority of nonabsorbable disaccharides versus placebo.16,17 It is a disaccharide composed of fructose and galactose, nonabsorbable, considered the first line therapy for patients with liver disease and HE, induces colonic acidification of the medium, which suppresses the growth of bacteria that produce ammonia, also has a cathartic effect, which reduce the serum ammonium. It has also been shown to prevent recapture of glutamine by the gut wall, which prevents it from being metabolized to ammonia.18 The oral dose or by nasogastric tube is 30 mL 2 to 4 times a day, reaching 2-3 soft stools daily. Rifaximin is a semisynthetic broad-spectrum antibiotic, rifamycin derivative, which is minimally absorbed in the intestine (< 0.4%) due to its pyridoimidazole ring, reducing systemic side effects and allows chronic use, inhibits bacterial RNA synthesis, reducing the growth of organisms producing ammonium.19,20 The maximum dose is 1,200 mg/day divided every 8-12 h. Other treatment options are sodium benzoate, L-ornithine-L-aspartate and vegetable protein based diet, with more weak evidence.5,11,21–23
If treatment is optimized and the patient continues with HE the approach should include a contrast enhanced abdominal CT to rule out portosystemic shunts.1,3,12 The etiology of this type of encephalopathy is caused by high levels of ammonia that are shunted from portal to systemic circulation, these type of shunts could be congenital, spontaneous or post TIPS procedure. In the present case a sponta- neous porto onfalo femoral shunt with a diameter of 12.34 mm diameter was discovered.
It has been demonstrated that clinical encephalopathy correlate with the diameter of the shunt (> 10 mm).1 Treatment options to close this communication can be either surgical (ligation) or minimal invasive (placement of sclerosing or embolizating agents).24–26 There are reports of shunt embolization with coils.1–3 Ballon retrograde embolization (BRTO) was described in 1996 by Kanagawa to control bleeding from gastric varices, it also has been used for closing portosystemic shunt in patients with chronic recurrent EH.27 Sclerosing agents may be used in liquid or foam. Mukund, et al., evaluated a series of 7 cases with occlusion of large shunts by BRTO with the application of sodium tetradecyl sulfate [STS], and he find that only 28% (2 patients) showed a response of encephalopathy one month after closure and 85% (6 patients) improved after 4 months.3
Complications of shunt closure could be porto collateral flow redistribution and growth of esophageal or gastric varices and a increased risk of bleeding.3 Tashiro, et al., reported a series of 6 patients treated with shunt ligation plus splenectomy, with the aim of reducing increased portal venous pressure (PVP). Only one patient required BRTO 8 months after surgery.2 The use of Amplatzer device for occlusion of this type of shunts is not widely reported in the literature, Boixadera, et al., reported a case where the Amplatzer device was successfully used for embolization of a spontaneous, large size mesocavo shunt. The Amplatzer Vascular Plug II device compared to coils, has several advantages: can be more accurately placed in the vessel, has a lower risk of migration, and time of occlusion is fast. It is unknown the impact on the redistribution of porto collateral flow by producing an abrupt and immediate closure of the shunt.28
ConclusionsThe presence of persistent hepatic encephalopathy despite the proper treatment and patient compliance should force to rule out spontaneous shunts. The use of Amplatzer devices for closing spontaneous shunts is rarely described in the literature. Closure of large collaterals is technically challenging and is a low risk procedure.
Assessment of the etiology and triggering factors of the persistent hepatic encephalopathy allows selecting patients who will benefit from shunt closure, as in this case. It should always take into account the risk of flow redistribution and the increased po- tential of development of varices with the potential bleeding risk.
Abbreviations- •
AF: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BRTO: balloon-occluded retrograde transvenous obliteration.
- •
CNS: central nervous system.
- •
CT: computed tomography.
- •
EEG: electroencephalogram.
- •
GGT: gamma-glutamyltransferasa.
- •
HE: hepatic encephalopathy.
- •
MELD: Model for End-stage Liver Disease.
- •
MR: magnetic resonance.
- •
PVP: portal venous pressure.
- •
STS: sodium tetradecyl sulfate.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
- •
USG: ultrasonography.