Cirrhosis is a primary cause of liver-related mortality and morbidity. The basic process driving chronic liver disease to cirrhosis is accelerated fibrogenesis. Although the pathogenesis of liver cirrhosis is a multifactorial process, the essential step in the evolution of liver fibrosis is the activation of hepatic stellate cells, which are the main source of collagen produced in the extracellular matrix. This activation process is mediated by multiple growth factors, cytokines, and chemokines. One of the hepatic stellate cell-activating signaling molecules (and also one associated with cell injury and fibrosis) is osteopontin (OPN). OPN concentration in the plasma has been found to be predictive of liver fibrosis in various liver diseases. OPN concentrations correlate significantly with the stage of fibrosis, liver insufficiency, portal hypertension, and the presence of hepatocellular cancer. However, due to its versatile signaling functions, OPN not only contributes to the development of liver cirrhosis, but is also implicated in the pathogenesis of other chronic hepatic diseases such as viral hepatitis, both alcoholic and non-alcoholic steatohepatitis, drug-induced liver injury, and hepatocellular cancer. Thus, the targeting of OPN pathways seems to be a promising approach in the treatment of chronic liver diseases.
Cirrhosis is a major cause of liver-related mortality and morbidity, and one with a rising incidence rate [1]. The basic process leading to the progression from chronic liver disease to cirrhosis is liver fibrogenesis, which results from chronic liver injury related to a variety of different factors such as: chronic viral hepatitis, chronic alcohol intake, non-alcoholic fatty liver disease (NAFLD), as well as other conditions including (among others) both immune system-related and hereditary metabolic liver diseases. The natural history of cirrhosis is characterized by an asymptomatic course until the increasing portal vein pressure goes over the safe threshold, which is then followed by a worsening of liver functions and the typical clinical symptoms. The most important clinical complications of liver cirrhosis include variceal bleeding, hepatic encephalopathy, ascites, and hepatorenal syndrome. Cirrhosis is also associated with an increased risk for the development of liver cancer [2]. The onset of fibrosis is mostly latent, with progression from the early fibrosis stage to cirrhosis usually occurring after an interval of 15 to 20 years. The progression to cirrhosis is characterized by liver cell necrosis and apoptosis, regeneration, and finally with the deposition of an extracellular matrix (ECM) [3]. The central event in this fibrogenesis process is the activation of hepatic stellate cells (HSCs), complemented by other cells that include fibroblasts and myofibroblasts (to mention the most important cell populations involved). The ECM-producing cells interact with other cells (mainly damaged hepatocytes) and provoke scarring as a response to chronic injury [4]. The interaction is mediated by multiple growth factors, cytokines, and chemokines. One such cell signaling molecule associated with cell injury and fibrosis is a secreted phosphoprotein 1 called osteopontin (OPN, OMIM No. *166490) [5]. OPN, discovered in 1979 [6], is a multi-functional protein, expressed under various physiological conditions in the kidneys and bones [7]. In pathological situations, OPN expressions have been attributed to inflammation, angiogenesis, fibrosis, and carcinogenesis in varied organs [8]. In the liver, OPN contributes to the migration of non-parenchymal cells into necrotic areas [9], and it also serves as an important cytokine - contributing to fibrogenesis [5,10] (Fig. 1). OPN concentration in the plasma was found to predict liver fibrosis in different liver diseases, including: non-alcoholic steatohepatitis (NASH) [11], alcoholic liver disease [12], as well as in both viral hepatitis B (HBV) [13] and viral hepatitis C (HCV) [14] (Table 1). Although this topic has been previously reviewed [8,15], data from the last few years has further expanded our knowledge on the role of OPN in liver diseases (Table 2). Therefore, the main focus of this review was to assess current data on OPN, as well as chronic liver diseases associated with advanced fibrogenesis.
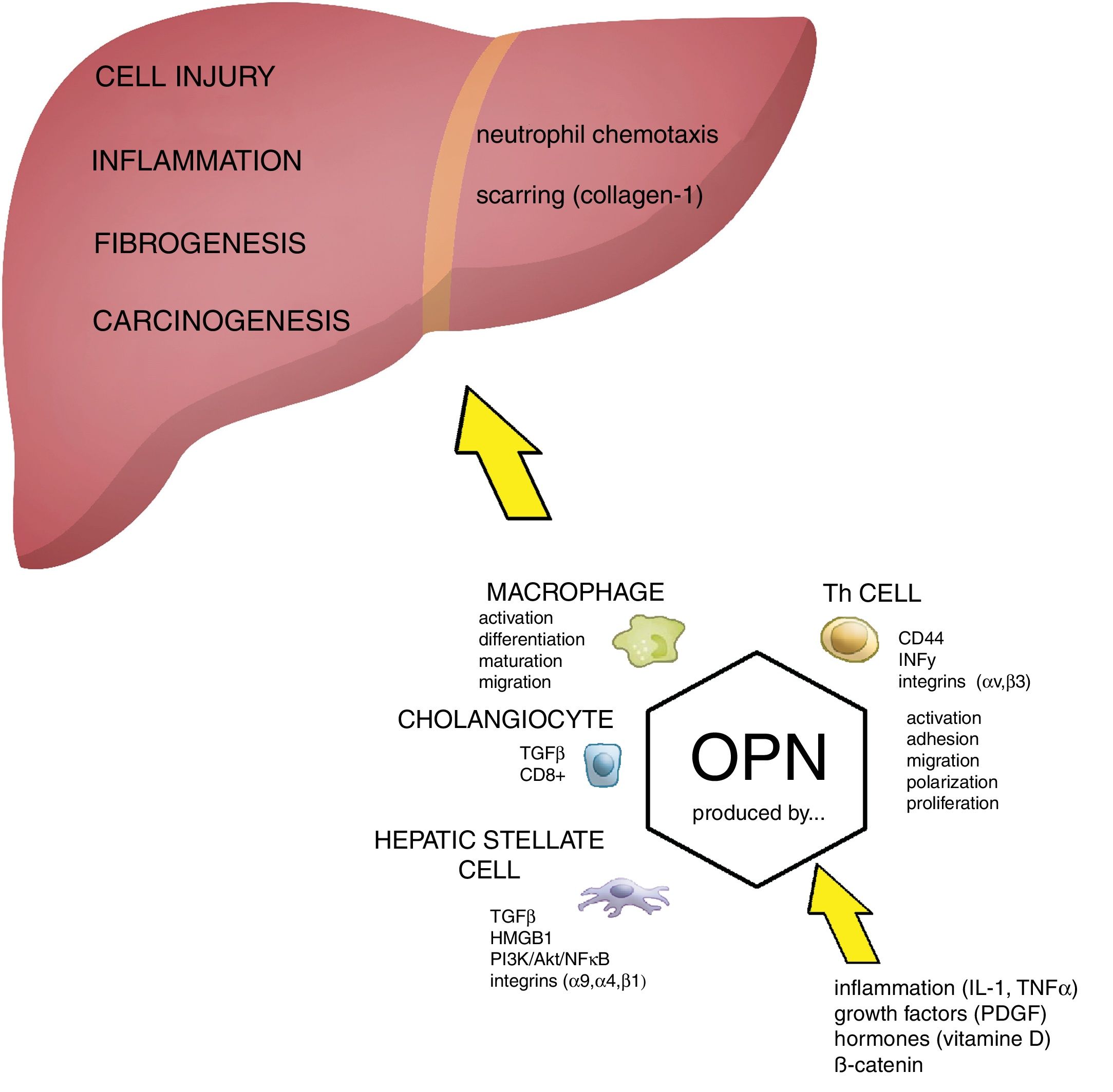
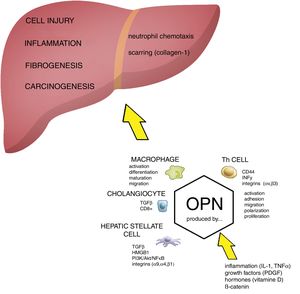
The role of OPN in the pathogenesis of liver damage. Osteopontin (OPN) is involved in regulation of numerous pathological conditions including cell injury, inflammation, fibrogenesis and carcinogenesis. OPN interacts with many signaling pathways including integrins, growth factors and cytokines. OPN acts as a chemoattractant for neutrophils and macrophages. During cell injury and inflammation OPN activates hepatic stellate cells and it has been directly implicated in fibrogenesis (scarring). Akt, protein kinase B; HMGB1, high mobility group box 1 protein; HSC, hepatic stellate cells; IL-1, interleukin 1; OPN, osteopontin; PDGF, platelet-derived growth factor; PI3K, phosphatidylinositol 3-kinase; NFκB, nuclear factor kappa-B; PKC, protein kinase C; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
The role of OPN in liver diseases.
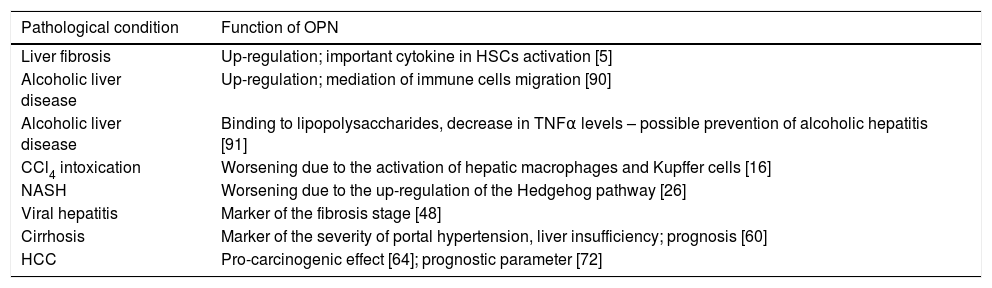
| Pathological condition | Function of OPN |
|---|---|
| Liver fibrosis | Up-regulation; important cytokine in HSCs activation [5] |
| Alcoholic liver disease | Up-regulation; mediation of immune cells migration [90] |
| Alcoholic liver disease | Binding to lipopolysaccharides, decrease in TNFα levels – possible prevention of alcoholic hepatitis [91] |
| CCl4 intoxication | Worsening due to the activation of hepatic macrophages and Kupffer cells [16] |
| NASH | Worsening due to the up-regulation of the Hedgehog pathway [26] |
| Viral hepatitis | Marker of the fibrosis stage [48] |
| Cirrhosis | Marker of the severity of portal hypertension, liver insufficiency; prognosis [60] |
| HCC | Pro-carcinogenic effect [64]; prognostic parameter [72] |
Clinical data indicating the role of osteopontin in various liver diseases.
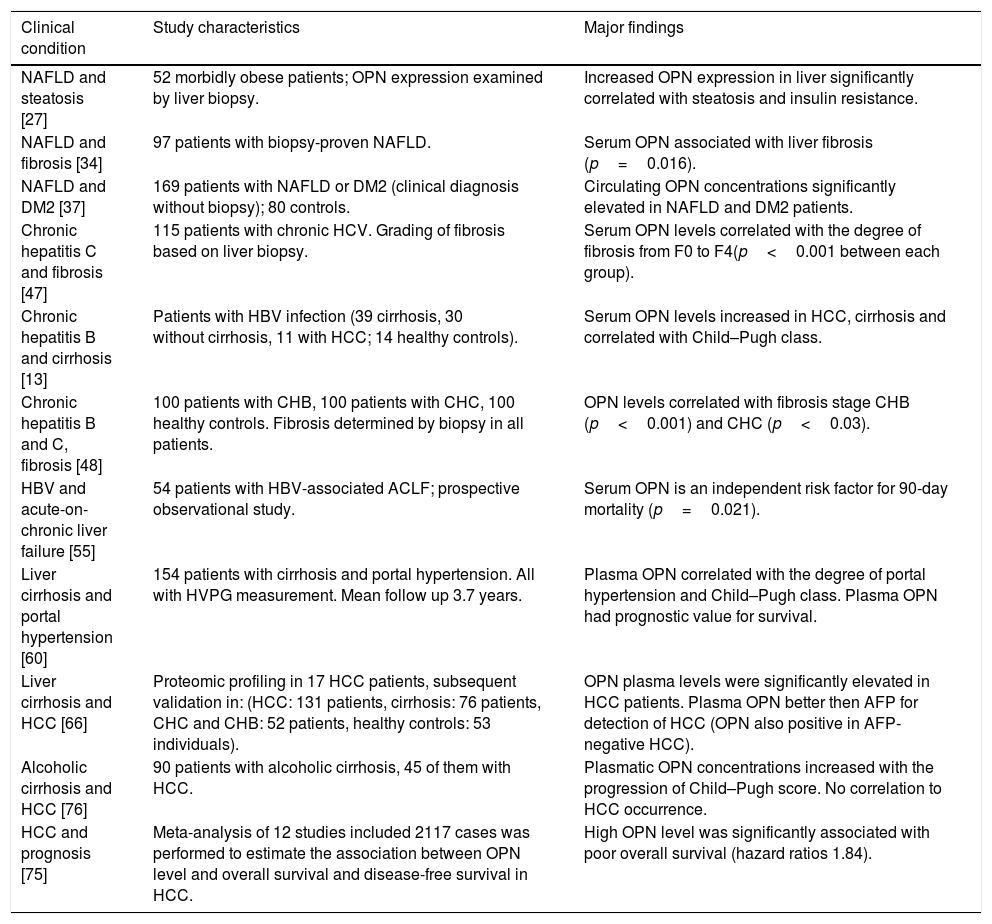
| Clinical condition | Study characteristics | Major findings |
|---|---|---|
| NAFLD and steatosis [27] | 52 morbidly obese patients; OPN expression examined by liver biopsy. | Increased OPN expression in liver significantly correlated with steatosis and insulin resistance. |
| NAFLD and fibrosis [34] | 97 patients with biopsy-proven NAFLD. | Serum OPN associated with liver fibrosis (p=0.016). |
| NAFLD and DM2 [37] | 169 patients with NAFLD or DM2 (clinical diagnosis without biopsy); 80 controls. | Circulating OPN concentrations significantly elevated in NAFLD and DM2 patients. |
| Chronic hepatitis C and fibrosis [47] | 115 patients with chronic HCV. Grading of fibrosis based on liver biopsy. | Serum OPN levels correlated with the degree of fibrosis from F0 to F4(p<0.001 between each group). |
| Chronic hepatitis B and cirrhosis [13] | Patients with HBV infection (39 cirrhosis, 30 without cirrhosis, 11 with HCC; 14 healthy controls). | Serum OPN levels increased in HCC, cirrhosis and correlated with Child–Pugh class. |
| Chronic hepatitis B and C, fibrosis [48] | 100 patients with CHB, 100 patients with CHC, 100 healthy controls. Fibrosis determined by biopsy in all patients. | OPN levels correlated with fibrosis stage CHB (p<0.001) and CHC (p<0.03). |
| HBV and acute-on-chronic liver failure [55] | 54 patients with HBV-associated ACLF; prospective observational study. | Serum OPN is an independent risk factor for 90-day mortality (p=0.021). |
| Liver cirrhosis and portal hypertension [60] | 154 patients with cirrhosis and portal hypertension. All with HVPG measurement. Mean follow up 3.7 years. | Plasma OPN correlated with the degree of portal hypertension and Child–Pugh class. Plasma OPN had prognostic value for survival. |
| Liver cirrhosis and HCC [66] | Proteomic profiling in 17 HCC patients, subsequent validation in: (HCC: 131 patients, cirrhosis: 76 patients, CHC and CHB: 52 patients, healthy controls: 53 individuals). | OPN plasma levels were significantly elevated in HCC patients. Plasma OPN better then AFP for detection of HCC (OPN also positive in AFP-negative HCC). |
| Alcoholic cirrhosis and HCC [76] | 90 patients with alcoholic cirrhosis, 45 of them with HCC. | Plasmatic OPN concentrations increased with the progression of Child–Pugh score. No correlation to HCC occurrence. |
| HCC and prognosis [75] | Meta-analysis of 12 studies included 2117 cases was performed to estimate the association between OPN level and overall survival and disease-free survival in HCC. | High OPN level was significantly associated with poor overall survival (hazard ratios 1.84). |
Under experimental conditions, increased OPN expression in the liver was first reported in animals after carbon tetrachloride intoxication [16]. Expression of OPN mRNA was increased in the macrophages, Kupffer cells, as well as in HSCs. Moreover, using recombinant human OPN, the authors proved a dose-dependent OPN-mediated augmentation in migration of the Kupffer cells. Since that first paper, increasing evidence has confirmed the pathological role of OPN in the progression of chronic liver diseases, fibrosis, and cirrhosis. Significantly, it has been demonstrated that under pathological conditions hepatocytes can act as an important source of OPN [17]. Furthermore, under experimental conditions, it has been demonstrated that OPN serves as an important cytokine in the ECM protein network, contributing to hepatic fibrogenesis [5]. Using transgenic mice with an overexpression of OPN in their liver cells, it was demonstrated that these animals developed fibrosis, even in the absence of any other profibrogenic factors. The possible role of OPN under conditions of deregulated cell replication and collagen ECM deposition appears to lead to the stabilization of cells in tissues developed de novo[18].
Despite both experimental and clinical data showing the possible role of OPN in liver fibrogenesis, a detailed mechanism of OPN's function in promoting liver fibrosis is incompletely understood, with several different possible mechanisms having been proposed.
Pritchett et al. [19] demonstrated that the sex-determining region Y-box 9, an important transcription factor, is expressed on activated HSCs, and is responsible for stimulation of OPN production. Chen et al. [20] investigated the molecular mechanism underpinning OPN regulation of the activation of HSCs in human tissue samples as well as in cell cultures. In cell culture, recombinant OPN upregulated expression of collagen type I, and the authors also demonstrated that OPN suppression of miR-129-5p resulted in the activation of HSCs. On the contrary, upregulation of miR-129-5p decreased the expression of collagen type I. The authors concluded that OPN has a clear pro-fibrogenic effect in HSCs mediated via the downregulation of miR-129-5p.
Consistent with these findings, Arffa et al. [21] showed that three microRNAs (miR-181a, miR-10b, and miR-221) are capable of downregulating OPN mRNA, with a subsequent reduction of fibrogenesis, as demonstrated in a study on thioacetamide-induced liver injury.
OPN has been shown to act as both an autocrine and paracrine factor in provoking liver tissue scarring [17]. This effect is mediated through the regulation of the high-mobility group box-1 (HMGB1; a nuclear non-histone chromosomal protein). OPN, whose gene is located upstream of HMGB1, stimulates the acetylation of intracellular HMGB1 in HSCs, leading to upregulation of collagen-I. In another study, OPN was suggested as regulating profibrogenic TGF-β [22], pointing to a wide cross-talk between OPN and pathways involved in the fibrogenesis process.
In an experimental mouse model of biliary injury, OPN expression and fibrosis were increased in relationship to the number of enhanced intrahepatic CD8+ lymphocytes. In contrast, an antibody-mediated depletion of CD8+ lymphocytes led to downregulated hepatic expression of OPN, as well as to diminished biliary injury and fibrosis; suggesting the possible role of the immune system in OPN-mediated liver injury [23].
The biological function of OPN might also be attributed to products of OPN degradation. For example, the cleavage of OPN by thrombin exposes an additional integrin-binding motif, promoting overall cell adherence. Cui et al. [24] described how the thrombin-cleaved OPN level correlated positively with the degree of liver fibrosis in both clinical and experimental settings. In OPN-deficient mouse models, thrombin-cleaved OPN peptides deteriorated liver fibrosis; whereas the neutralization of thrombin-cleaved OPN mitigated hepatic fibrosis in control mice. Compared with full length OPN, thrombin-cleaved OPN had an increased capability to promote HSC activation, proliferation, and migration.
The role of OPN in NAFLD pathogenesis was investigated by Syn et al. [25], who showed that NASH-related cirrhosis was associated with activation of the Hedgehog pathway. In turn, this led to the activation of liver progenitor cells to proliferate, undergo epithelial-mesenchymal transformation; producing factors activating neighboring progenitor cells as well as ECM-producing cells. In their follow-up study, Syn et al. [26] reported that in both in vivo and in vitro experiments OPN promoted pro-fibrogenic processes via modulation of the Hedgehog pathway. Experimental fibrosis in mice, induced by a methionine choline-deficient diet, was associated with active Hedgehog signaling and increased OPN expression. In contrast, OPN-deficient animals exhibited less fibrosis. Interestingly, in cultures of HSCs, Hedgehog pathway agonists upregulated; whereas antagonists decreased the expression of OPN. The fibrogenic process in HSCs could be sustained by recombinant OPN; whereas it was inhibited by the neutralization of OPN. OPN was markedly upregulated in patients with NASH, and correlated with the activity of the Hedgehog pathway as well as the degree of fibrosis; OPN thus mirrored the activity of liver fibrogenesis. Another study indicated that increased expression of OPN in NAFLD could be attributed to the accumulation of triacylglyceroles, as fat loading in HepG2 cells culture sustained OPN expression [27].
Finally, the role of OPN in liver injury seems not only to be negative. Patouraux et al. [28] described how liver ischemia in mice and subsequent re-perfusion led to the upregulation of hepatic and serum concentrations of OPN; yet also significantly increased liver cell necrosis. Also, higher ALT and AST activities were found in OPN-deficient mice compared to the control animals. Thus, OPN moderately prevented experimental animals from liver ischemia-reperfusion injury; presumably due to the partial anti-apoptotic ability of OPN, as well as inhibition of nitric oxide production by macrophages. Consistent with this observation, a protective effect of OPN in both renal and cardiac ischemic injuries has also been reported [29].
OPN delayed resolution of liver fibrosis because of sustained deposition of collagen-I, as was proven in mice after thioacetamide-induced fibrosis [10]. De Souza et al. [30] induced liver fibrosis in mice by the administration of carbon tetrachloride and ethanol. Subsequent treatment with bone marrow-derived monocytes led to a reduction of liver fibrosis, accompanied with a marked reduction of OPN expression.
Several studies have demonstrated that OPN neutralization abrogates fibrogenesis [22,31]. In another animal study of rats, utilizing a mesalazine treatment (a new agent with a promising OPN-lowering function) along with thioacetamide-induced liver fibrosis markedly diminished the expression of OPN [32]. These findings will encourage further targeting of OPN as an effective treatment approach in liver fibrosis [33].
3Clinical consequences of OPN in liver diseases3.1OPN and NAFLD/NASHAn ever increasing body of evidence suggests the involvement of OPN in both NAFLD and NASH development. As reported by Bertola et al., increased OPN expression in the liver correlated significantly with steatosis and insulin resistance in morbidly obese individuals [27]. Similarly, in their cross-sectional study on NAFLD patients, Glass and co-authors [34] demonstrated a significant association of both hepatic OPN mRNA expression and serum OPN concentrations with liver fibrosis. They suggested that OPN was a potential non-invasive biomarker of liver fibrosis in NAFLD. It is well known that liver fibrogenesis in NASH is accelerated by the adipokine leptin, which promotes liver fibrosis by directly activating HSC via the hedgehog pathway [35]; and this pathway is also under the control of OPN [36]. In a recent study on type 2 diabetes mellitus patients [37] increased serum OPN concentrations were also associated with NAFLD and multiple metabolic markers. In addition, the prevalence of NAFLD and NASH is increased in polycystic ovary syndrome [38,39], OPN is believed to be one of the key drivers in the development of fat deposition within these patients [40,41].
Clinical data on the role of OPN in the pathogenesis of NAFLD is supported by observations of the regulatory actions of OPN on cholesterol and phospholipid synthesis in the liver [42]. OPN inhibits the expression of CYP7A1 [42], the rate-limiting enzyme in bile acid biosynthesis, which is believed to contribute significantly to development of both NAFLD and metabolic syndrome [43,44].
3.2OPN and viral hepatitisOPN plays an important role in the control of immune system functions, and its dysfunctional signaling has been linked to development of various autoimmune diseases [45]. It is therefore not surprising that OPN was reported to be strongly associated with fulminant hepatitis, mostly of viral origin [46]. Several clinical studies have confirmed this association. Matsue et al. [47] compared plasmatic concentrations of OPN to the grade of liver fibrosis in 115 subjects with chronic HCV infection, and found a correlation of plasmatic OPN concentrations with the stage of liver fibrosis. Similarly, in patients with chronic HBV infection, plasmatic concentrations of OPN predicted the presence of liver cirrhosis [13]. Another large study performed on 200 patients with chronic HCV and B infections compared the plasma concentrations of OPN with the stage of fibrosis. It found increased OPN concentrations in both the HCV and HBV infections, with a positive correlation to the fibrosis stages [48]. The area under the curve (AUC), sensitivity, and specificity of OPN in predicting any stage of fibrosis were 0.99 (96% and 100% in patients with HBV infection), and 0.974 (96.5% and 100% in patients with HCV infection); clearly indicating that OPN might be considered a non-invasive biomarker for liver fibrosis in patients with chronic viral hepatitis. It has also been reported that genetic variants of the OPN gene strongly predict inflammatory activity in chronic HCV infection patients [49]; affected the response to HCV antiviral therapy [50], as well as the risk for development of HBV-related HCC [51]. Vice versa, HCV infection via induction of OPN expression was reported to activate downstream signaling pathways important for: epithelial to mesenchymal transition, migration, and invasiveness of the hepatocytes; pointing to the carcinogenic potential of OPN in liver cells of chronically infected HCV patients [52]. Importantly, OPN has been demonstrated to directly stimulate HCV replication and assembly [53,54]. In another study on chronic HBV patients, OPN was demonstrated to be a negative prognostic factor for the development of acute-on-chronic liver failure (ACLF) [55]. All of this data convincingly demonstrates the importance of OPN in hepatic inflammation, its interplay with inflammatory processes, as well as its cross-talk with pathways implicated in the development of complications of chronic hepatitis such as advanced fibrogenesis and carcinogenesis.
3.3OPN, liver cirrhosis and portal hypertensionThe underlying process driving the liver toward cirrhosis is the acceleration of fibrogenesis. An unavoidable consequence of advanced cirrhosis is portal hypertension, which leads to the risk of variceal bleeding, ascites development, renal failure, hepatic encephalopathy, as well as other complications. As discussed above, multiple studies have shown that plasma OPN concentration may serve as a marker for assessment of the severity of the fibrosis [12]. It might be anticipated that systemic OPN concentrations could be related to the severity of portal hypertension; and hence serve as a non-invasive biomarker for complications of advanced liver disease. Up to now in routine clinical settings portal hypertension has been evaluated by an invasive evaluation of the hepatic venous pressure gradient (HVPG) [56]. The HVPG is a prognostic parameter for the survival of cirrhotic patients [57], and seems to mirror the evolution of liver disease in the pre-cirrhotic stage. Recently, non-invasive markers have been proposed as surrogates for the invasive evaluation of portal pressure [58].
The first report on OPN regarding an assessment of portal hypertension was published in 2015 by Pereira et al. [59]. They reported that serum concentrations of OPN correlated with the pressure in the splenic vein, as well as the stage of liver fibrosis in patients with schistosomiasis. However, these findings were related to a specific disease, which is rare in western countries, as well as having used a very unusual evaluation of portal pressure (the measurement of splenic vein pressure).
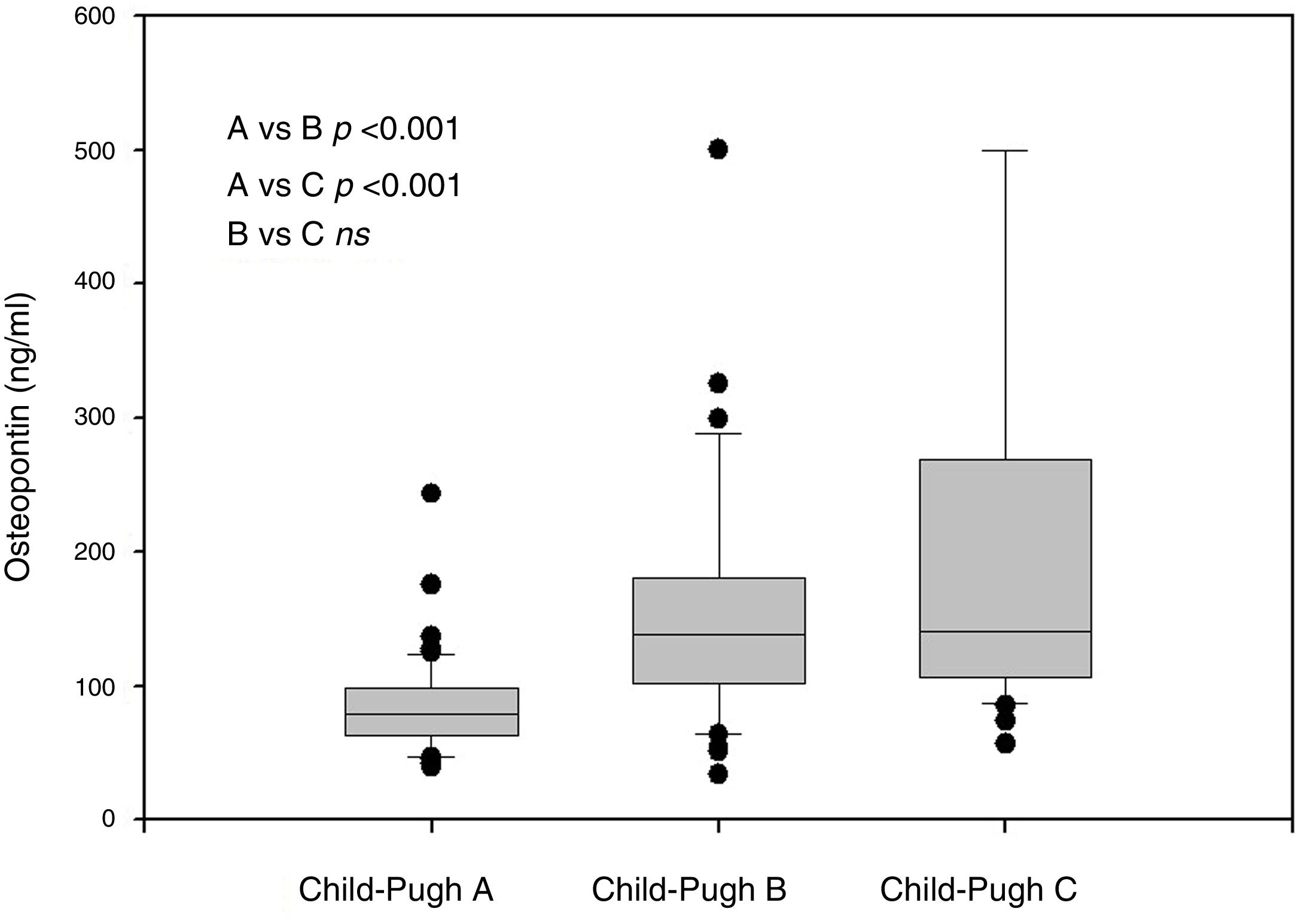
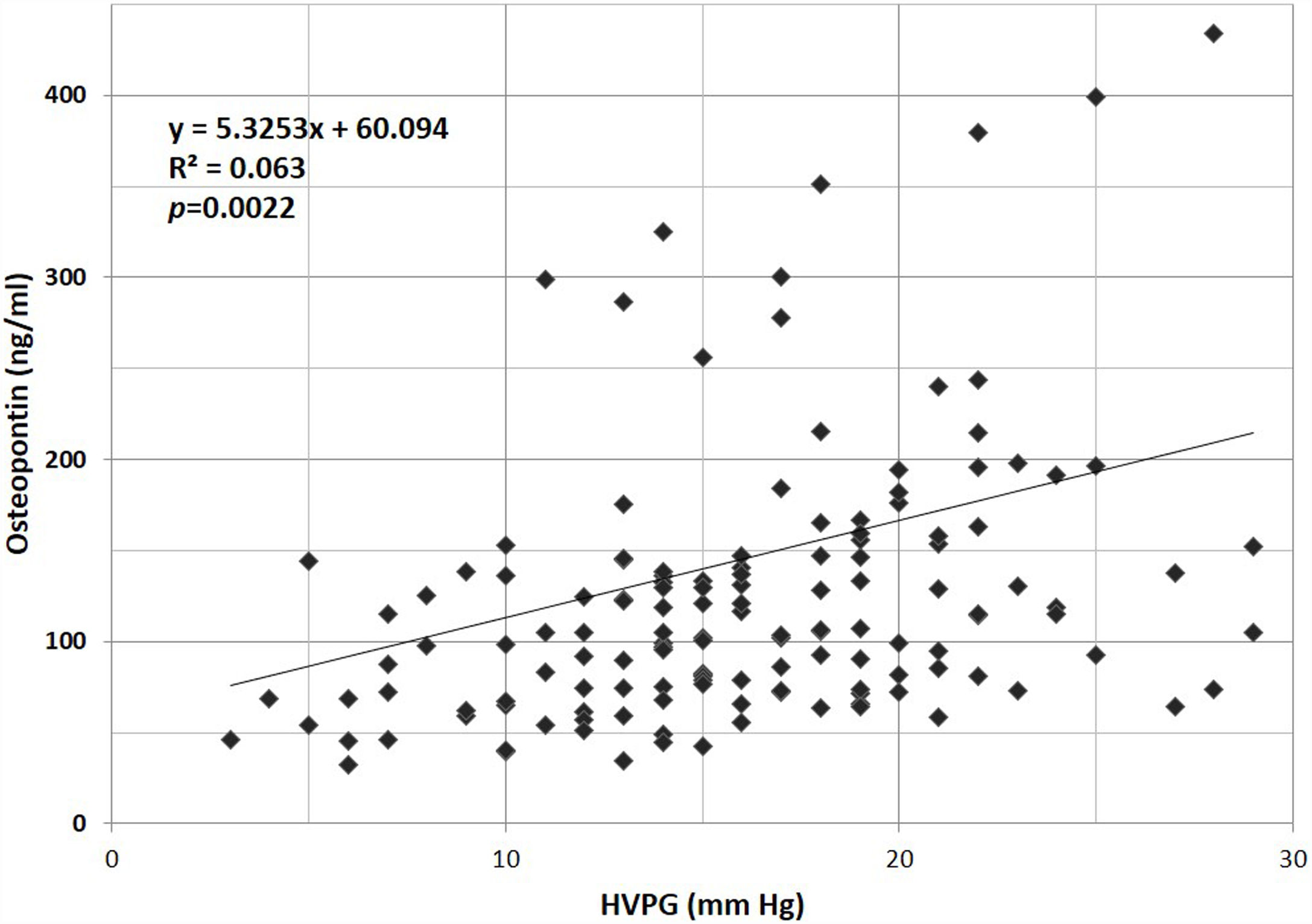
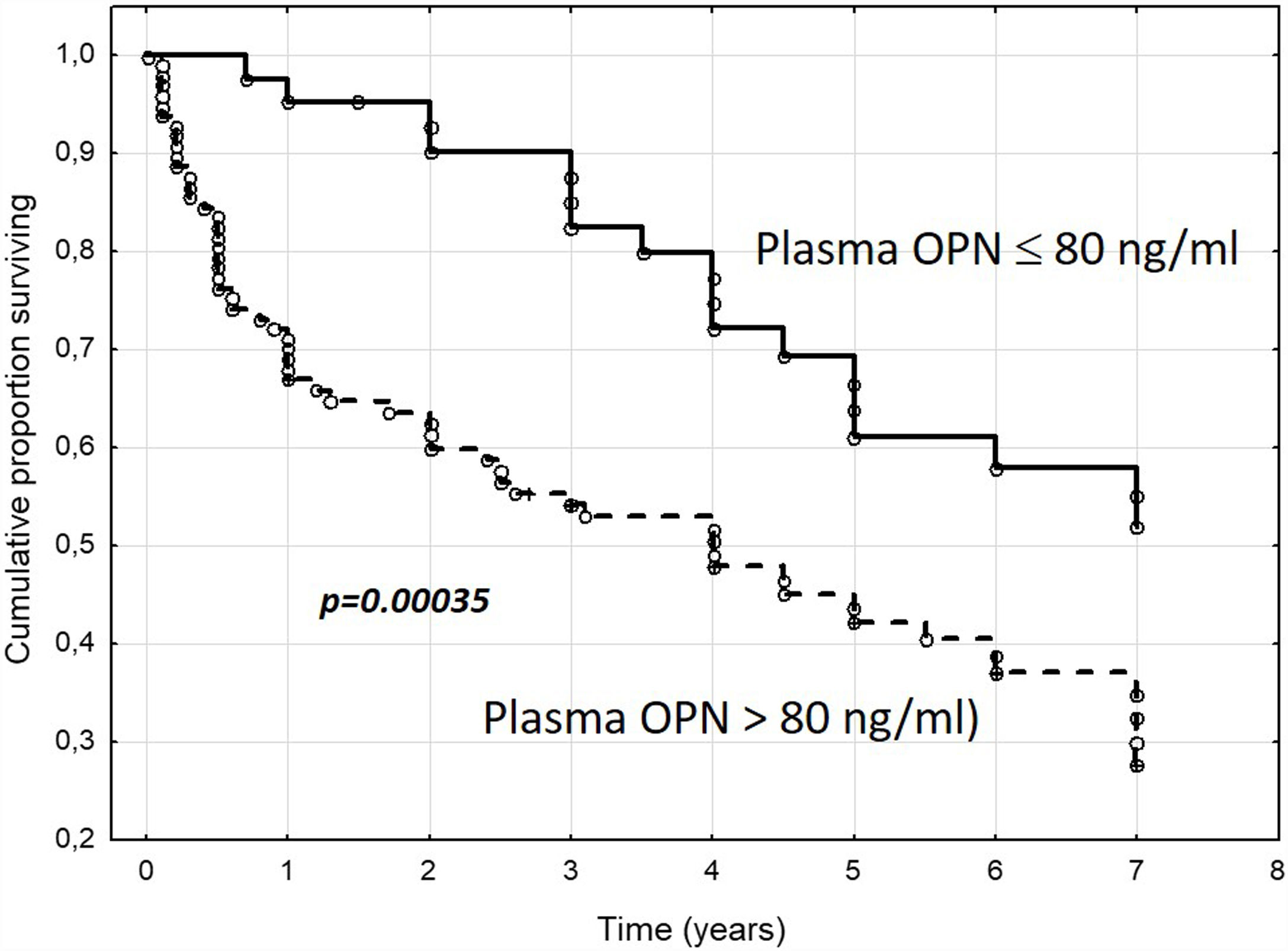
In our own study, the evaluation of the role of OPN in portal hypertension in a group of 154 cirrhotic patients [60] was based on a long-term follow-up interval, along with a detailed hemodynamic evaluation. First, we described the correlation between the plasma concentration of OPN with the severity of liver insufficiency (Fig. 2). Second, we found that plasmatic concentrations of OPN closely correlated with HVPG values (Fig. 3), and that an OPN plasmatic concentration over 80ng/ml discriminated subjects with clinically significant portal hypertension (i.e., HVPG >10mm Hg), with 75% sensitivity and 63% specificity; suggesting that plasmatic concentrations of OPN could be used as a biomarker of clinically significant portal hypertension. Most importantly, OPN was a powerful indicator for the prognosis in cirrhotic patients, and in a manner similar to the HVPG value significantly determined the survival rate. The cut-off value of 80ng/ml discriminated different groups with various probabilities of survival. This was also observed in the group of well-compensated cirrhotic patients, suggesting that OPN evaluation could be implemented into clinical work-ups (Fig. 4). In addition, the validity of OPN for patient prognosis was significantly increased when HVPG was considered in combination with it.
Plasma OPN in cirrhotic patients with different Child–Pugh classes (A, B, C) (Reproduced with permission from Bruha et al., Ref. [60]).
Relationship between hepatic venous pressure gradient and plasmatic concentrations of osteopontin in cirrhotic patients. HVPG, hepatic venous pressure gradient. (Reproduced with permission from Bruha et al., Ref. [60]).
Cumulative proportion of surviving patients with liver cirrhosis according to plasma osteopontin concentrations (cut-off 80ng/ml). OPN, osteopontin; n=154 patients, mean follow-up=3.7±2.6 years. (Reproduced with permission from Bruha et al., Ref. [60]).
Due to specific adhesive domains that interact with both the CD44 surface receptor and surface integrins in many different cells, OPN has an important role in the migration and adhesion of tumor cells [61,62] as well as contributing to carcinogenesis [63]. Indeed, studies have demonstrated that OPN is actually involved in the progression of different tumors, including hepatocellular cancer (HCC) [64] and cholangiocarcinoma [65].
In a proteomic study, plasma OPN was found to be significantly elevated in HCC patients [66].
Egyptian authors [67] studied the expressions of the OPN gene in liver tissue from patients with liver fibrosis as well as HCC due to chronic HCV infection. Compared to patients without fibrosis, OPN gene expression was higher in subjects with fibrosis, and was even more increased in HCC patients. Moreover, OPN gene expression correlated with the histopathological grade of HCC.
Another study by Egyptian authors investigated serum concentrations of OPN in patients with chronic HCV infection at a different stage of liver disease, including HCC in cirrhotic livers [68]. The serum concentrations of OPN were significantly higher in patients with HCC compared to patients with other liver diseases and with no HCC. They also observed a positive significant correlation between OPN and α1-fetoprotein (AFP), leading to their conclusion that the OPN concentrations might be used as a marker for HCC detection in cirrhotic patients with chronic HCV infection.
This relationship between OPN serum concentrations and HCC was confirmed by Abdel-Hafiz et al. [69], who correlated the serum OPN concentration in patients with both HCV-positivity and HCC to the expression of OPN in tumor and non-tumor liver tissue. They found that OPN concentrations were remarkably higher in HCC patients compared to the control group. Additionally, as in the previous study, a close correlation with the expression of AFP was also observed.
Recently, Nabih et al. suggested OPN as a tumor biomarker for screening of HCC in HCV cirrhosis. In their study, plasma OPN concentrations had shown greater sensitivity for HCC diagnosis compared to AFP [70]. These observations on the associations between OPN and AFP were also confirmed in a recent meta-analytic study [71].
In another study, Zhang et al. [72] evaluated the prognostic significance of preoperative plasmatic concentrations of OPN in cirrhotic patients who had undergone HCC resection. In patients with a recurrence of HCC during follow up, significantly increased concentrations of OPN were found, when compared to those patients in remission. Further, the plasmatic OPN concentration was an independent prognostic parameter for survival. The prognostic value of OPN in HCC patients was highlighted by Huang et al., who found an association between OPN and a high metastatic potential of HCC [73].
Consistently, the prognostic significance of OPN plasmatic concentration has also been described in cirrhotic patients with an early stage of HCC [74]. The prognostic value of both serum and tissue OPN levels on the progression of HCC were confirmed in a recent meta-analytic study, covering more than 2100 HCC patients from 12 clinical studies [75]. Indeed, OPN tissue expression as well as systemic concentration have been associated with overall survival, stage, as well as tumor size [75].
It is known that HCC risk correlates to the degree of portal hypertension; therefore, increased concentrations of OPN could reflect the presence of advanced cirrhosis and portal hypertension rather than the occurrence of HCC itself.
This association was studied by Simao et al. [76], who examined 90 consecutive patients with alcoholic cirrhosis. OPN concentrations notably increased in parallel with a progression in the Child–Pugh score. When patients with HCC were compared to those without HCC, a correlation was observed between OPN concentrations and both the stage of HCC as well as liver dysfunction. The authors concluded that OPN could reflect liver dysfunction rather than the appearance of HCC. Thus, the use of OPN as a marker for HCC should be cautiously considered. Indeed, Abdel-Hafiz et al. [69] found increased serum concentrations of OPN in HCV-induced cirrhotic patients with HCC; but found no significant difference between the expression of OPN in tumor vs. tumor-free liver samples.
3.5OPN as a prognostic factor in advanced liver diseaseThe most important prognostic scoring systems of liver cirrhosis used in routine practice are Child–Pugh and Model for End-Stage Liver Disease (MELD) scores [77]. An increasing body of evidence has shown the relationship of MELD and Child–Pugh scores to OPN. The relationship between OPN and MELD score was studied in 54 patients with HBV-associated ACLF. Liu et al. [55] reported a positive correlation between OPN and MELD score and proved serum OPN to be an independent prognostic factor patients with HBV-associated ACLF. Saha et al. [78] measured OPN in 89 patients with acute alcoholic hepatitis (AH) and found a correlation between the MELD score and plasmatic concentrations of OPN. The authors also identified OPN as an independent predictor of 90-day mortality in patients with acute AH. Cabiati et al. [79] studied the correlation between the OPN concentrations and MELD score in 10 HCV-positive patients with HCC undergoing liver transplantation and found that both OPN mRNA expressions in liver tissue samples and plasmatic levels of OPN correlated positively with the MELD score.
The correlation between the OPN concentrations and Child–Pugh score was studied also in our study on a group of 154 cirrhotic patients [60]. Plasma OPN concentrations increased significantly with the increased Child–Pugh stage in patients with cirrhosis. Kim et al. [80] measured OPN in patients with HCC, patients with chronic liver diseases and healthy subjects. Within the HCC patient group, plasma OPN concentrations increased significantly with the advancing degree of Child–Pugh class as well as tumor stage. On contrary, the other study did not find any correlation between Child–Pugh score and plasma OPN levels in patients with HCC in cirrhotic liver [81]. Thus, OPN as a marker for HCC should be considered with caution [76].
3.6OPN and other hepatic diseases, and the possible links to liver metabolismIn addition to the direct links of OPN to liver pathology, it is interesting to note that OPN signaling is inhibited by apolipoprotein D (ApoD) [82], a strong bilirubin-binding protein in human plasma [83]. Hence, ApoD has been identified as a potential anti-carcinogenic molecule [82,84]. Although the data remains insufficient, it is possible that bilirubin bound to ApoD might be responsible for these antiproliferative effects, as was previously reported in breast cancer some years ago [85].
In addition to these effects, OPN is associated with liver regeneration due to the activation of hepatic stem cells [86]; and it probably also has a pathogenic role in acute liver failure [87]. As OPN strongly interferes with the immune functions (see above), it is also implicated in the development of autoimmune hepatitis [45]. Furthermore, according to a very recent study, OPN seems to be a very promising biomarker of drug-induced liver injury [88]. Finally, due to its calcium-binding properties, OPN has been suggested as an important pathogenic factor in the formation of pigment gallstones, as was immunohistochemically confirmed in a recent human study [89]. All of these diverse observations indicate the wide impacts of OPN signaling involved in the development of various liver diseases.
4ConclusionsOPN is an important signaling cytokine in the developmental process toward liver disease, despite the fact that all of the OPN-affected pathways still await more full scientific elucidation. OPN has been predominantly related to liver fibrosis under different pathological conditions such as NAFLD, chronic viral hepatitis B and C, as well as alcoholic liver disease. OPN could serve as a surrogate diagnostic marker of liver fibrosis, cirrhosis, portal hypertension, HCC, as well being as a prognostic parameter in liver cirrhosis (Table 2). The use of anti-OPN directed treatment seems to be promising; although further detailed studies are definitely needed in order to discover the full potential of this potential therapeutic approach.
FundingSupported by a grant from the Czech Ministry of Health (Grant No. RVO-VFN64165/2019) and by a grant No. PROGRES Q25/LF1 given by Charles University in Prague.AbbreviationsAFP α1-fetoprotein alanine transaminase apolipoprotein D aspartate transaminase extracellular matrix hepatitis B virus hepatocellular cancer hepatitis C virus high-mobility group box-1 hepatic stellate cells hepatic venous pressure gradient osteopontin non-alcoholic fatty liver disease non-alcoholic steatohepatitis transforming growth factor beta
The authors declare having no conflicts of interest.




![Plasma OPN in cirrhotic patients with different Child–Pugh classes (A, B, C) (Reproduced with permission from Bruha et al., Ref. [60]). Plasma OPN in cirrhotic patients with different Child–Pugh classes (A, B, C) (Reproduced with permission from Bruha et al., Ref. [60]).](https://static.elsevier.es/multimedia/16652681/0000001900000004/v2_202007040825/S1665268120300016/v2_202007040825/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Relationship between hepatic venous pressure gradient and plasmatic concentrations of osteopontin in cirrhotic patients. HVPG, hepatic venous pressure gradient. (Reproduced with permission from Bruha et al., Ref. [60]). Relationship between hepatic venous pressure gradient and plasmatic concentrations of osteopontin in cirrhotic patients. HVPG, hepatic venous pressure gradient. (Reproduced with permission from Bruha et al., Ref. [60]).](https://static.elsevier.es/multimedia/16652681/0000001900000004/v2_202007040825/S1665268120300016/v2_202007040825/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Cumulative proportion of surviving patients with liver cirrhosis according to plasma osteopontin concentrations (cut-off 80ng/ml). OPN, osteopontin; n=154 patients, mean follow-up=3.7±2.6 years. (Reproduced with permission from Bruha et al., Ref. [60]). Cumulative proportion of surviving patients with liver cirrhosis according to plasma osteopontin concentrations (cut-off 80ng/ml). OPN, osteopontin; n=154 patients, mean follow-up=3.7±2.6 years. (Reproduced with permission from Bruha et al., Ref. [60]).](https://static.elsevier.es/multimedia/16652681/0000001900000004/v2_202007040825/S1665268120300016/v2_202007040825/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





