The optimal timing to treat recurrent hepatitis-C virus (HCV) after liver transplantation (LT) remains uncertain. We compared the outcome of early (acute phase) and deferred (chronic phase) antiviral treatment for recurrent HCV infection in this population. Consecutive HCV genotype-1 infected LT patients receiving antiviral therapy between 2001-2010 were retrospectively classified according to histology at treatment start into the early or deferred treatment group. Measured endpoints included sustained virological response (SVR) rates and long-term survival. The study cohort comprised 105 patients: 60 (57%) received early treatment (ET) and 45 (43%) deferred treatment (DT). The median interval from LT to antiviral start was 3 (1-9) and 18 months (11-74) in ET and DT respectively. The SVR rate was similar in both treatment groups (23% ET and 36% DT; p = 0.27). After a median follow-up of 5.8 years, all-cause and liver-related mortality were similar in both groups. Variables independently associated with mortality included pre-treatment bilirubin > 2 mg/dL (HR 6.1, 95%CI: 2.8-13.7; p < 0.001), donor age > 60 (HR 3.1, 95%CI: 1.4-6.7; p = 0.01), and failure to achieve SVR (HR 10.3, 95%CI: 1.3-18.3; p = 0.03). In conclusion, early treatment of recurrent HCV is safe, but does not lead to higher SVR rates. In HCV-infected LT recipients, elevated bilirubin, older donor age, and failure to achieve SVR are independently associated with increased mortality.
Cirrhosis secondary to hepatitis C virus (HCV) infection has become the most common indication for liver transplantation (LT). However, graft reinfection is almost universal,1,2 progression to cirrhosis occurs in 10% to 30% of patients 5 to 7 years following the procedure, and there is a high risk of clinical decompensation with poor survival.3–7 Management of recurrent HCV infection after LT has significantly improved during the past decade. Combined treatment with pegylated interferon (Peg-IFN) and ribavirin leads to sustained viral response (SVR) in 30 to 50% of patients.8–13 The optimal time to begin antiviral therapy (AVT) after LT is uncertain. The indication for treatment is usually established based on the fibrosis stage at one year.10,14 Few studies have evaluated the utility of treatment during the acute phase of recurrent HCV. Although this strategy has proven safe,15 reported SVR rates vary considerably from 19 to 35%.15,16
This retrospective study was conducted to compare the efficacy, safety, and long-term outcome associated with AVT given during the acute phase of recurrent HCV infection (early treatment) with that of AVT administered during established chronic hepatitis C (deferred treatment).
Material and MethodsThe Strengthening the Report of Observational Studies recommendations for reporting observational Studies17 were applied for the manuscript design. The study was approved by the Ethics Committee of our institution and all patients gave written informed consent.
Cohort recruitment and data collectionWe reviewed the medical records of all consecutive adult HCV-infected patients who had undergone LT at Hospital Universitari Vall d’Hebrón, a third-level teaching hospital in Barcelona (Spain), between 2001 and 2010. The patient data was retrieved from a regularly updated database designed to assess the long-term outcome of LT. For each patient, the demographic, clinical, and analytical data at baseline, and the follow-up data during and after treatment were recorded by the attending physicians at each outpatient visit. These prospectively collected data were retrospectively analyzed.
Study designThis is a retrospective, observational study of prospectively monitored patients receiving AVT for recurrent HCV following LT. The primary endpoint of the analysis was SVR. Secondary endpoints included safety and tolerability of AVT and longterm survival.
Antiviral treatment criteriaThe diagnosis of HCV recurrence was established on persistent ALT elevation, histological features and detectable HCV-RNA.
- •
Early treatment group. Between April 2001 and October 2002, all LT patients with acute, recurrent HCV were given prompt treatment, regardless of their clinical or histological status, as participants in a prospective study.15 Subsequently, the indication for treatment was based on clinical and histological criteria. Patients who had more severe clinical recurrence in the opinion of the attending physician during the first year post-LT underwent liver biopsy and received treatment if there was histological evidence of HCV recurrence.
- •
Deferred treatment group. Patients with clinically mild recurrence and an Ishak fibrosis score ≥ 218 in biopsies performed per protocol at 12 months after LT and thereafter were treated.
For the purposes of this study, patients who had received AVT were retrospectively classified according to liver histology at the time of treatment initiation into two groups: the early treatment group (ET) included patients with evidence of acute lobular hepatitis without fibrosis, and the deferred treatment group (DT) included patients with established chronic active hepatitis.
Patients with HIV coinfection, non-1 HCV genotype, or fibrosing cholestatic hepatitis, and those in whom viral load data at baseline or weeks 4 or 12 were missing, were excluded from the study.
Liver histologyBiopsies were obtained percutaneously. Liver specimens were fixed in formalin and embedded in paraffin. Two-micron sections were stained with hematoxylin-eosin and Masson’s trichrome for histological assessment. A single experienced pathologist (HA), blinded to the clinical data, examined the samples. Fibrosis stage was assessed according to Ishak’s scoring system.18
ImmunosuppressionIn 2004, the immunosuppression protocol for HCV-infected LT recipients at our center was changed from tacrolimus (Fk) (Prograf, Astellas Pharma GmbH, Munich, Germany) plus steroids to Fk plus mycophenolate mofetil (MMF) (CellCept, Roche Pharmaceuticals Inc, NJ, USA) with or without low-dose steroid therapy when required, as reported.19 Fk was started at standard doses and adjusted to achieve a 10-15 ng/mL blood trough level during the first weeks. MMF was started at 1-2 g/day, depending on white blood cell count. Methylprednisolone (when indicated) was administered daily, starting at 20-mg doses and slowly tapering over the ensuing 6 months. Patients with pre-transplant serum creatinine > 1.5 mg/dL were initially given basiliximab (Simulect, Novartis Pharma AG, Basel, Switzerland), plus MMF and steroids, with subsequent introduction of low-dose Fk. Tacrolimus was switched to cyclosporine (Sandimmun Neoral, Novartis Pharma AG, Basel, Switzerland) when necessary due to side effects.
Antiviral treatment regimen and response definitionsAntiviral treatment consisted on Peg-IFN α-2b (Peg-Intron, Schering-Plough, Inc., Kenilworth, NJ) and ribavirin (Rebetol, Schering-Plough, Inc., Kenilworth, NJ) were administered for 24 weeks. If HCV-RNA tested positive at that time, treatment was stopped, and if negative, the patient completed 48 weeks of treatment except in 3 partial responders in whom treatment was extended to 72 weeks.
Peg-IFN was initiated at 1.5 µg/kg and was reduced to 1 µg/kg when neutrophils dropped below 1.5 x 109/L or platelets dropped below 50 x 109/L. When neutrophil count was less than 1.0 x 109/L, filgrastim (Neupogen 30, Amgen SA, Barcelona) was initiated. Ribavirin dose has been weight-adjusted to 800-1,200 mg/day. The initial dose was reduced if hemoglobin dropped below 9 g/dL, and weekly darbepoetin-α (Aranesp, Amgen SA, Barcelona) was started if hemoglobin failed to increase within 2 weeks.
Rapid virological response (RVR) was defined as undetectable HCV-RNA at week 4, and early virological response (EVR), as a ≥ 2 log10 viral load decrease at 12 weeks. End-of-treatment response (ETR) was defined as undetectable serum HCV-RNA at the end of therapy. Sustained virological response (SVR) was defined as undetectable serum HCV-RNA 24 weeks after treatment completion.20
Viral load testing From 2001 to 2006, HCV-RNA was detected by a qualitative polymerase chain reaction (PCR) assay (Cobas Amplicor HCV Test v2.0; Roche Molecular Diagnostics, Barcelona, Spain; detection limit 50 IU/mL) and, when positive, measured by a quantitative PCR assay (Cobas Amplicor HCV Monitor Test v2.0; Roche Molecular Diagnostics, Barcelona, Spain; detection limit 600 IU/mL). Since 2007, a real-time PCR-based test (Cobas Ampliprep/Cobas TaqMan; Roche Molecular Diagnostics, Barcelona, Spain; detection limit 15 IU/mL) has been used for HCV quantitation. HCV genotyping was performed by line probe assay (Inno-LiPA II; Innogenetics, Antwerp, Belgium).
Genotyping of IL28B polymorphismsGenomic DNA from LT recipients and their donors was extracted from formalin-fixed, paraffin-embedded biopsies of liver explants (recipients) and grafts (donors) obtained at LT, using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). Genotyping of rs12979860 was performed using a real-time PCR with allele-specific Taq-Man probes, as described previously.21,22 IL28B rs12979860 was defined as CC, CT, or TT.
Statistical analysisThe patients’ descriptive statistics are reported. Data are presented as medians and range, and categorical variables as frequency and percentage. Normality was determined by the Shapiro-Wilk test.
Differences between categorical variables were assessed by the chi-square test or Fisher exact test. Continuous variables were compared using the Mann-Whitney test. The propensity score was not used because it was assumed at study design that there would be differences between the groups.
Multivariate logistic regression analysis was performed with variables showing a significance level of p < 0.1 and those considered clinically significant by the investigators. Results are expressed as odds ratios (OR) with 95% confidence intervals (CIs). Survival analysis was performed with a KaplanMeier and Cox-regression model including variables showing a significance level of p < 0.1 and those considered clinically significant by the investigators. Results are expressed as hazard ratios (HR) with 95% CIs. A two-sided p value of < 0.05 was required for statistical significance. Data were analyzed with SPSS software (19.0, SPSS Inc., Chicago IL, USA).
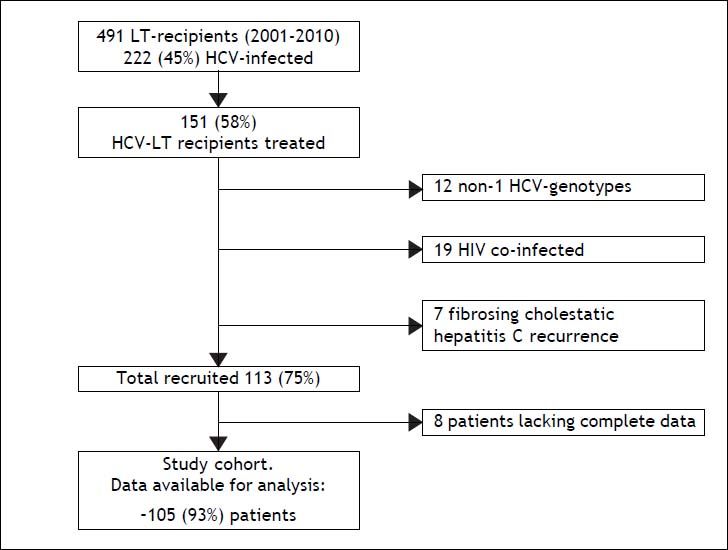
ResultsDuring the 9-year study period, 491 patients underwent LT at our institution, 222 of them had HCV infection (45%), and 151 (68%) received AVT. Of the 151 patients considered for enrollment, 46 were excluded (19 HIV co-infection, 7 fibrosing cholestatic hepatitis, 12 HCV genotype other than 1, and 8 lacking complete data), yielding a final cohort of 105 patients (Figure 1).
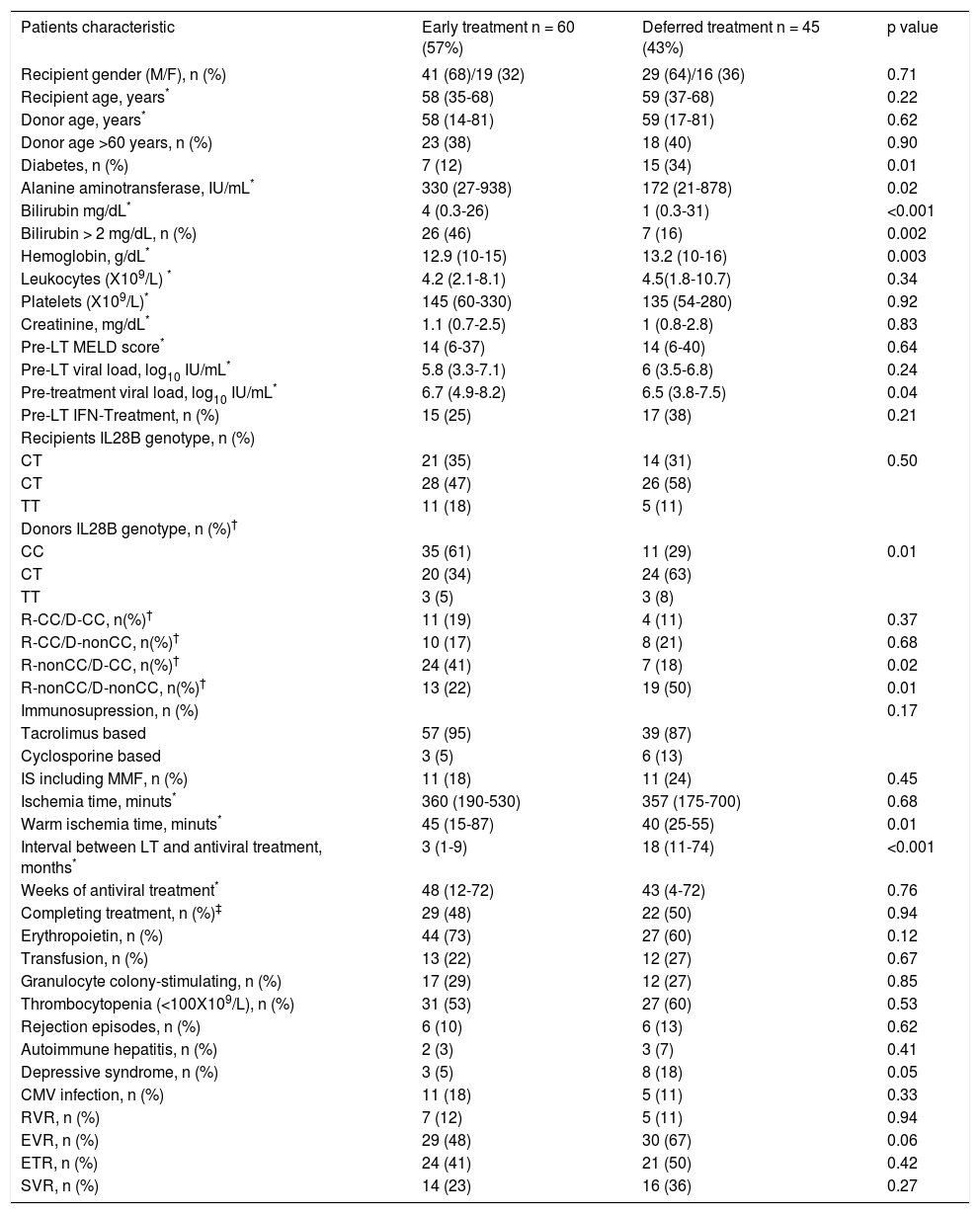
Baseline data by treatment group are summarized in table 1. There were 60 patients in the ET group and 45 in the DT group. Twenty-four (40%) of the 60 ET patients received treatment within a prospective study.15 The groups were similar at baseline except for higher ALT, bilirubin and HCV RNA, lower hemoglobin levels, and smaller percentage of diabetic patients in the ET group. Warm ischemia time was also longer in ET patients. The interval between LT and start of AVT was significantly shorter in ET than in DT (3 vs. 18 months, respectively; p < 0.001).
Baseline and on treatment characteristics of the study cohort according to the time of treatment.
| Patients characteristic | Early treatment n = 60 (57%) | Deferred treatment n = 45 (43%) | p value |
|---|---|---|---|
| Recipient gender (M/F), n (%) | 41 (68)/19 (32) | 29 (64)/16 (36) | 0.71 |
| Recipient age, years* | 58 (35-68) | 59 (37-68) | 0.22 |
| Donor age, years* | 58 (14-81) | 59 (17-81) | 0.62 |
| Donor age >60 years, n (%) | 23 (38) | 18 (40) | 0.90 |
| Diabetes, n (%) | 7 (12) | 15 (34) | 0.01 |
| Alanine aminotransferase, IU/mL* | 330 (27-938) | 172 (21-878) | 0.02 |
| Bilirubin mg/dL* | 4 (0.3-26) | 1 (0.3-31) | <0.001 |
| Bilirubin > 2 mg/dL, n (%) | 26 (46) | 7 (16) | 0.002 |
| Hemoglobin, g/dL* | 12.9 (10-15) | 13.2 (10-16) | 0.003 |
| Leukocytes (X109/L) * | 4.2 (2.1-8.1) | 4.5(1.8-10.7) | 0.34 |
| Platelets (X109/L)* | 145 (60-330) | 135 (54-280) | 0.92 |
| Creatinine, mg/dL* | 1.1 (0.7-2.5) | 1 (0.8-2.8) | 0.83 |
| Pre-LT MELD score* | 14 (6-37) | 14 (6-40) | 0.64 |
| Pre-LT viral load, log10 IU/mL* | 5.8 (3.3-7.1) | 6 (3.5-6.8) | 0.24 |
| Pre-treatment viral load, log10 IU/mL* | 6.7 (4.9-8.2) | 6.5 (3.8-7.5) | 0.04 |
| Pre-LT IFN-Treatment, n (%) | 15 (25) | 17 (38) | 0.21 |
| Recipients IL28B genotype, n (%) | |||
| CT | 21 (35) | 14 (31) | 0.50 |
| CT | 28 (47) | 26 (58) | |
| TT | 11 (18) | 5 (11) | |
| Donors IL28B genotype, n (%)† | |||
| CC | 35 (61) | 11 (29) | 0.01 |
| CT | 20 (34) | 24 (63) | |
| TT | 3 (5) | 3 (8) | |
| R-CC/D-CC, n(%)† | 11 (19) | 4 (11) | 0.37 |
| R-CC/D-nonCC, n(%)† | 10 (17) | 8 (21) | 0.68 |
| R-nonCC/D-CC, n(%)† | 24 (41) | 7 (18) | 0.02 |
| R-nonCC/D-nonCC, n(%)† | 13 (22) | 19 (50) | 0.01 |
| Immunosupression, n (%) | 0.17 | ||
| Tacrolimus based | 57 (95) | 39 (87) | |
| Cyclosporine based | 3 (5) | 6 (13) | |
| IS including MMF, n (%) | 11 (18) | 11 (24) | 0.45 |
| Ischemia time, minuts* | 360 (190-530) | 357 (175-700) | 0.68 |
| Warm ischemia time, minuts* | 45 (15-87) | 40 (25-55) | 0.01 |
| Interval between LT and antiviral treatment, months* | 3 (1-9) | 18 (11-74) | <0.001 |
| Weeks of antiviral treatment* | 48 (12-72) | 43 (4-72) | 0.76 |
| Completing treatment, n (%)‡ | 29 (48) | 22 (50) | 0.94 |
| Erythropoietin, n (%) | 44 (73) | 27 (60) | 0.12 |
| Transfusion, n (%) | 13 (22) | 12 (27) | 0.67 |
| Granulocyte colony-stimulating, n (%) | 17 (29) | 12 (27) | 0.85 |
| Thrombocytopenia (<100X109/L), n (%) | 31 (53) | 27 (60) | 0.53 |
| Rejection episodes, n (%) | 6 (10) | 6 (13) | 0.62 |
| Autoimmune hepatitis, n (%) | 2 (3) | 3 (7) | 0.41 |
| Depressive syndrome, n (%) | 3 (5) | 8 (18) | 0.05 |
| CMV infection, n (%) | 11 (18) | 5 (11) | 0.33 |
| RVR, n (%) | 7 (12) | 5 (11) | 0.94 |
| EVR, n (%) | 29 (48) | 30 (67) | 0.06 |
| ETR, n (%) | 24 (41) | 21 (50) | 0.42 |
| SVR, n (%) | 14 (23) | 16 (36) | 0.27 |
Prevalences of rs12979860 CC, CT and TT genotypes in recipient samples were 33%, 52%, and 15% respectively, whereas in donor samples, genotype CC was the most common (47%), followed by genotype CT (45%) and TT (8%) (p = 0.001).
When categorized according to treatment group, recipient rs12979860 genotypes were similarly distributed in the two groups (Table 1). However, significantly more patients in the ET group received livers from donors with rs12979860 CC genotype (61%), than patients in the DT group (29%) (p = 0.01).
There were no differences concerning side effects, type of immunosuppression used, haematological support, rejection, or CMV infection.
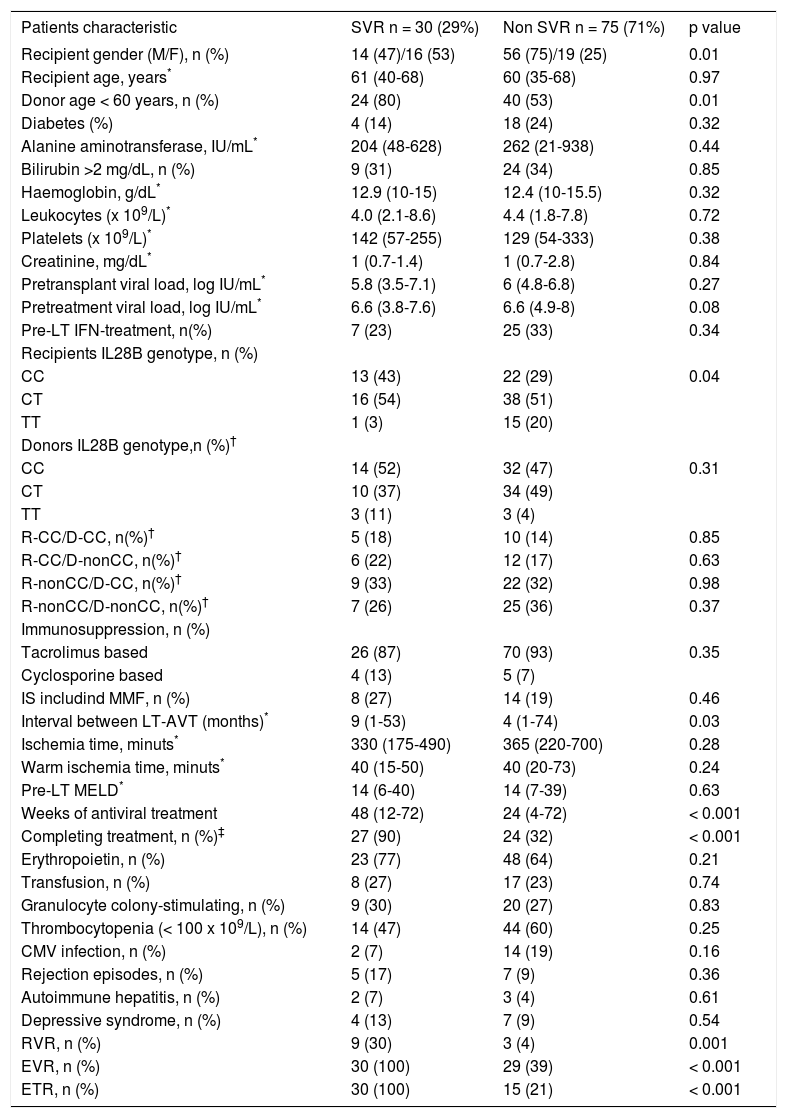
Treatment outcomeThere were no differences between groups in the percentage of patients who completed treatment, or required erythropoietin, blood transfusion or granulocyte colony-stimulating factor, or in the number of rejection episodes or de novo autoimmune hepatitis cases. The interval between LT and treatment start was longer in patients achieving SVR (Table 2).
Baseline and on treatment characteristics of the study cohort according to sustained virological response.
| Patients characteristic | SVR n = 30 (29%) | Non SVR n = 75 (71%) | p value |
|---|---|---|---|
| Recipient gender (M/F), n (%) | 14 (47)/16 (53) | 56 (75)/19 (25) | 0.01 |
| Recipient age, years* | 61 (40-68) | 60 (35-68) | 0.97 |
| Donor age < 60 years, n (%) | 24 (80) | 40 (53) | 0.01 |
| Diabetes (%) | 4 (14) | 18 (24) | 0.32 |
| Alanine aminotransferase, IU/mL* | 204 (48-628) | 262 (21-938) | 0.44 |
| Bilirubin >2 mg/dL, n (%) | 9 (31) | 24 (34) | 0.85 |
| Haemoglobin, g/dL* | 12.9 (10-15) | 12.4 (10-15.5) | 0.32 |
| Leukocytes (x 109/L)* | 4.0 (2.1-8.6) | 4.4 (1.8-7.8) | 0.72 |
| Platelets (x 109/L)* | 142 (57-255) | 129 (54-333) | 0.38 |
| Creatinine, mg/dL* | 1 (0.7-1.4) | 1 (0.7-2.8) | 0.84 |
| Pretransplant viral load, log IU/mL* | 5.8 (3.5-7.1) | 6 (4.8-6.8) | 0.27 |
| Pretreatment viral load, log IU/mL* | 6.6 (3.8-7.6) | 6.6 (4.9-8) | 0.08 |
| Pre-LT IFN-treatment, n(%) | 7 (23) | 25 (33) | 0.34 |
| Recipients IL28B genotype, n (%) | |||
| CC | 13 (43) | 22 (29) | 0.04 |
| CT | 16 (54) | 38 (51) | |
| TT | 1 (3) | 15 (20) | |
| Donors IL28B genotype,n (%)† | |||
| CC | 14 (52) | 32 (47) | 0.31 |
| CT | 10 (37) | 34 (49) | |
| TT | 3 (11) | 3 (4) | |
| R-CC/D-CC, n(%)† | 5 (18) | 10 (14) | 0.85 |
| R-CC/D-nonCC, n(%)† | 6 (22) | 12 (17) | 0.63 |
| R-nonCC/D-CC, n(%)† | 9 (33) | 22 (32) | 0.98 |
| R-nonCC/D-nonCC, n(%)† | 7 (26) | 25 (36) | 0.37 |
| Immunosuppression, n (%) | |||
| Tacrolimus based | 26 (87) | 70 (93) | 0.35 |
| Cyclosporine based | 4 (13) | 5 (7) | |
| IS includind MMF, n (%) | 8 (27) | 14 (19) | 0.46 |
| Interval between LT-AVT (months)* | 9 (1-53) | 4 (1-74) | 0.03 |
| Ischemia time, minuts* | 330 (175-490) | 365 (220-700) | 0.28 |
| Warm ischemia time, minuts* | 40 (15-50) | 40 (20-73) | 0.24 |
| Pre-LT MELD* | 14 (6-40) | 14 (7-39) | 0.63 |
| Weeks of antiviral treatment | 48 (12-72) | 24 (4-72) | < 0.001 |
| Completing treatment, n (%)‡ | 27 (90) | 24 (32) | < 0.001 |
| Erythropoietin, n (%) | 23 (77) | 48 (64) | 0.21 |
| Transfusion, n (%) | 8 (27) | 17 (23) | 0.74 |
| Granulocyte colony-stimulating, n (%) | 9 (30) | 20 (27) | 0.83 |
| Thrombocytopenia (< 100 x 109/L), n (%) | 14 (47) | 44 (60) | 0.25 |
| CMV infection, n (%) | 2 (7) | 14 (19) | 0.16 |
| Rejection episodes, n (%) | 5 (17) | 7 (9) | 0.36 |
| Autoimmune hepatitis, n (%) | 2 (7) | 3 (4) | 0.61 |
| Depressive syndrome, n (%) | 4 (13) | 7 (9) | 0.54 |
| RVR, n (%) | 9 (30) | 3 (4) | 0.001 |
| EVR, n (%) | 30 (100) | 29 (39) | < 0.001 |
| ETR, n (%) | 30 (100) | 15 (21) | < 0.001 |
A higher percentage of DT patients had depressive symptoms (18% DT vs. 5% ET; p = 0.05).
A similar percentage of patients in both groups achieved RVR and EVR (Table 1) and there were no differences in the SVR rate (23% ET vs. 36% DT; p = 0.27).
For the overall cohort, univariate analysis identified the following pre-treatment variables as associated with SVR: female recipient (p = 0.01), IL28B genotype CC (p = 0.04), and graft donor younger than 60 years (p = 0.01) (Table 2). The on-treatment variables associated with SVR included, the weeks of AVT (p < 0.001), completing treatment (p < 0.001), and achieving an RVR (p = 0.001), EVR (p < 0.001), and ETR (p < 0.001). On multivariate analysis, the fact of achieving an RVR (OR 13.6, CI 95% 1.7-107.5, p = 0.01) and completing treatment (OR 14.8, CI 95% 2.1-103.8, p = 0.01) were the only variables independently associated with SVR.
In the same analysis by treatment group, completing treatment (p < 0.001), achieving an RVR (p = 0.01), and achieving an EVR (p < 0.001) were associated with SVR in the ET group, whereas completing treatment (p = 0.002), achieving an RVR (p = 0.04), achieving an EVR (p < 0.001), and receiving a graft from a donor younger than 60 years (p = 0.03), were associated with SVR in the DT group.
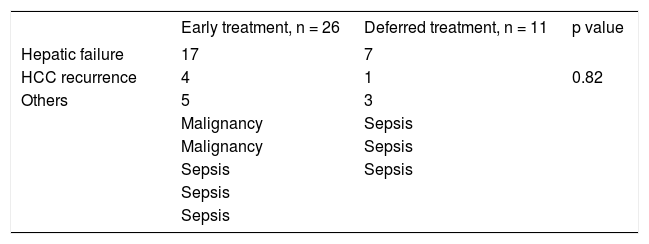
Survival analysisThe study cohort was followed up for a median of 5.8 years (range 0.4-13.5). In the overall cohort, 37 patients (35%) died during follow-up: 24 (65%) due to liver failure after a median of 2.1 years (range 0.4-9.9), 5 (13%) due to HCC recurrence after a median of 1.6 years (range 0.7-7.6), and the remaining 8 (22%) due to liver-unrelated causes after a median of 4 years post-LT (range 0.5-6.5).
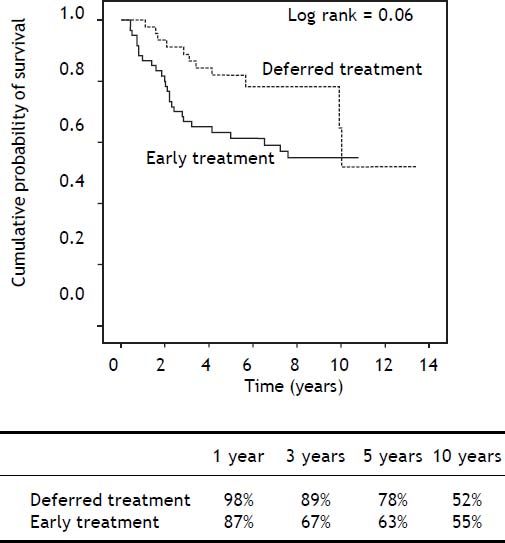
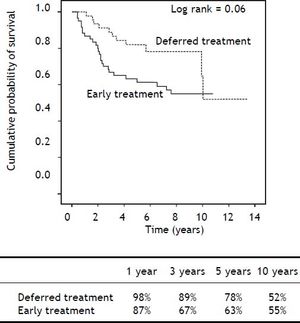
After a median follow-up of 6.9 years (range 0.4-11) in the ET group and 5.6 years (range 1.1-13.5) in the DT group (p = 0.71), Kaplan-Meier analysis showed a trend towards shorter survival in the ET group during the first years, but with similar longterm survival in both groups (Log-rank p = 0.06) (Figure 2). Importantly, liver-related mortality was similar in both groups (p = 0.82) (Table 3).
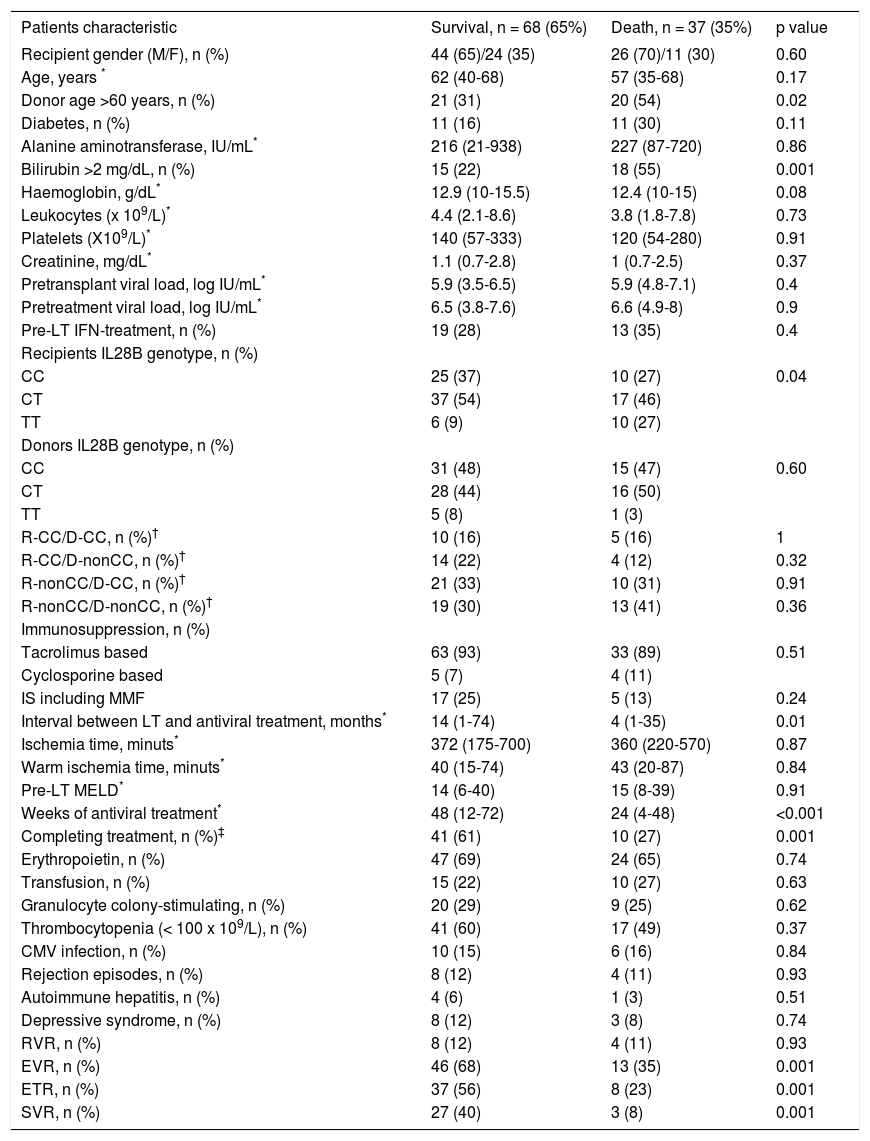
The variables associated with mortality in the overall cohort included graft donor > 60 years old (p = 0.02), recipient IL28B genotype TT (p = 0.03), pre-treatment bilirubin > 2 mg/dL, (p = 0.001), weeks of AVT (p < 0.001), premature treatment discontinuation (p = 0.001); and failure to achieve EVR (p = 0.001), ETR (p = 0.001), and SVR (p = 0.001) (Table 4).
Baseline and on treatment characteristics of the study cohort of 105 patients according to mortality.
| Patients characteristic | Survival, n = 68 (65%) | Death, n = 37 (35%) | p value |
|---|---|---|---|
| Recipient gender (M/F), n (%) | 44 (65)/24 (35) | 26 (70)/11 (30) | 0.60 |
| Age, years * | 62 (40-68) | 57 (35-68) | 0.17 |
| Donor age >60 years, n (%) | 21 (31) | 20 (54) | 0.02 |
| Diabetes, n (%) | 11 (16) | 11 (30) | 0.11 |
| Alanine aminotransferase, IU/mL* | 216 (21-938) | 227 (87-720) | 0.86 |
| Bilirubin >2 mg/dL, n (%) | 15 (22) | 18 (55) | 0.001 |
| Haemoglobin, g/dL* | 12.9 (10-15.5) | 12.4 (10-15) | 0.08 |
| Leukocytes (x 109/L)* | 4.4 (2.1-8.6) | 3.8 (1.8-7.8) | 0.73 |
| Platelets (X109/L)* | 140 (57-333) | 120 (54-280) | 0.91 |
| Creatinine, mg/dL* | 1.1 (0.7-2.8) | 1 (0.7-2.5) | 0.37 |
| Pretransplant viral load, log IU/mL* | 5.9 (3.5-6.5) | 5.9 (4.8-7.1) | 0.4 |
| Pretreatment viral load, log IU/mL* | 6.5 (3.8-7.6) | 6.6 (4.9-8) | 0.9 |
| Pre-LT IFN-treatment, n (%) | 19 (28) | 13 (35) | 0.4 |
| Recipients IL28B genotype, n (%) | |||
| CC | 25 (37) | 10 (27) | 0.04 |
| CT | 37 (54) | 17 (46) | |
| TT | 6 (9) | 10 (27) | |
| Donors IL28B genotype, n (%) | |||
| CC | 31 (48) | 15 (47) | 0.60 |
| CT | 28 (44) | 16 (50) | |
| TT | 5 (8) | 1 (3) | |
| R-CC/D-CC, n (%)† | 10 (16) | 5 (16) | 1 |
| R-CC/D-nonCC, n (%)† | 14 (22) | 4 (12) | 0.32 |
| R-nonCC/D-CC, n (%)† | 21 (33) | 10 (31) | 0.91 |
| R-nonCC/D-nonCC, n (%)† | 19 (30) | 13 (41) | 0.36 |
| Immunosuppression, n (%) | |||
| Tacrolimus based | 63 (93) | 33 (89) | 0.51 |
| Cyclosporine based | 5 (7) | 4 (11) | |
| IS including MMF | 17 (25) | 5 (13) | 0.24 |
| Interval between LT and antiviral treatment, months* | 14 (1-74) | 4 (1-35) | 0.01 |
| Ischemia time, minuts* | 372 (175-700) | 360 (220-570) | 0.87 |
| Warm ischemia time, minuts* | 40 (15-74) | 43 (20-87) | 0.84 |
| Pre-LT MELD* | 14 (6-40) | 15 (8-39) | 0.91 |
| Weeks of antiviral treatment* | 48 (12-72) | 24 (4-48) | <0.001 |
| Completing treatment, n (%)‡ | 41 (61) | 10 (27) | 0.001 |
| Erythropoietin, n (%) | 47 (69) | 24 (65) | 0.74 |
| Transfusion, n (%) | 15 (22) | 10 (27) | 0.63 |
| Granulocyte colony-stimulating, n (%) | 20 (29) | 9 (25) | 0.62 |
| Thrombocytopenia (< 100 x 109/L), n (%) | 41 (60) | 17 (49) | 0.37 |
| CMV infection, n (%) | 10 (15) | 6 (16) | 0.84 |
| Rejection episodes, n (%) | 8 (12) | 4 (11) | 0.93 |
| Autoimmune hepatitis, n (%) | 4 (6) | 1 (3) | 0.51 |
| Depressive syndrome, n (%) | 8 (12) | 3 (8) | 0.74 |
| RVR, n (%) | 8 (12) | 4 (11) | 0.93 |
| EVR, n (%) | 46 (68) | 13 (35) | 0.001 |
| ETR, n (%) | 37 (56) | 8 (23) | 0.001 |
| SVR, n (%) | 27 (40) | 3 (8) | 0.001 |
Multivariate analysis using a Cox regression model (see variables included in table 3) identified the following mortality-related variables: pretreatment bilirubin > 2 mg/dL (HR 6.1, CI 95% 2.7-13.7, p < 0.001), graft donor older than 60 years (HR 3.1, 95% CI 1.4-6.7, p = 0.01), and failure to achieve SVR (HR 10.3, CI 95% 1.2-18.3, p = 0.03) (Figure 3).
Analysis of mortality-associated variables according to treatment group identified pre-treatment bilirubin > 2 mg/dL (HR 4.9, 95% CI 1.9-12.7, p = 0.001) and graft donor older than 60 years (HR 3.2, 95% CI 1.3-7.9, p = 0.01) in the ET group, whereas only pre-treatment bilirubin > 2 mg/dL (HR 8.7, 95% CI 1.9-39.9, p = 0.01) was independently associated with mortality in the DT group.
Subgroup analysisIn an exploratory analysis removing patients from the first study,15 the results in terms of efficacy of therapy were similar to those found using the whole cohort, 17% SVR in ET group vs. 36% in DT group, p = 0.06. No differences regarding related variables were found.
Regarding IL28b genotype we obtained same results; more patients in the ET group received livers from donors with rs12979860 CC genotype (65%), than patients in the DT group (29%) (p = 0.01).
Kaplan-Meier analysis showed shorter survival in the ET group (log-rank p = 0.01).
Multivariate analysis using a Cox regression model identified the following mortality-related variables: pre-treatment bilirubin > 2 mg/dL (HR 17.9, CI 95% 5.6-57.6, p < 0.001), graft donor older than 60 years (HR 3.2, 95% CI 1.1-9.5, p = 0.04), failure to achieve SVR (HR 14.5, CI 95% 1.5-141.8, p = 0.02) and diabetes (HR 3.3, CI 95% 1.0-10.5, p = 0.04).
Analysis of mortality-associated variables according to treatment group identified pre-treatment bilirubin >2 mg/dL (HR 013.0, 95% CI 2.4-69.9, p = 0.003) in ET group.
DiscussionIn most centers, treatment of recurrent HCV is started when chronic hepatitis is established and progressive fibrosis has been documented.23 This strategy has the advantage of treating when the patient’s clinical status after LT is stable and the immunosuppression requirement and risk of acute rejection are presumably lower. In a previous study,15 however, we found that antiviral treatment for recurrent HCV in the acute stage is safe and effective, although the limited sample size and comparison with patients who did not receive AVT precluded identification of patients who might obtain the most benefit from this strategy.
In the present study, our expanded experience with early treatment of recurrent HCV is compared with treatment of established chronic HCV recur-rence in a large cohort of patients over a decade in time. Our results indicate that the two treatment strategies are similar with regard to tolerability and safety, except for a higher frequency of interferon-related depressive symptoms in patients with established recurrent HCV. Regarding the potential risk of interferon-induced acute rejection,24,25 our results do not support the notion that antiviral therapy in the early phase of recurrence increases the risk of rejection.
It is worthwhile to stress that therapy used in the early post-transplantation phase had similar rate of discontinuations as when used in a delayed phase, receiving similar cumulative dosage of ribavirin and Peg-IFN in both groups; moreover, we found that treatment intolerance was not an important limiting factor in the early post-LT phase. Hematological side effects were similar between groups and controlled with growth factors or transfusions when required.
It is well known that in the early transplant period doses of IS are higher, whether it has have an impact in the rate of achievement of SVR in our ET group cannot be establish, what we can gauge is that the type of IS and proportion of patients receiving MMF was similar, however we cannot reject the possibility of a negative impact of the higher IS, which could have balanced the positive impact of the absence of fibrosis.
The lower frequency of IL28B CC genotype in the LT recipients than in HCV-negative donors is consistent with the role of the CC variant in spontaneous HCV clearance and sensitivity to interferon; these characteristics would lead to over-representation of non-CC variants in the chronic hepatitis C population.26,27 Although the distribution of recipient IL28B genotypes was similar in both treatment groups, the finding of a higher frequency of CC genotype in grafts transplanted to ET recipients deserves further comment. This finding is consistent with the results of recent reports in patients with post-LT recurrent HCV.28,29 These authors found faster disease progression in patients who had received a CC donor graft, suggesting that although donor IL28B genotype CC associates with higher SVR rates after antiviral therapy, in the absence of viral eradication, it is associated with poorer outcome and survival. The authors concluded that these patients should be systematically offered antiviral treatment.28
In the present study, the only variables independently associated with SVR were achieving RVR and completing treatment. We found a significantly higher SVR rate in recipients with genotype CC (p = 0.04), but there was no association with donor genotype (p = 0.30). Nonetheless, recipient genotype was not significantly associated with treatment response in the multivariate analysis. Similar discrepancies between recipient and donor IL28B genotype and SVR have been reported by others.30–32 Although recipient IL28B genotype was not independently associated with treatment response in our study, the limited number of patients and the high number of other variables influencing treatment outcome limit the interpretation of this finding. Further studies are needed to clarify this issue. There were no differences in the proportion of patients achieving SVR regarding the group of treatment; however, including only more sick patients in the ET as the result of removing patients from the first study,15 there was a trend to a higher SVR in the DT group, despite not achieving statistical significance.
We identified a subgroup of LT recipients with a poor prognosis: patients with pre-treatment bilirubin > 2 mg/dL who received a graft from a donor older than 60 years, and failed to achieve SVR. In an attempt to select the most severe patients as accurately as possible we remove patients from the first study,15 this strategy added diabetes as a variable associated with mortality and showed that patients treated in the ET group had a higher mortality than patients treated in the DT, we firmly believe that this is a consequence of the severity of the recurrence and not of the timing.
Patients identified as being at higher risk of severe recurrence might benefit from early treatment with direct-acting antiviral (DAA) containing regimens.33–38 In this regard, our results and those of others28,29 suggest that donor IL28B genotype should be considered a potential prognostic marker for early severe recurrence until further information is obtained.
Although data is scarce in the LT setting,35–37 DDA containing regimens have shown higher SVR (20-71%) rates in LT recipients. With these regimens side effects are high; anemia was the most common (92%), with a rate of infection of 27% with 8% of fatal outcomes, and treatment was discontinued in 43% of patients. However, it is likely that patients with more severe recurrence will benefit from DAA-containing regimens. Accordingly, patients at higher risk of severe recurrence should be promptly identified since they might benefit from earlier DAA-containing antiviral therapy and all efforts should be directed at completing treatment to increase SVR rates.
Our study has several limitations. First, because of its retrospective nature, decisions on the indication for treatment were made by clinicians on a case-by-case basis. Furthermore, except for the ET patients who had been included in the previous study,15 most patients in this group had clinically more severe recurrent HCV than those in the DT group. Therefore, we were unable to assess the utility of prompt treatment in LT recipients with mild recurrence. Nonetheless, the fact that patients were prospectively monitored at a single center by the same physicians according to homogeneous management criteria would likely decrease the impact of potential biases. In addition, due to the observational nature of the study, extrapolation of the results to the liver transplant population at large is difficult. Ideally, an extensive multicenter study comparing DAA-containing treatment given during acute versus established recurrence would allow more accurate treatment guidelines to be developed. Finally, we mention that fibrosis was not compared between groups because this factor was absent in patients treated in the acute phase of recurrence, in accordance with the study design.
In conclusion, early treatment of recurrent HCV genotype 1 in LT recipients is safe and achieves similar rates of viral eradication as deferred treatment. Patients with clinically severe HCV recurrence and those who received a graft from an older donor should be considered candidates for an early, intensive therapeutic intervention to increase HCV eradication and optimize graft and patient survival.
AcknowledgmentsThe authors thank Celine Cavallo for English language support, Anna Oliveira and Angie Rico for technical support, and Esther Delgado for secretarial work.
Abbreviations- •
95CI: 95% confidence interval.
- •
AVT: antiviral treatment.
- •
CMV: cytomegalovirus.
- •
DT: deferred treatment.
- •
DAA: direct acting antivirals.
- •
ET: early treatment.
- •
ETR: end of treatment response.
- •
EVR: early virological response.
- •
Fk: tacrolimus.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
HR: hazard ratio.
- •
IL28B: interleukin 28B.
- •
LT: liver transplantation.
- •
MELD: model of end-stage liver disease.
- •
MMF: mycophenolate mofetil.
- •
OR: odds ratios.
- •
PCR: polymerase chain reaction.
- •
PEG-INF: pegylated interferon.
- •
RVR: rapid virologic response.
- •
SVR: sustained virological response.
Isabel Campos-Varela is a recipient of a ‘Río Hortega’ fellowship grant from the Instituto de Salud Carlos III and a Juan Rodes grant from the Asociación Española para el Estudio del Hígado. CIBERehd is supported by Instituto de Salud Carlos III. This work has been partially supported by a grant (PI10/ 01505) from the Spanish Ministry of Health.
Author ParticipationIsabel Campos-Varela, Lluis Castells, and Juan Ignacio Esteban participated in making the study design, and writing the article; and Marta Bes, Francisco Rodríguez-Frías, Silvia Sauleda participated in performing the research, Cristina Dopazo, Helena Allende, María Teresa Salcedo, Ramón Charco, Jaime Guardia and Rafael Esteban reviewed the article.
Conflict of Interest DisclosureDr. Rafael Esteban has attended advisor meetings with Abbott, Boehringer Ingelheim, Bristol Myers-Squibb, Glaxo, Gilead, Janssen Merck, and Novartis; has provided lectures on behalf of Abbott, Boehringer Ingelheim, Bristol Myers-Squibb, Glaxo, Gilead, Janssen, Merck, Novartis and Roche; and has served on a Data Safety Monitoring Board for Novartis. The authors have no other potential conflicts of interest to disclose regarding the manuscript.