We report the case of a 37-year-old woman with no relevant medical history. She was admitted to the hospital for epigastric pain related with food intake for 4 days; the pain did not improve with symptomatic management. A laparoscopic cholecystectomy due to acute lithiasic cholecystitis was performed. However, after 4 days, postoperative painless jaundice was evident; thus, endoscopic retrograde cholangiopancreatography was performed, which revealed an amputation of intrapancreatic common bile duct, as well as secondary intra- and extrahepatic bile duct dilatation. Brushing of the distal portion of the common bile duct revealed a well-differentiated adenocarcinoma. Therefore, a Whipple procedure with pylorus preservation was performed. Pathologic diagnosis of a papillary in situ adenocarcinoma with two microscopic foci of microinvasion was established. The pathologic Tumor-Node-Metastasis (TNM) stage was pT1, pN0, pM0, R0. The patient is asymptomatic and disease-free 24 months after surgery. In general, adenocarcinomas of the extrahepatic bile ducts are uncommon and have a poor prognosis. However, symptomatic patients with early disease stages are even rarer and can be cured surgically.
Carcinomas of the extrahepatic bile ducts (EHBD) are uncommon. They represent 0.16% of all invasive carcinomas in males and 0.15% in females in the United States (U.S.) and constitute the third leading cause of EHBD obstruction.1,2 Nearly 2,500 new cases are reported each year in the U.S., and their incidence is 0.88 per 100,000 inhabitants.1,2
Carcinomas have a major incidence in males, with a 1.3:1 ratio.1 Mean age at diagnosis is 63.3 years (range, 31-81 years). On evaluation of a specific population, Brugen, et al. reported on 43 patients: 93% were Caucasian; 5% Afro-American, and 2%, Hispanic.3 Carcinomas can arise in any part of the EHBD. For prognostic and therapeutic purposes, it has been useful to divide the extrahepatic ductal system into three parts: upper (hepatic ducts and the common hepatic duct); middle (common hepatic duct and proximal common bile duct), and lower (intra- or peripancreatic zone of the common bile duct).1,4 Over 50% are located in the upper third, 18% in the middle third, and 22%, in the lower third.1,4,5
Because these tumors are rarely diagnosed in their early stages, we present a case of an in situ and intramucosal carcinoma of the lower third of common bile duct.
Clinical CaseA 37-year-old woman was admitted to the Médica Sur Hospital in Mexico City with stabbing, epigastric abdominal pain associated with consumption of food during 4 days. The patient did not have relevant medical history and was previously healthy. A clinical diagnosis of acute lithiasic cholecystitis was established; therefore, a laparoscopic cholecystectomy was performed. Intraoperatively, the gallbladder was enlarged, had an edematous wall, and contained multiple gallstones. Moreover, external compression of the common hepatic duct by an impacted stone in cystic duct was observed.
During the postoperative period, the patient evolved favorably, but at 48 h, jaundice was documented. The liver tests showed hyperbilirubinemia at the expense of direct bilirubin. After a 24-h observation period, the following new changes in liver function tests were reported: Total bilirubin, 5.8 (previously 3.9); Direct bilirubin, 3.97; indirect bilirubin, 1.88; SGPT, 197; SGOT, 120; FA, 220, and GGT, 271.
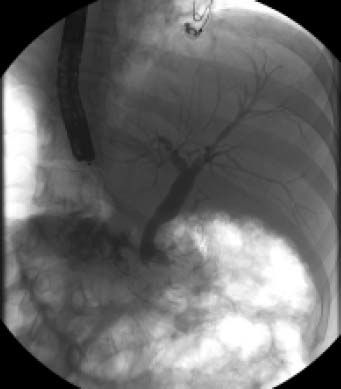
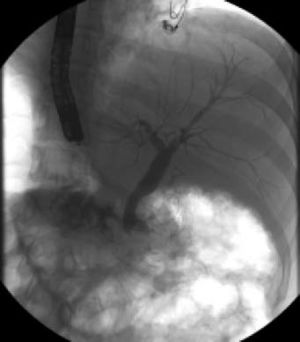
Cholangiopancreatographic magnetic resonance (MR) was performed without relevant findings. Endoscopic retrograde cholangiopancreatography (ERCP) revealed stenosis and amputation of the intrapancreatic common bile duct (Figure 1). A sphincterotomy with brushing of the lower third of the common bile duct was performed. Because of the pathological report, a pyloric-preserving Whipple surgery was performed.
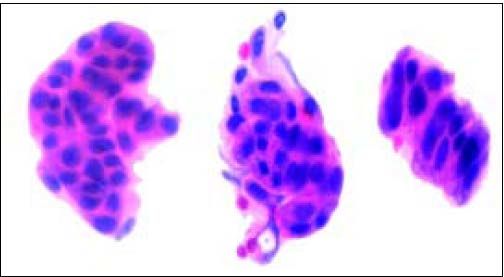
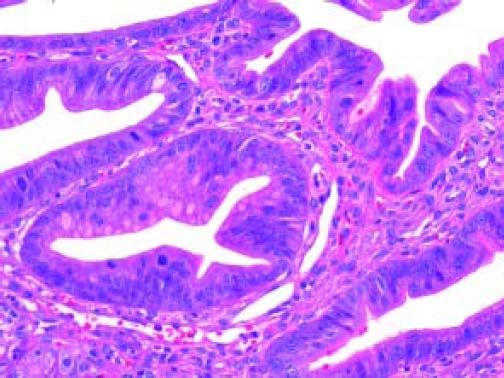
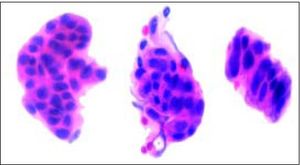
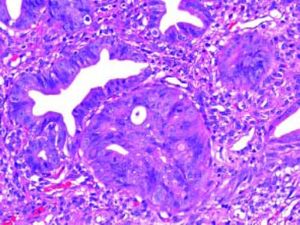
Gross and Microscopic FindingsBrush cytology exhibited groups and papillary structures of biliary epithelial columnar cells with moderate and high-grade atypia (Figure 2). Diagnosis of well-differentiated adenocarcinoma was established.
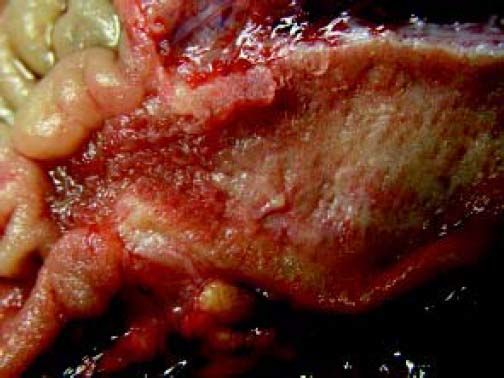
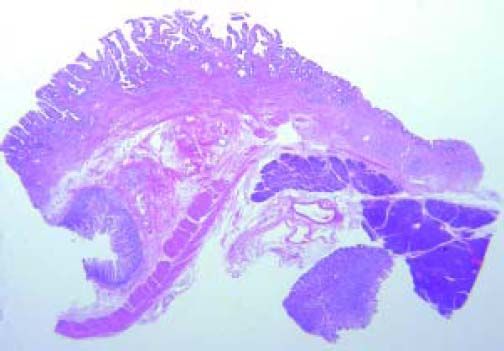
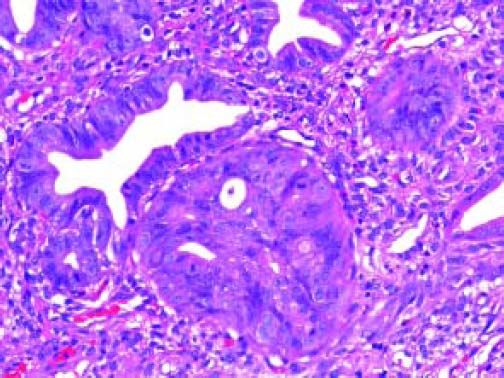
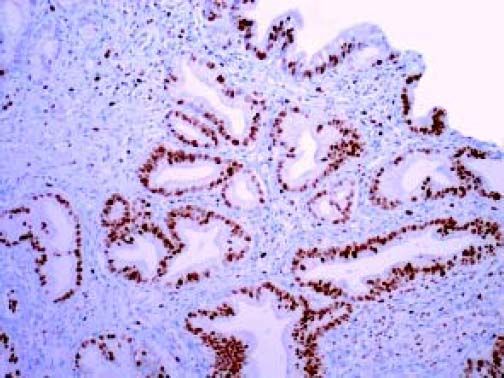
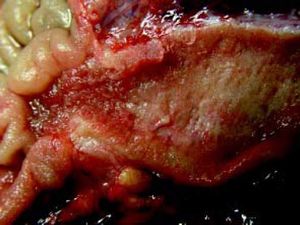
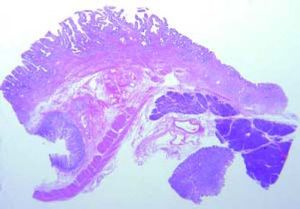
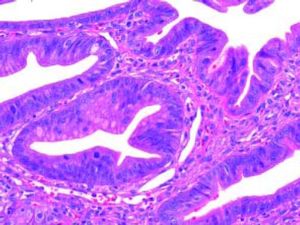
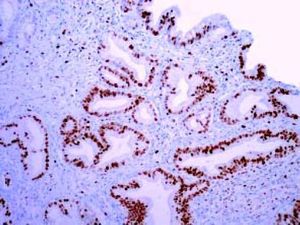
The Whipple specimen demonstrated fibrosis of the wall of the intrapancreatic portion of the common bile duct. A mucosal lesion with micropapillary projections that extends into lumen and ampulla of Vater was observed. This mucosal lesion measured 1.3 cm at its greatest dimension (Figure 3). Microscopically, the lesion was composed of papillary structures lined predominantly with biliary type epithelium with moderate- and high-grade dysplasia/in situ adenocarcinoma, characterized by nuclear pseudostratification, prominent nucleoli, and mitotic figures (Figures 4 and 5). The tumor showed two foci of cribiform structures with stromal microinvasion (Figure 6); these areas were strongly positive for MUC1. Moreover, foci of intestinal differentiation composed of a few goblet cells that expressed CDX2 were also observed. The proliferative index measured by Ki-67 was 95% (Figure 7). Surgical margins and 14 lymph nodes were negative. Perineural, lymphatic, and vascular invasion were not identified. A diagnosis of papillary in situ adenocarcinoma with two foci of intramucosal carcinoma was established. Tumor-Node-Metastasis (TNM) stage was pT1, pN0, pMX, R0.
The patient is asymptomatic and disease-free 24 months after Whipple surgery.
DiscussionCarcinomas of the EHBD are rare, and only < 10% will be cured surgically. Unfortunately, at diagnosis, the majority of patients present with unresectable tumors, and a surgical procedure can be performed in only 25-30% of patients.1,5
These neoplasms have been associated with different risk factors. The most important predisposing factors for dysplasia and invasive carcinomas include Primary sclerosing cholangitis (PSC), abnormal choledochopancreatic junction and fluke infestation.1,6–8 The presence of gallstones is a wellknown risk factor for gallbladder cancer but the role of this entity in cancers of the extrahepatic bile duct is less established. Chronic biliary diseases may lead to long-term irritation and inflammation with resultant fibrosis and dysplasia, and may have contributed to the development of cancer in this patient with no other risk factor.9,10
Invasive adenocarcinomas are observed in 7-14% of patients with PSC.1,6,7 But on thinking of infectious parasites, Clonorchis sinensis and Opisthorchis viverrini should be considered. In the Orient, infestations by Clonorchis sinensis and Opisthorchis viverrini are the most common cause of carcinoma of the EHBD.11
The main symptoms are abdominal pain (right upper quadrant pain), pruritus, and weight loss during from days to weeks, but this can be variable. On physical examination, jaundice (93%), acholia, choluria, and hepatomegaly are observed.1,3
Laboratory tests will show an obstructive pattern, with prominent elevation of bilirubins and alkaline phosphatase, mild elevation of transaminases, and prolongation of prothrombin time in some patients.
If ERCP is performed, cytology and biopsy for early diagnosis of the disease should be performed.
The use of other modalities, such as SpyGlass®, increase sensitivity.15
Histologically, dysplasia and carcinoma in situ have been recognized adjacent to invasive carcinomas of the EHBD (10-75%), and can be multicentric in the majority of patients.1,16 High-grade dysplasia/carcinoma in situ of the EHBD have also been described in association with PSC and less frequently with ulcerative colitis.1,17–19 Incidence varies according to the series from about 0 to 1.8%.1,18,19 The incidence of high-grade dysplasia/ carcinoma in situ in the bile duct mucosa adjacent to carcinomas arising on a background of primary sclerosing cholangitis is higher (60%).19 Carcinomas of the EHBD probably evolve through a dysplasia-carcinoma sequence.
Well and moderately differentiated adenocarcinomas are the most common invasive type of the EHBD. The morphology of these carcinomas is heterogeneous.20,21 The most common variants of adenocarcinoma are biliary and intestinal.1
Total resection is possible in only 25-30% of lesions in distal origin. In the case of a distal lesion, a Whipple procedure should be performed.22
The overall 5-year survival rate for these neoplasms is 28%. Bile duct tumors confined to the wall have a 10-year survival of 19%, but if there is regional invasion or metastasis, survival can be worse (9 and 1%, respectively). If pancreatic invasion is documented, prognosis is poor.23 Prognosis of carcinomas that arise in the upper third of the EHBH is worse than for those localized in the remaining portions.1,23
Another point for consideration is that the majority of tumors are multifocal. Up to 5% of patients with EHBD carcinomas may have a synchronic neoplasm of the gallbladder, and an examination of the entire biliary tree is recommended.25,26
Finally, perineural and lymphatic invasion is common in this type of carcinoma, but at early stages, it is less frequent, up to 11%, and should be evaluated specifically.23
In conclusion, we present the case of a woman with a papillary in situ and intramucosal carcinoma of the EHBD that was completely resected, and at this time the woman is disease-free 24 months after surgery.