Introduction and aim. Serum glypican-3 (GPC3) has been explored as a non-invasive biomarker of hepatocellular carcinoma (HCC). However, controversy remains on its diagnostic accuracy. Therefore, we aimed to conduct a systematic review and metaanalysis to evaluate the differential diagnostic accuracy of serum GPC3 between HCC and liver cirrhosis (LC) cases.
Material and methods. After the strict filtering and screening of studies from NCBI, PUBMED, Clinical Trials, Cochrane library, Embase, Prospero and Web of Science databases, 11 studies were selected. All studies provided the sensitivity and specificity of GPC3 and the alpha-fetoprotein (AFP) in the HCC and LC diagnosis. The sensitivity and specificity, and the area under the receiver operating characteristic curve (AUC) were determined and compared between GPC3 and AFP, which was set as a positive control.
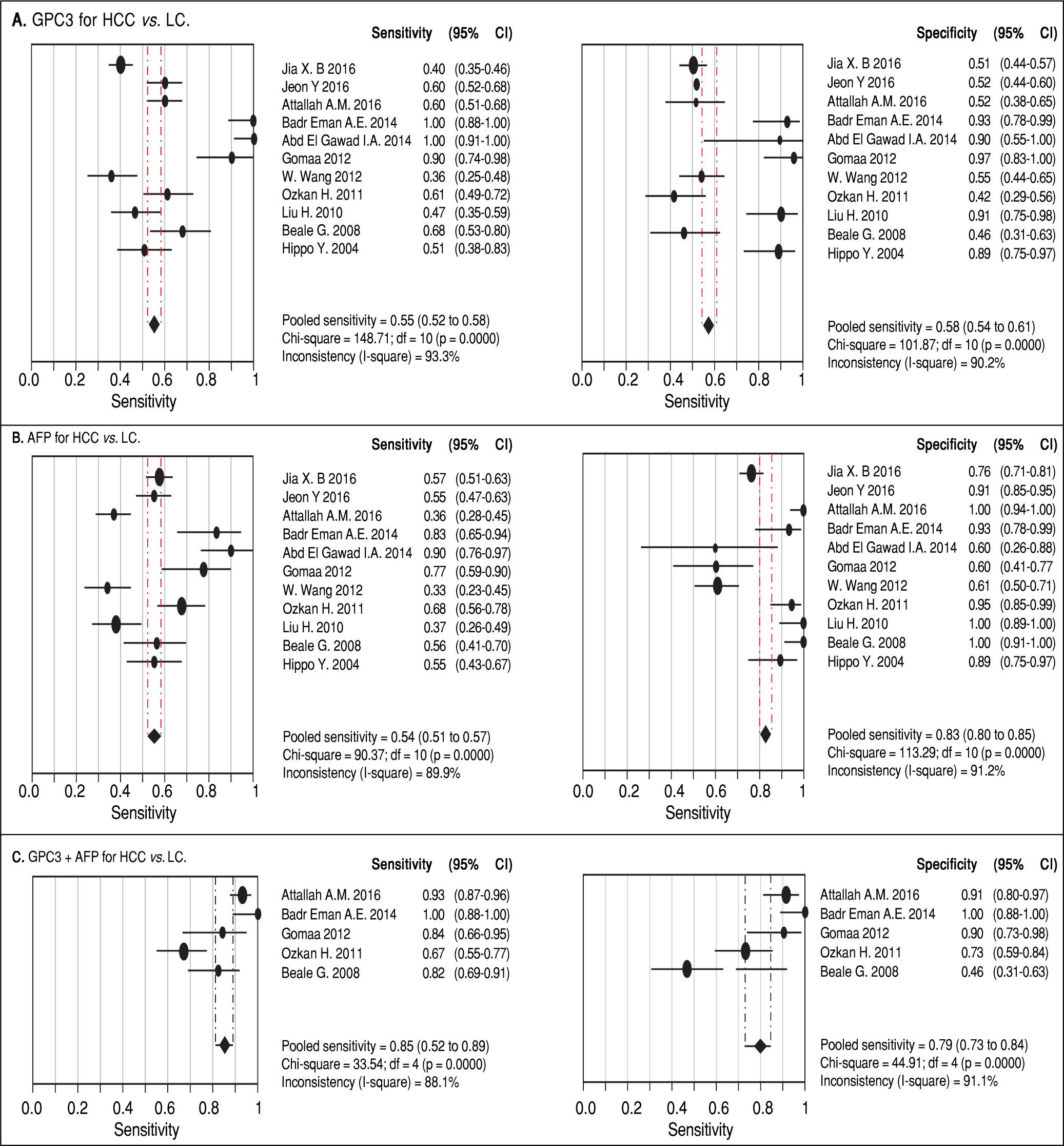
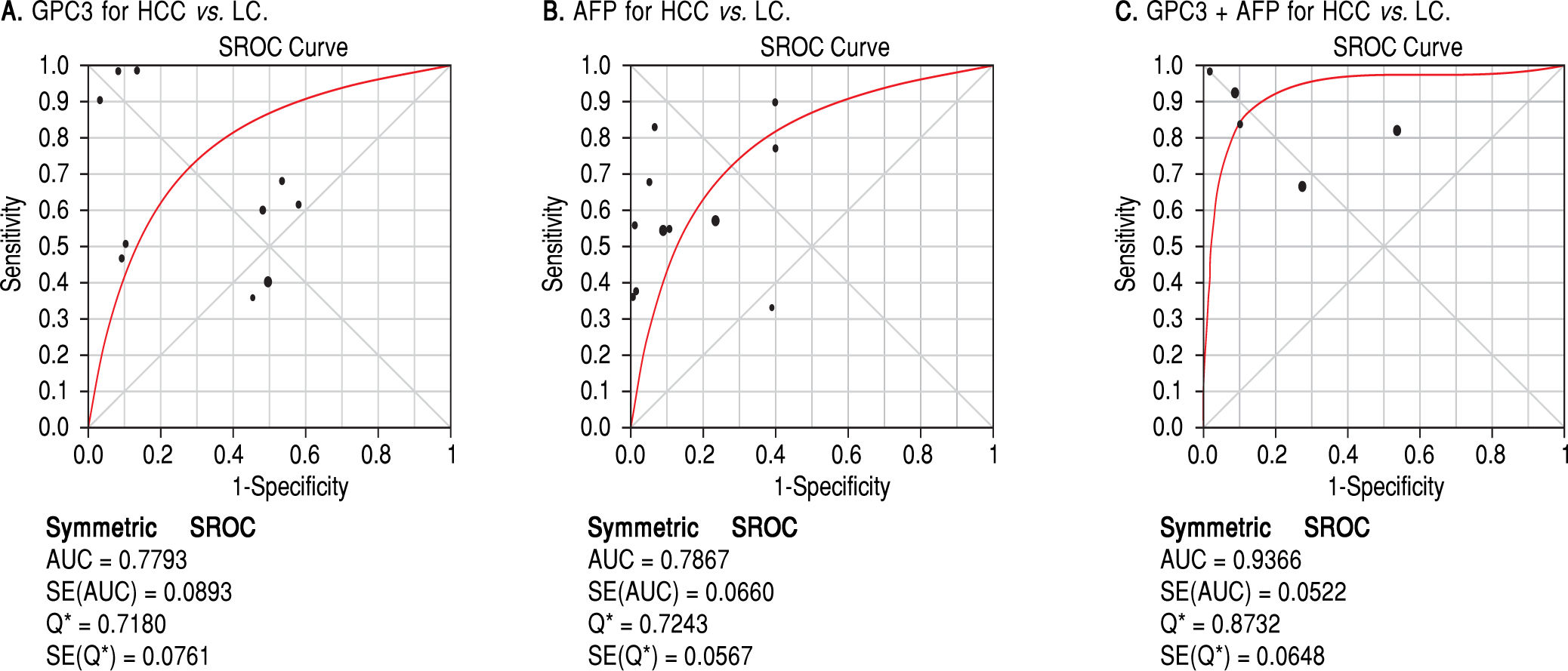
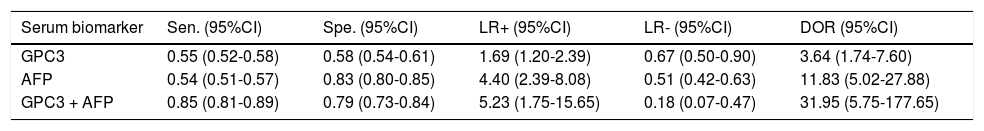
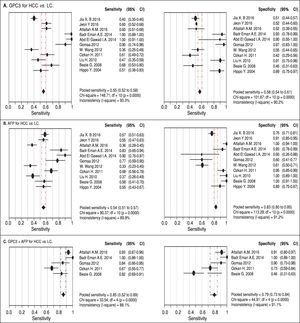
Results. Pooled sensitivity (95% CI) and specificity (95% CI) were 0.55 (0.52-0.58) and 0.58 (0.54-0.61) for GPC3, 0.54 (0.51-0.57) and 0.83 (0.80-0.85) for AFP, and 0.85 (0.81-0.89) and 0.79 (0.73-0.84) for GPC3 + AFP, respectively. The AUCs of GPC3, AFP and GPC3 + AfP were 0.7793, 0.7867 and 0.9366, respectively. GPC3 had a nearly similar sensitivity as AFP, while the specificity and AUC of GPC3 was lower than that of AFP. The combination of GPC3 and AFP yielded a better sensitivity and AUC than GPC3 or AFP.
Conclusion. Serum GPC3 is inferior to AFP in the differential diagnosis between HCC and LC. However, the combination of GPC3 and AFP exhibited a much better performance.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and the second leading cause of site-specific cancer-related death. Usually, HCC is asymptomatic at the early stage. However, it is always detected at the advanced stage when diagnosed, which limits treatment options. Thus, the early diagnosis of HCC is of great significance to enable early therapeutic intervention and prolong the survival period.1 In this context, the serological level of alpha-fetoprotein (AFP) has been used as a classical marker of HCC. However, AFP has modest sensitivity and specificity for HCC diagnosis,2,3 which makes its application questionable. Indeed, AFP has been excluded from the present guidelines of the American Association for the Study of Liver Disease and the European Association for the Study of the Liver due to its modest accuracy.4 Therefore, there is an urgent need to explore a surrogate serological marker with higher sensitivity and specificity for HCC diagnosis.
Glypican-3 (GPC3) is a transmembrane proteoglycan anchored to the cell membrane by glycosylated phosphatidylinositol (GPI). GPC3 has been verified to be closely correlated to the growth, proliferation, invasion and metastasis of cancer cells.5,6 Several studies have addressed the involvement of GPC3 in various types of tumors, including HCC.7-10 Interestingly, increasing evidence indicates that approximately 40% of HCC patients are positive for GPC3 and negative for AFP.3 This might indicate the promising role of GPC3 in HCC diagnosis and its superiority over AFP. Notably, there is no correlation between the level of GPC3 and AFP,11,12 which indicates that both parameters are functionally independent. Given that increasing evidence has indicated the application of serum GPC3 as a noninvasive marker for HCC diagnosis, many investigators have explored this issue in comprehensive settings with respect to the diagnostic efficiency of GPC3, compared to AFP.
Several liver diseases, such as viral hepatitis, autoimmune hepatitis, alcoholic hepatitis and primary biliary cirrhosis, have progressed to liver cirrhosis (LC), which is a major predisposing factor of HCC risk. It is noteworthy that although the vast majority of HCC cases developed from cirrhotic livers, not all cirrhotic livers always end with HCC. In previous meta-analysis studies,13-18 no meta-analysis study employed cirrhotic patients as controls. Therefore, the novelty of the present systemic review and meta-analysis over all previous studies originates from the use of LC cases as the control for the study. Through this approach, we were able to precisely and comprehensively assess the accuracy of GPC3 in the differential diagnosis between HCC and LC, compared to AFP.
Material and MethodsLiterature search strategyTwo independent investigators (Chang Su and Dahai Xu) conducted an electronic literature search on seven databases, which include the NCBI, PUBMED, Clinical Trials, Cochrane library, Embase, Prospero and Web of Science databases. The search was updated as of November 10, 2017. The entry terms used for the literature search were as follows:
- •
HCC. Liver neoplasm, hepatic neoplasm, hepatocellular cancer, hepatic cancer, and liver cancer.
- •
GPC3. Glypican, glypican 3, glypican3, and glypican-3.
- •
AFP. Alpha-Fetoprotein and alpha Fetoprotein. No limit was set on publication time, study design and publishing format, and only publications in the English language were searched.
Inclusion criteria:
- •
Studies that accurately diagnosed the experimental group with HCC
- •
Studies that measured serum GPC3 and AFP protein.
- •
Studies that determined the HCC diagnostic sensitivity and specificity of GPC3 and AFP.
Exclusion criteria:
- •
Letters, reviews, case reports, abstracts, editorials, and expert opinions.
- •
Studies that lack sufficient data to obtain the sensitivity and specificity of GPC3 and AFP in HCC and LC.
- •
Studies on experimental models, such as laboratory animals and cultured cells.
- •
Studies that considered specimens other than blood.
- •
Studies that evaluated serum maker levels by messenger RNA, DNA, or DNA polymorphisms.
- •
Studies that focused on diseases other than primary hepatocellular carcinoma.
Different articles with the same authors and data were checked to avoid duplicates, and the most recent or most complete study was selected.
Data extractionAfter selecting all the eligible studies, two investigators (Liang Sun and Yuanyuan Gao) independently extracted the data. The collected data included the first author’s name, the country of origin of the patients, publication year, name of the journal, study design, number of patients, age and gender, assay type, cut-off value, and raw data (True Positive, TP; False Positive, FP; False Negative, FN; True Negative, TN). For disagreements, a third investigator (Youjun Li) was consulted to make the judgment.
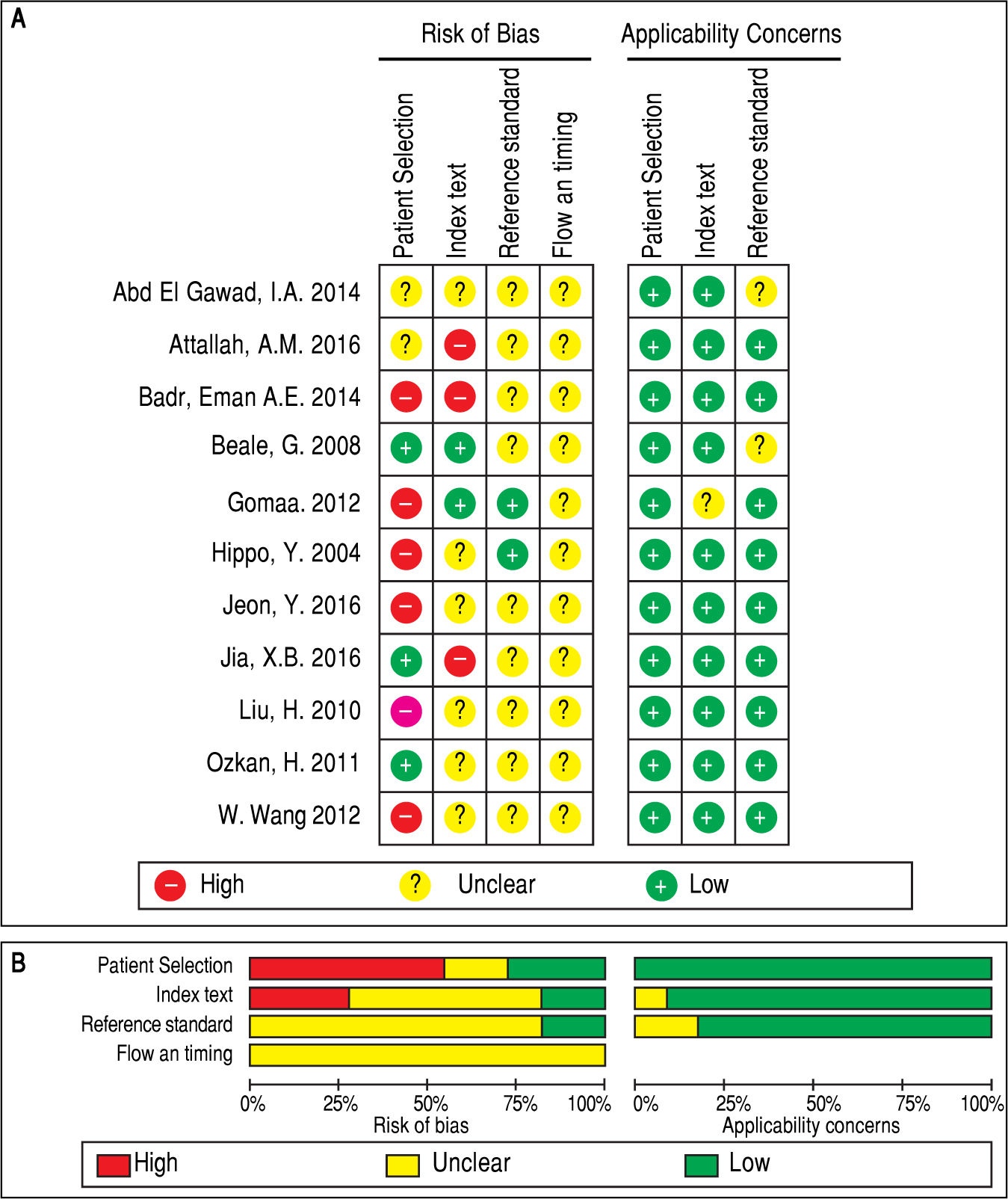
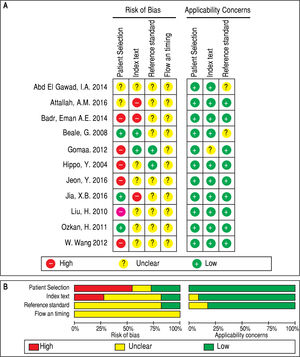
Assessment of quality of the selected articlesTwo independent investigators (Chang Su and Dahai Xu) assessed the quality of the included studies according to the Quality Assessment of Studies of Diagnostic Accuracy II (QUADAS-2).14 Signaling questions on the risk of bias in the assessment check list were labeled as ‘‘yes’’, ‘‘no’’, or ‘‘unclear’’. Items that assessed applicability risk were labeled as ‘‘high’’, ‘‘low’’, or ‘‘unclear’’. If the study design was cross-sectional, the risk of bias of the patient selection domain was labeled as ‘‘high risk’’. A third investigator (Youjun Li) was consulted for disagreements.
Statistical analysisStatistical analyses were performed using Review Manager 5.3, Stata 12 and Meta-Disc 1.4 software. Sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR-), diagnostic odds ratio (DOR), 95% confidence intervals (95% CIs) and the area under the receiver operating characteristic curve (AUC) were determined. Forest plots and receiver operating characteristic curves (ROCs) were used to determine the diagnostic performance. The heterogeneity of the retrieved data was evaluated using the I2-value. An I2-value < 50% and a P-value > 0.1 was considered with insignificant heterogeneity. If the heterogeneity was not identified, the fixed-effects model was used for the meta-analysis, and if the heterogeneity was identified, the random-effects model (DerSimonian-Laird) was used. Publication bias was measured by Egger’s test using Stata 12 (StataCorp LP, College Station, TX, USA). P < 0.05 was considered statistically significant. In order to analyze the source of heterogeneity, threshold analysis was conducted using Meta-Disc 1.4 and meta-regression was conducted using Stata 12.
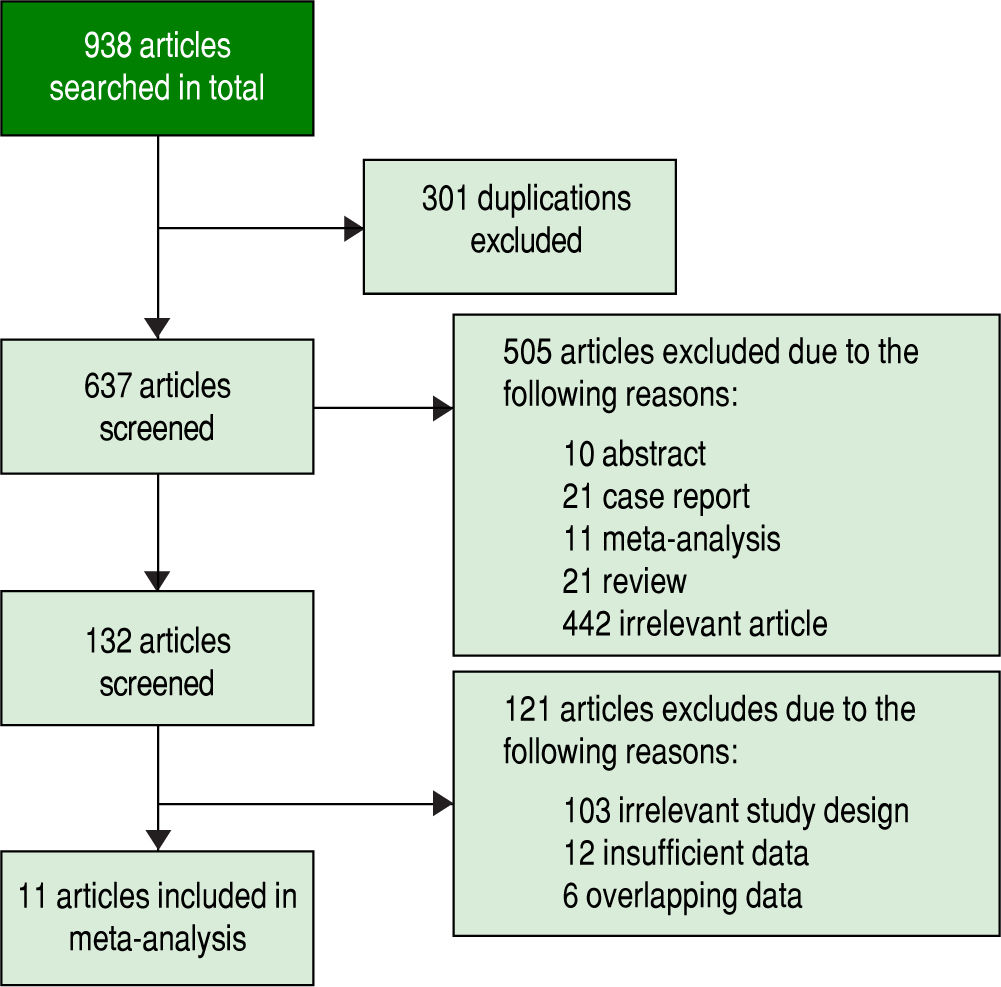
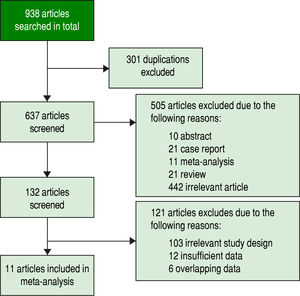
ResultsStudy selectionInitially, 938 potentially relevant articles were retrieved from the databases mentioned above. Then, due to duplication, 301 articles were excluded. Next, after reviewing the titles and abstracts, 10 abstracts, 21 case reports, 11 meta-analyses, 21 reviews and 442 irrelevant articles (subjects were not human and the specimens were not serum, or GPC3 was determined based of the expression level of its mRNA or DNA) were excluded. Subsequently, by reading the full text, 121 studies were excluded due irrelevant design or insufficient data to calculate the sensitivity and specificity of GPC3. Finally, a total of 11 studies were eligible8-11,19-25 (Figure 1). All included studies were approved by the Ethics Committee, and an informed consent was obtained from all subjects.
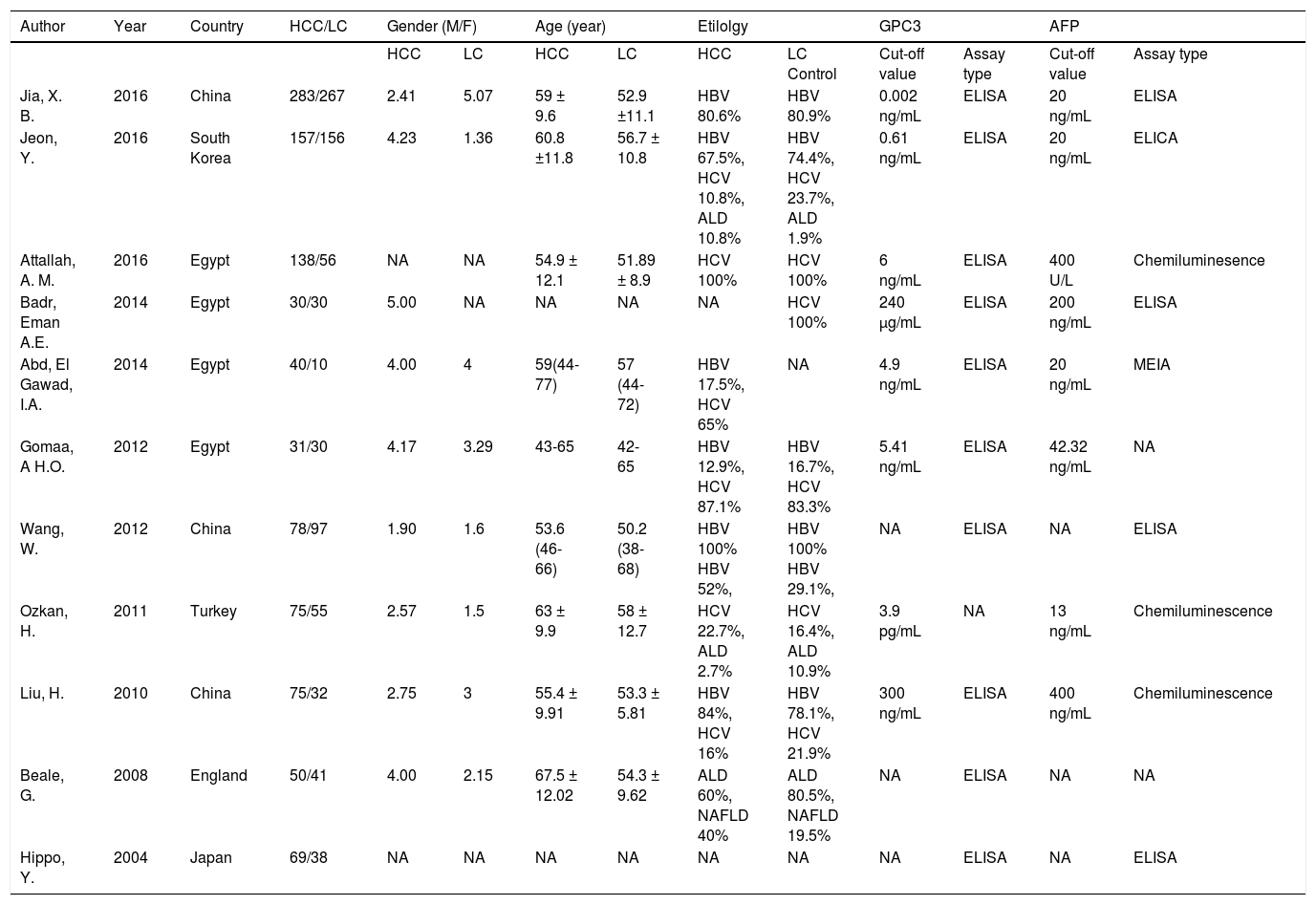
Features and methodologyThe clinical features and methodology of these eligible studies are summarized in table 1.
Clinical features of the included studies
| Author | Year | Country | HCC/LC | Gender (M/F) | Age (year) | Etilolgy | GPC3 | AFP | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC | LC | HCC | LC | HCC | LC Control | Cut-off value | Assay type | Cut-off value | Assay type | ||||
| Jia, X. B. | 2016 | China | 283/267 | 2.41 | 5.07 | 59 ± 9.6 | 52.9 ±11.1 | HBV 80.6% | HBV 80.9% | 0.002 ng/mL | ELISA | 20 ng/mL | ELISA |
| Jeon, Y. | 2016 | South Korea | 157/156 | 4.23 | 1.36 | 60.8 ±11.8 | 56.7 ± 10.8 | HBV 67.5%, HCV 10.8%, ALD 10.8% | HBV 74.4%, HCV 23.7%, ALD 1.9% | 0.61 ng/mL | ELISA | 20 ng/mL | ELICA |
| Attallah, A. M. | 2016 | Egypt | 138/56 | NA | NA | 54.9 ± 12.1 | 51.89 ± 8.9 | HCV 100% | HCV 100% | 6 ng/mL | ELISA | 400 U/L | Chemiluminesence |
| Badr, Eman A.E. | 2014 | Egypt | 30/30 | 5.00 | NA | NA | NA | NA | HCV 100% | 240 µg/mL | ELISA | 200 ng/mL | ELISA |
| Abd, El Gawad, I.A. | 2014 | Egypt | 40/10 | 4.00 | 4 | 59(44-77) | 57 (44-72) | HBV 17.5%, HCV 65% | NA | 4.9 ng/mL | ELISA | 20 ng/mL | MEIA |
| Gomaa, A H.O. | 2012 | Egypt | 31/30 | 4.17 | 3.29 | 43-65 | 42-65 | HBV 12.9%, HCV 87.1% | HBV 16.7%, HCV 83.3% | 5.41 ng/mL | ELISA | 42.32 ng/mL | NA |
| Wang, W. | 2012 | China | 78/97 | 1.90 | 1.6 | 53.6 (46-66) | 50.2 (38-68) | HBV 100% HBV 52%, | HBV 100% HBV 29.1%, | NA | ELISA | NA | ELISA |
| Ozkan, H. | 2011 | Turkey | 75/55 | 2.57 | 1.5 | 63 ± 9.9 | 58 ± 12.7 | HCV 22.7%, ALD 2.7% | HCV 16.4%, ALD 10.9% | 3.9 pg/mL | NA | 13 ng/mL | Chemiluminescence |
| Liu, H. | 2010 | China | 75/32 | 2.75 | 3 | 55.4 ± 9.91 | 53.3 ± 5.81 | HBV 84%, HCV 16% | HBV 78.1%, HCV 21.9% | 300 ng/mL | ELISA | 400 ng/mL | Chemiluminescence |
| Beale, G. | 2008 | England | 50/41 | 4.00 | 2.15 | 67.5 ± 12.02 | 54.3 ± 9.62 | ALD 60%, NAFLD 40% | ALD 80.5%, NAFLD 19.5% | NA | ELISA | NA | NA |
| Hippo, Y. | 2004 | Japan | 69/38 | NA | NA | NA | NA | NA | NA | NA | ELISA | NA | ELISA |
QUADAS-2 quality assessment was conducted to evaluate the quality of the included studies (Figure 2). All studies were retrospectively designed, and none of these studies were randomized controlled trials (RCTs). Merely two studies were consecutive investigations.8,10 Thus, the patient selection domain of other studies was labeled as ‘high risk’. Since there was no fixed diagnostic standard in each study, the flow and timing domain was labeled as ‘unclear’, while most of the studies included the cut-off levels of either serum GPC3 or AFP.8-11,19,20’22’23’25 Two studies21,24 did not include the cutoff value for GPC3, while two studies21,24 did not include the cutoff value for AFP. Notably, the baseline-pretreatment level of serum GPC3 was determined in four studies,8,9,19,24 and the remaining seven studies did not mention whether the sample was collected before therapy.10,11,20-23,25 On the other hand, some studies indicated that their thresholds were not pre-specific (the cut-off point was fixed before the test), because their cut-off values were determined based on the ROC analysis.8-10,19 Hence, the index test was set as ‘high risk’. Since the temperature of the serum sample storage is critical for assay accuracy, three different temperatures (-20¯C, -70¯C and -80¯C) were considered.8,9,11,19,21,24 Studies with high risk of bias were not excluded to investigate the heterogeneity in the following step.8-10,19,21-23,25,26
Diagnostic accuracy of serum GPC3 and AFP for HCC diagnosisSensitivity, specificity, LR +, LR-, DOR and I2 were calculated for all the included studies (Table 2 and Figure 3). The sensitivity of GPC3 was almost the same as that of AFP, but the specificity of GPC3 was lower than that of AFP. Moreover, the AUC of GPC3 (0.7793) was lower than that of AFP (0.7867) and GPC3 + AFP (0.9366) (Figure 4).
Diagnostic values of GPC3 and/or AFP for HCC vs. LC
| Serum biomarker | Sen. (95%CI) | Spe. (95%CI) | LR+ (95%CI) | LR- (95%CI) | DOR (95%CI) |
|---|---|---|---|---|---|
| GPC3 | 0.55 (0.52-0.58) | 0.58 (0.54-0.61) | 1.69 (1.20-2.39) | 0.67 (0.50-0.90) | 3.64 (1.74-7.60) |
| AFP | 0.54 (0.51-0.57) | 0.83 (0.80-0.85) | 4.40 (2.39-8.08) | 0.51 (0.42-0.63) | 11.83 (5.02-27.88) |
| GPC3 + AFP | 0.85 (0.81-0.89) | 0.79 (0.73-0.84) | 5.23 (1.75-15.65) | 0.18 (0.07-0.47) | 31.95 (5.75-177.65) |
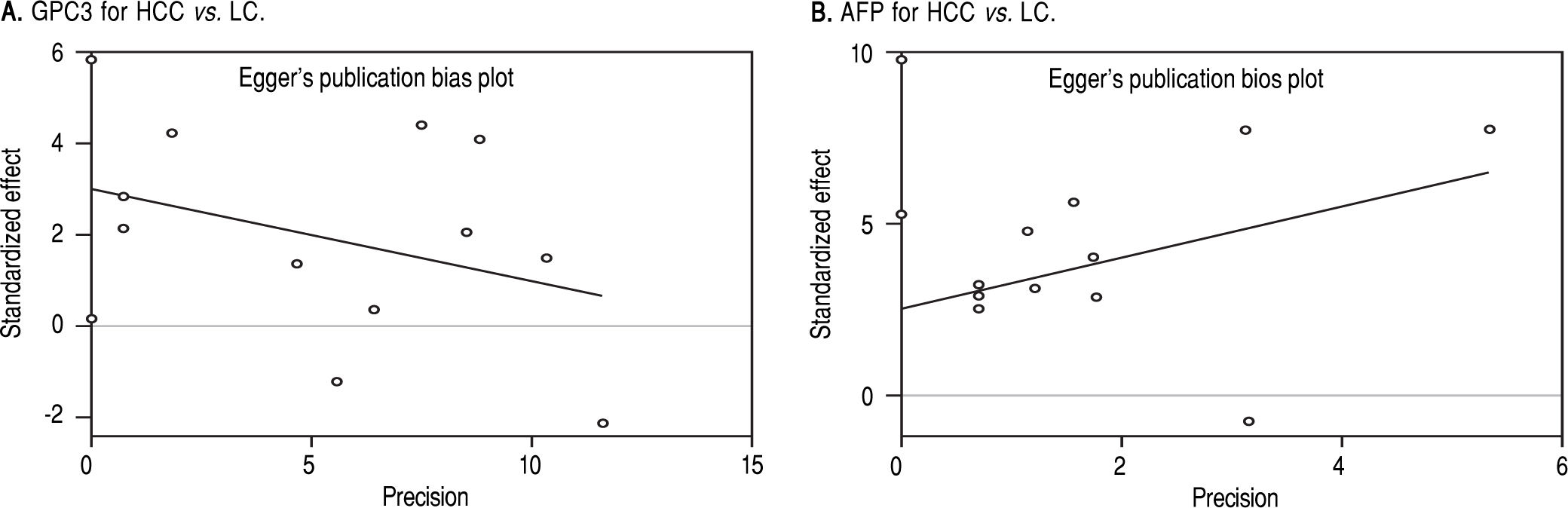
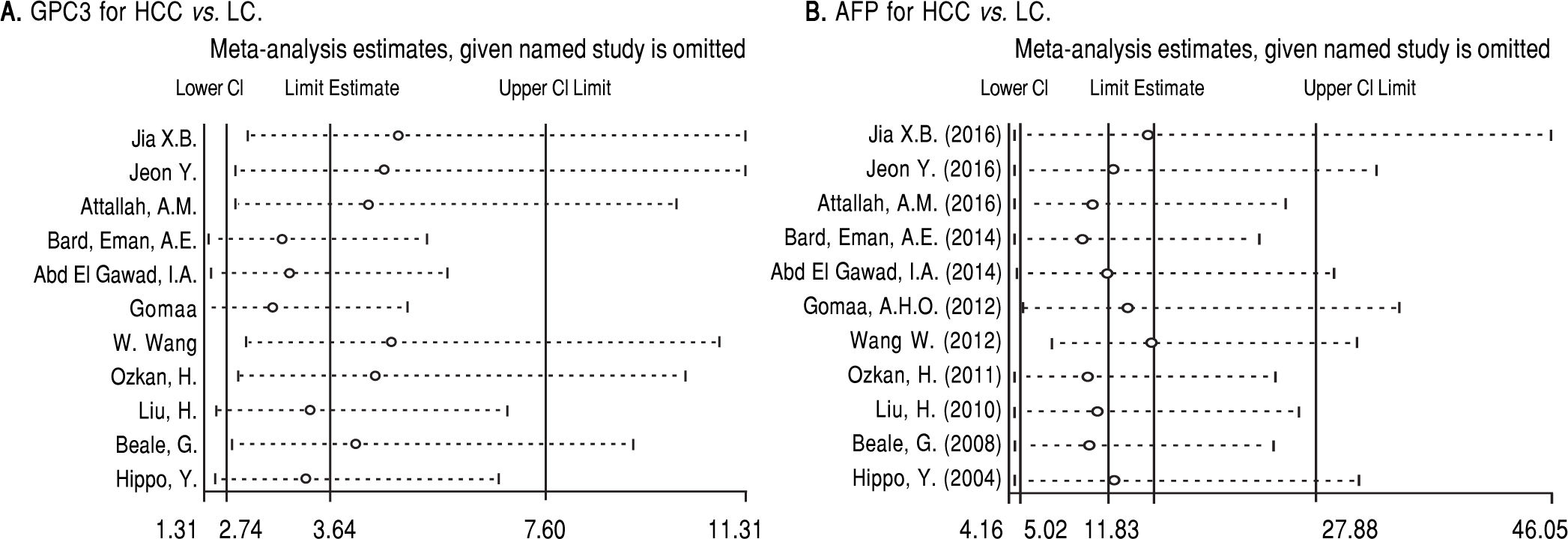
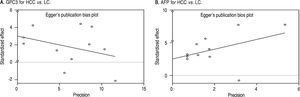
Based on Egger’s test, the included studies did not have publication bias (Coefficient = 3.00, P = 0.052 for GPC3; Coefficient = 2.56, P = 0.065 for AFP) (Figure 5). Furthermore, the diagnostic odds ratio revealed significant heterogeneity (I2 = 85.3%, P < 0.001 for GPC3; I2 = 86.8%, P < 0.001 for AFP). The threshold effect test (Spearman’s correlation coefficient was -0.209, P = 0.537, for GPC3; Spearman’s correlation coefficient was 0.392, P = 0.233, for AFP) indicated that the cut-off point was not the source of heterogeneity. In addition, the meta-regression analysis indicated that publication year, country, sample size, clinical characteristics methodology and the quality of articles were not correlated with heterogeneity. The sensitivity analysis indicated that the pooled estimates were stable and not influenced by a single study (Figure 6). A subgroup analysis was conducted by publication year, country, methodology, clinical features, and study quality. However, subject size was the only possible source of heterogeneity found (whether the number of patients in both HCC and LC groups were 3 40 or not). For GPC3, in the subgroup that had a larger sample size was 66.5%, while the heterogeneity in another subgroup was 77.2%. The present meta-analysis did not include any RCTs. Other covariates were also potential sources of heterogeneity, such as the differences in operating protocol, enrollment period, pathological grades and tumor burden.
DiscussionIn the present study, we found that the sensitivity of GPC3 was nearly the same as that of AFP, while the specificity of GPC3 was lower than that of AFP. However, the combination of GPC3 and AFP can significantly increase the sensitivity and AUC in the differential diagnosis between HCC and LC. However, the specificity of GPC3+AFP was lower than that of AFP.
Recently, GPC3 has become a focus in HCC studies. Many studies have explored the performance of GPC3 in liver tissues for HCC diagnosis,27-29 and some clinical trials on therapeutic agents against GPC3 have also been conducted.30-33 In the present investigation, we conducted a meta-analysis on studies that investigated the application of GPC3 as a diagnostic marker for HCC, and compared this with AFP. The novelty of this investigation originates from the use of LC patients as control subjects, which were compared to HCC. For the 11 eligible studies, the results infer that the performance of GPC3 to discriminate HCC from LC was unsatisfactory and lower than that of AFP. However, the combination of GPC3 and AFP produced a higher diagnostic accuracy than either of the makers when used separately.
GPC3 is implicated in cell growth, differentiation and migration.34 GPC3 is greatly expressed in HCC tissues, fetal livers and most HCC cell lines, compared to other normal human tissues.8,20,35 GPC3 has also been reported in other tumors, such as lung cancer, thyroid cancer and melanoma.36,37 Notably, serum GPC3 levels were significantly higher in HCC than in normal controls, as well as in liver cirrhosis, colorectal cancer, esophageal cancer, gastric cancer and hepatitis cases.2,19,20 Furthermore, GPC3 expression in HCC was positively associated with tumor size and pathological grade.3 In addition, Ohno, et al. reported that HCC patients with high GPC3 level had poor prognosis.38-40 Badr, et al. and Yeon, et al. found that the sensitivity of serum GPC3 in small size HCC was higher than that of AFP.19,41 Moreover, Zhang QY, et al. reported that GPC3 is superior to AFP for HCC diagnosis, with higher sensitivity and specificity.42Generally, controversy remains on the application of GPC3 in HCC diagnosis, since several studies have either supported 2,3,19,42,43or limited 9,11,44,45 the role of GCP3 in HCC diagnosis.
The results of the present meta-analysis support the observation of Jia, et al.,8 since there was no significant difference in the serum level of GPC3 between HCC and LC patients, indicating that GPC3 is not efficiently valid as a HCC serum biomarker.
It is important to note that the sensitivity, specificity and AUC of GPC3 determined in the present meta-analysis differed from those reported in other studies.13-18 This difference might be attributed to either the difference in the nature of the control cohort or the number of included studies.
To the best of our knowledge, the present meta-analysis is the first to set patients with LC as a control cohort. However, there were some limitations in the present study:
• A small number of included studies was included, which was attributed to the strict inclusion and exclusion criteria.
• There was an obvious heterogeneity, which might be contributed to the limited number of subjects.
• The quality of the included studies was unsatisfactory. Due to the absence of RCTs, the treatment effects may be overestimated.
Therefore, more high quality researches with different subgroups according to tumor etiology, tumor size and tumor stage are needed in the future to further verify the role of GPC3 in HCC diagnosis.
In conclusion, Serum GPC3 cannot be used as a substitute for AFP to differentially diagnose HCC and LC. However, the combination of both markers would be a better choice.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (#81372456) YJL.
Conflict of InterestThe authors declares that there is no conflict of interest regarding the publication of this article.