Introduction & Aim. The role of age as a predictor of mortality after transjugular intra hepatic portosystemic shunt (TIPS) is controversial. Age has been found to be an important predictor of post-TIPS mortality in some, but not all, studies and is not a component of the MELD score. The purpose of this study was to compare the 90-day survival of subjects with cirrhosis age ≥ 70 years with younger subjects undergoing TIPS.
Material and methods. A database of adult with cirrhosis undergoing TIPS from 2003-2011 was analyzed. The primary endpoint was survival 90-days post-TIPS. Survival was analyzed by the Kaplan-Meier method and proportional hazard modeling.
Results. 539 subjects met study criteria. 474 (88%) were between the ages of 24-69 and 65 (12%) were age 70-89 years. The groups were similar with respect to the indication for TIPS, mean MELD score and distribution of MELD score. Survival 90-days post-TIPS was 60% in the older cohort compared with 85% in the younger cohort (p < 0.001). Proportional hazards modeling controlled for comorbidities identified age ≥ 70 and MELD score as predictors of early post-TIPS survival. The hazard ratio associated with age increased monotonically, became significant at age ≥ 70 years (HR 3.22; 95% CI 1.81-5.74; p < 0.001) and exceeded the effect of MELD on survival.
Conclusions. Age ≥ 70 was associated with reduced survival within 90 days following TIPS. The findings from this study indicate that age is a relevant consideration in assessing the early mortality risk of TIPS.
The transjugular intrahepatic portosystemic (TIPS) shunt is an indispensable tool in the management of complications of portal hypertension and, based on randomized, controlled trials (RCTs), has an established role in the treatment of refractory ascites and secondary prevention of variceal bleeding.1,2 Prospective and retrospective studies have also provided insight into the procedure’s risk and benefits. In the case of refractory ascites, for example, four multi-center RCTs have shown TIPS to be superior to intermittent large volume paracentesis for relief of ascites but, in some studies, associated with higher rates of moderate-severe encephalopathy.3–6 In addition, all patients undergoing TIPS are at risk for procedure related complications and deterioration of liver function.
Critical to the mitigation of TIPS-related complications is proper patient selection. The role that age plays in selection criteria is complex. Though studies have identified age as a relevant predictor of mortality post-TIPS,2,7,8 the Model for End Stage Liver Disease (MELD) which was developed to assess 3-month post-TIPS mortality excludes age as a variable.9
The discordance in these studies can be related to methodological issues. Studies that associate age with increased risk have been retrospective, do not control for comorbidities and,in some cases, only report overall survival, which by its very nature, is strongly associated with age. In comparison, scoring systems like MELD that are silent to age are constructed from clinical data over a narrow range in ages; the participants in clinical studies of TIPS are, on average, in their fifth decade and many randomized trials of TIPS exclude subjects older than 70. Thus, even when not specifically excluded, older subjects will often comprise a small contribution to the study population.
To date, there has been no published analysis dedicated to elucidating the outcome of TIPS in patients age 70 years and older. Though the risks and benefits of TIPS are largely known, whether these parameters are applicable in older individuals is unknown.
Understanding the risks and benefits of TIPS in older individuals has its merits because it is reasonable to expect that an aging population will also be reflected in increasing number of older patients with liver disease related complications.10 In addition to demographic shifts, the increasing incidence of decompensated cirrhosis as a consequence of nonalcoholic fatty liver disease may also result in an older population with complications of portal hypertension.11
The purpose of this study was to delineate the outcome of TIPS in cirrhotic subjects 70 years of age and older compared with younger subjects. The study was undertaken as a single-center, cohort study to compare characteristics of these two groups of cirrhotic patients undergoing TIPS and proportional hazard modeling to determine the effect of age on post-TIPS survival.
Material and MethodsA database of all patients undergoing TIPS by our institution’s Vascular and Interventional Radiology Service was queried for subjects age 18 and older who underwent successful creation of a TIPS using a PTFE-covered Viatorr stent (W. L. Gore & Associates, Flagstaff, Arizona) from 2003-2011. Subjects are enrolled into the database at the time of TIPS which includes information on pre-TIPS laboratory data, the TIPS procedure and comorbidities. Post-TIPS survival times were determined by reviewing the institutions electronic medical record or the Social Security Administration Death Master File. Individuals were excluded from analysis if they had noncirrhotic portal hypertension or underwent either TIPS or liver transplantation at any time prior to the dates under study. The diagnosis of cirrhosis was based on liver biopsy or, when biopsy was unavailable, liver imaging with a compatible diagnosis and laboratory data. The beginning date was chosen because it reflected the year that the near exclusive use of PTFE-covered stents began. The institution’s Human Research Protection Office approved the study and a waiver of informed consent was granted. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or compatible ethical standards.
The primary study endpoint was death within 90 days following TIPS. Patient survival was censored if they were alive at the end of the study period, at time of liver transplantation or at date of last documented contact.
MELD score was computed from the equation: MELD score = (9.57 x loge creatinine [mg/dL]) + (3.78x loge bilirubin [mg/dL]) + 11.20 x loge INR) + 6.43.
In keeping with standard modifications to the MELD score, laboratory values < 1 were assigned a value of 1, the upper limit of creatinine was set to 4 mg/dL and subjects receiving renal replacement at least twice in the week prior to TIPS were assigned a creatinine of 4 mg/dL. Comorbidities were assessed using a modified Charlson Comorbidity Index developed for predicting post-liver transplant survival.12
TIPS was performed by gaining access to the right internal jugular vein under ultrasound guidance and a wire advanced centrally followed by placement of a vascular sheath. Under fluoroscopic guidance, a 5 French catheter is advanced into the right hepatic vein and over a stiff guide wire, the catheter is exchanged for a balloon occlusion catheter and wedged hepatic venography is performed to identify the main portal vein branches. The catheter is exchanged for a Colapinto needle (Cook Medical, Indianapolis, Indiana) and passes made to gain access to the portal vein, confirmed by carbon dioxide contrast injection. The parenchymal tract is then predilated with an 8 mm balloon. A long sheath is advanced into the portal vein over which the stent graft is advanced, deployed and dilated to the desired diameter. The technique for TIPS placement was identical in subjects regardless of age. The portosystemic gradient (PSG) was computed from pressure measurements taken from the right atrium and the portal vein pressure.
Normalized, parametric and continuous data were compared by Student t-test, categorical data was analyzed by χ2 testing or Fisher exact testing and non-normalized variables were compared with the Wilcoxon rank sum test.
Unadjusted patient survival was estimated by the Kaplan-Meier method with comparison between groups performed with the log-rank test. Cox proportional-hazards analysis was used to identify factors independently associated with patient survival at 90 days after TIPS placement. Variables with a p-value < 0.1 were entered in a multivariate analysis.Tests of the proportional hazards assumption was performed with scaled Schoenfeld residuals. Statistical analysis was performed with R version 3.0.213 and Survival package version 2.37-4.14
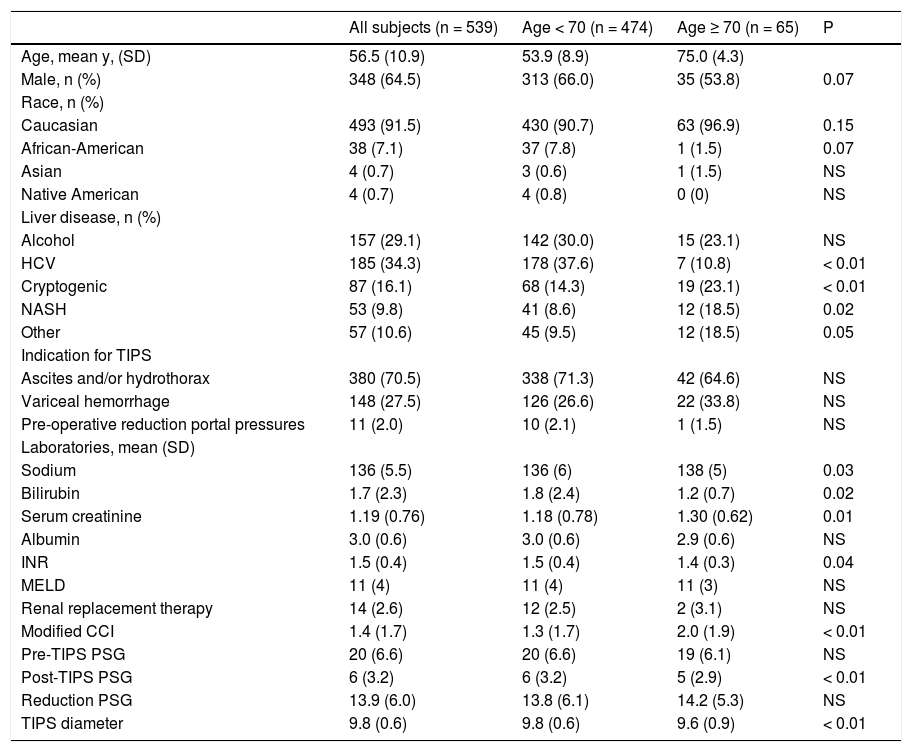
ResultsCharacteristics of the study cohortsBetween January 2003 and July 2011, 539 patients meeting the selection criteria underwent successful TIPS placement. 474 (88%) were between age 24-69 and 65 (12%) were age 70-89 years. An average of 60 TIPS were performed per year of which subjects age ≥ 70 years comprising 3-18% of TIPS placed each year. Demographic characteristics of the patient, the cause of liver disease, indication for TIPS and pre-TIPS laboratories are shown in table 1.
Demographic and clinical characteristics of the study population.
| All subjects (n = 539) | Age < 70 (n = 474) | Age ≥ 70 (n = 65) | P | |
|---|---|---|---|---|
| Age, mean y, (SD) | 56.5 (10.9) | 53.9 (8.9) | 75.0 (4.3) | |
| Male, n (%) | 348 (64.5) | 313 (66.0) | 35 (53.8) | 0.07 |
| Race, n (%) | ||||
| Caucasian | 493 (91.5) | 430 (90.7) | 63 (96.9) | 0.15 |
| African-American | 38 (7.1) | 37 (7.8) | 1 (1.5) | 0.07 |
| Asian | 4 (0.7) | 3 (0.6) | 1 (1.5) | NS |
| Native American | 4 (0.7) | 4 (0.8) | 0 (0) | NS |
| Liver disease, n (%) | ||||
| Alcohol | 157 (29.1) | 142 (30.0) | 15 (23.1) | NS |
| HCV | 185 (34.3) | 178 (37.6) | 7 (10.8) | < 0.01 |
| Cryptogenic | 87 (16.1) | 68 (14.3) | 19 (23.1) | < 0.01 |
| NASH | 53 (9.8) | 41 (8.6) | 12 (18.5) | 0.02 |
| Other | 57 (10.6) | 45 (9.5) | 12 (18.5) | 0.05 |
| Indication for TIPS | ||||
| Ascites and/or hydrothorax | 380 (70.5) | 338 (71.3) | 42 (64.6) | NS |
| Variceal hemorrhage | 148 (27.5) | 126 (26.6) | 22 (33.8) | NS |
| Pre-operative reduction portal pressures | 11 (2.0) | 10 (2.1) | 1 (1.5) | NS |
| Laboratories, mean (SD) | ||||
| Sodium | 136 (5.5) | 136 (6) | 138 (5) | 0.03 |
| Bilirubin | 1.7 (2.3) | 1.8 (2.4) | 1.2 (0.7) | 0.02 |
| Serum creatinine | 1.19 (0.76) | 1.18 (0.78) | 1.30 (0.62) | 0.01 |
| Albumin | 3.0 (0.6) | 3.0 (0.6) | 2.9 (0.6) | NS |
| INR | 1.5 (0.4) | 1.5 (0.4) | 1.4 (0.3) | 0.04 |
| MELD | 11 (4) | 11 (4) | 11 (3) | NS |
| Renal replacement therapy | 14 (2.6) | 12 (2.5) | 2 (3.1) | NS |
| Modified CCI | 1.4 (1.7) | 1.3 (1.7) | 2.0 (1.9) | < 0.01 |
| Pre-TIPS PSG | 20 (6.6) | 20 (6.6) | 19 (6.1) | NS |
| Post-TIPS PSG | 6 (3.2) | 6 (3.2) | 5 (2.9) | < 0.01 |
| Reduction PSG | 13.9 (6.0) | 13.8 (6.1) | 14.2 (5.3) | NS |
| TIPS diameter | 9.8 (0.6) | 9.8 (0.6) | 9.6 (0.9) | < 0.01 |
There was a higher frequently of HCV infection and more men in the younger cohort. In comparison, there was a higher frequency of cryptogenic and NASH-related cirrhosis in the older cohort. The percentage of African Americans was greater in the younger group than older group; though Caucasians comprised the majority of subjects in both groups.
The majority of the TIPS that were placed were for the management of refractory ascites and/or hepatic hydrothorax (70.5%). This indication encompassed a range of subjects including both those with refractory and diuretic intolerant ascites based on the International Ascites Club Definition15 and subjects without refractory ascites but in whom TIPS was considered the appropriate therapy in the judgment of the treating physician. The remaining indication for TIPS were for either acute variceal hemorrhage or secondary prevention of portal hypertensive bleeding (27.5%) or were performed to assist in the decompression of abdominal varices prior to abdominal surgery (2.0%). The indications for TIPS were similar in both groups.
The older cohort had a higher serum sodium, higher creatinine, lower bilirubin, lower INR and higher modified Charlson comorbidity index compared to the younger cohort. The proportion receiving renal replacement therapy, mean MELD score at TIPS,serum albumin and reduction of the portosystemic gradient with TIPS were similar between groups. 88% of all study subjects underwent TIPS with MELD scores ≤ 15. Among the younger group, 88.4% had MELD scores <15 compared with 89.2% in the older cohort. The distribution of MELD scores above and below 15 were similar in both groups (χ2=1). Also similar between groups was the mean reduction in the portosystemic gradient (14 mmHg). The majority of subjects achieved a final TIPS diameter of 10 mm though a higher percentage in the older group received an 8 mm TIPS.
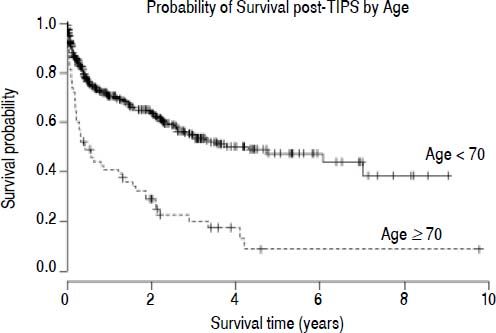
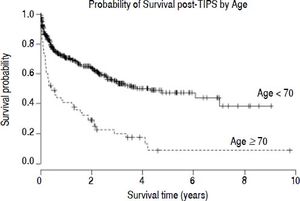
Survival analysisThe estimated survival in all TIPS recipients was 81.7, 73.0 and 66.7% at 90-days, 6 months and 1 year, respectively. In those ≥ 70, there was a decreased probability of survival post-TIPS; their survival was 60.0, 49.1 and 40.9% at 90-days, 6 months and 1 year, respectively. In comparison, survival in the younger cohort was 84.9, 76.5 and 70.7% at 90-days, 6 months and 1 year, respectively (χ2 = 43.6, p < 0.001) (Figure 1). The mean time of follow up was 1.6 years in those < 70 years and 1.2 years in those ≥ 70 years. The number of subjects lost to follow up was low; no subjects were lost to follow up in the older age group and only 13 subjects were lost to follow up in the group < 70 years. Thirteen subjects in the younger age group received liver transplants in the first 90 days post-TIPS.
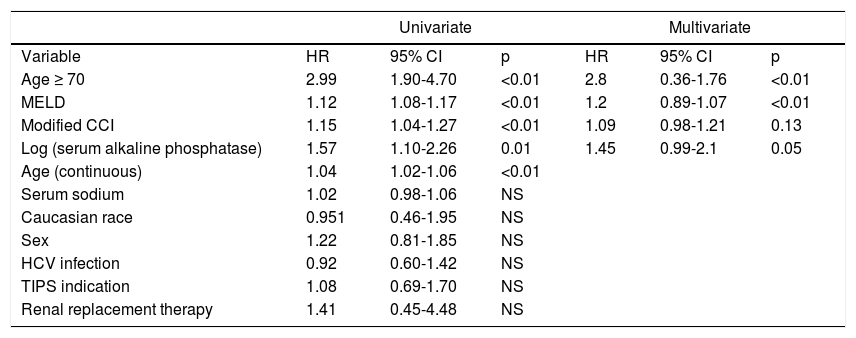
In univariate analysis (Table 2), age as a continuous variable, age ≥ 70, MELD, serum alkaline phosphatase (logarithmically transformed) and the modified Charlson Comorbidity Index (CCI) yielded statistically significant hazard ratios for death within 90 days post-TIPS. The hazard ratios for age were 1.04 (95% CI 1.02 - 1.06; p < 0.001) as a continuous variable and 2.99 (95% CI 1.90 - 4.70, p < 0.001) as a dichotomous value. Hazard ratios for the other significant covariates were: MELD 1.12 (95% CI 1.07 - 1.17; p < 0.001), modified CCI 1.14 (95% CI 1.031 - 1.267; p = 0.008) and log-transformed alkaline phosphatase 1.57 (95% CI 1.10 - 2.26; p = 0.014). None of the other variables resulted in statistically significant hazard ratios; INR, bilirubin and creatinine are captured in MELD and were not independently tested.
Univariate and multivariate proportional hazards analysis of the association between clinical variables and 90-day post-TIPS mortality.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age ≥ 70 | 2.99 | 1.90-4.70 | <0.01 | 2.8 | 0.36-1.76 | <0.01 |
| MELD | 1.12 | 1.08-1.17 | <0.01 | 1.2 | 0.89-1.07 | <0.01 |
| Modified CCI | 1.15 | 1.04-1.27 | <0.01 | 1.09 | 0.98-1.21 | 0.13 |
| Log (serum alkaline phosphatase) | 1.57 | 1.10-2.26 | 0.01 | 1.45 | 0.99-2.1 | 0.05 |
| Age (continuous) | 1.04 | 1.02-1.06 | <0.01 | |||
| Serum sodium | 1.02 | 0.98-1.06 | NS | |||
| Caucasian race | 0.951 | 0.46-1.95 | NS | |||
| Sex | 1.22 | 0.81-1.85 | NS | |||
| HCV infection | 0.92 | 0.60-1.42 | NS | |||
| TIPS indication | 1.08 | 0.69-1.70 | NS | |||
| Renal replacement therapy | 1.41 | 0.45-4.48 | NS | |||
CI: confidence interval. CCI: Charlson comorbidity index. HCV: hepatitis C virus. PSG:portosystemic gradient.
Given the threshold effect detected with age, a multivariate model was constructed incorporating age as a dichotomous variable, MELD, modified CCI and log-transformed alkaline phosphatase. In this model, only age and MELD remained significant. Hazard ratios for a model incorporating only these covariates were: age (dichotomous) 2.80 (95% CI 1.76 - 4.45; p < 0.01) and MELD score 1.2 (95% CI 1.07 - 1.18; p < 0.01) (Table 2).
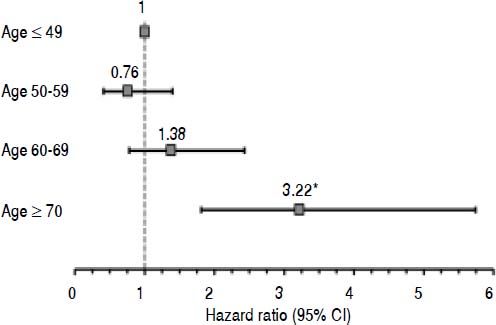
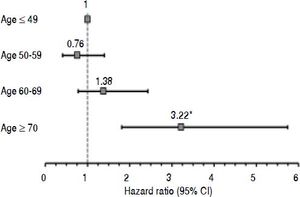
To further assess the relationship between age and postTIPS survival, a multivariate model was constructed with the subjects stratified into four age groups: 19 - 49, 50 - 59, 60 - 69 and ≥ 70 years. The adjusted hazard ratio for age group when controlled for MELD increased monotonically above age 59 and became statistically significant in those ≥ 70 years with a hazard ratio of 3.23 (95% CI 1.81 - 5.74; p = 0.001) (Figure2).
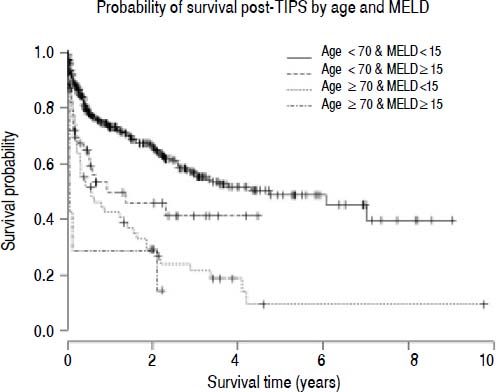
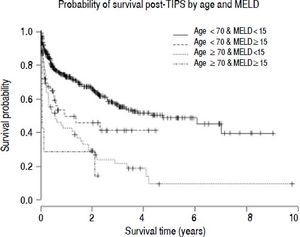
To investigate the relationship between MELD and age, survival was compared between the threshold age of 70 and threshold MELD score of 15. The probability of survival 90-days post-TIPS was 86.8% in those age <70 and MELD <15 (n = 419), 69.6% in those age <70 and MELD ≥ 15 (n = 58), 63.8% in those age ≥ 70 and MELD <15 (n=55) and 28.6% in those age ≥ 70 and MELD ≥ 15 (n=7) (χ2=48; p < 0.001 log-rank test for trend) (Figure 3).
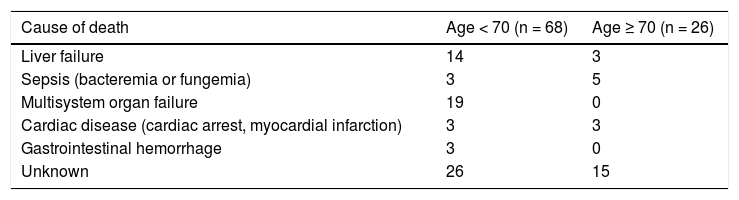
There were a total of 94 deaths within the first 90-days post TIPS. Sixty-eight deaths were in the subjects < 70 years and 26 in those ≥ 70. The cause of death, where known, is listed in table 3.
ConclusionsIn this study, we sought to demonstrate that older age is associated with decreased survival within 3 month following TIPS placement. The study was limited to those with cirrhosis undergoing TIPS for the first time. By limiting the survival analysis to the 90-day time interval, it was hoped that this would minimize the general effect of age on survival and permit a comparison with MELD, arguably the dominant model for the prediction of post-TIPS survival.
The older cohort comprised 65 subjects age 70 years or older and represented 12% of those undergoing TIPS. The choice of a threshold of age 70 was based on the fact that three of the four randomized, controlled trials of TIPS for refractory ascites either excluded subjects over age 72-75 or lacked subjects > 74 years. Further, preliminary data analysis suggested this threshold would provide sufficient subjects and events to demonstrate a statistically valid result. Further, by evaluating age as a dichotomous variable allowed us to demonstrate both similarities (MELD distribution, comorbidities) and differences (TIPS indication) between these two groups.
Age, even when controlled for MELD, was found to be strongly associated with 90-day post-TIPS mortality risk, particularly in those ≥ 70 years. The subjects of each group were well matched for MELD score (both mean scores and distribution), indication for TIPS and comorbidities supporting the conclusion that age was the cause for the difference in survival and not a surrogate for another variable. Comorbidities were assessed by a modified Charlson Comorbidity Index. This index was developed in pre-liver transplant subjects to predict post-transplant survival.12 Though the modified CCI was statistically greater in the older cohort, the numerical difference was small and it was not a relevant covariate in the final proportional hazards model.
The groups did differ with respect to their sex distribution and causes of liver disease, however, neither sex nor cause of liver disease, including hepatitis C infection, were found to influence survival. A difference was seen between groups with respect to laboratory values of creatinine, bilirubin, INR and serum sodium, though the mean values in the two groups were very similar, within standard laboratory reference ranges and did not result in significant differences in MELD score.
There is also no reason to believe that there is a substantial selection bias between the two cohorts. Other than the assessment of mesenteric vessel patency, our institution does not employ a templated pre-TIPS assessment nor require echocardiography to exclude pulmonary hypertension; this approach is consonant with society level guidelines.16 It is also reasonable to assume that bias introduced in patient selection would work toward minimizing the difference between groups as it is unlikely that demonstrably frail older patients would be considered for TIPS in comparison to younger subjects with similar symptoms of portal hypertension and severity of illness.
The hazard ratio associated with age increased in a monotonic fashion above age 59, crossed the threshold of statistical significance above age 69 and exceeded the effect of MELD in the older age group. Illustrative of this relationship among age, MELD and survival is the finding that the median survival of younger subjects with MELD scores ≥ 15 was greater than those in the older cohort with MELD scores <15.
To be sure, the inclusion of age as a relevant variable in post-TIPS survival has been reported in other retrospective single site,17,18 multi-site studies19 and meta-analysis of prospective studies.2 There are, however, studies in which the effect of age is statistically insignificant in comparison to bilirubin and other measures of liver function, such as Child-Pugh score.7,20 Those studies with a positive association often rely on a small number of subjects over age 65 from which their conclusions are drawn and fail to account for comorbidities, which would be critical to control for in any study in comparing age groups. As an example, in the most recent publication by Parvinian, et al., age but not MELD was associated with a greater 90 day post-TIPS mortality risk among those with MELD scores of 18-25.8 This publication, however, failed to control for comorbidities and only included 23 subjects over the age of 54.
What is unique about this study is that it both provides a greater degree of granularity with respect to the clinical characteristics and of the older cohort and a more precise relationship between age and early post-TIPS mortality. Further, it is the only study to control for comorbidities that might bias outcome.
Though the results of this study argue strongly that older age is a relevant consideration in assessing mortality risk of TIPS, it is premature to modify patient selection for TIPS based on this data alone, particularly for indications in which there is high-level outcome data supporting its benefit. Similarly, practitioners may wish to exercise caution in placing TIPS in elderly subjects when the indication is less secure or the benefits more speculative. Examples of this latter group include TIPS for decompression of abdominal varices prior to abdominal surgery or difficult to control but not refractory ascites in elderly, high MELD subjects.
More importantly, the data from this study should be taken into consideration as indications for TIPS expand beyond refractory ascites. For example, García-Pagán, et al. has demonstrated a survival advantage with early TIPS in subjects with variceal bleeding and Child-Pugh class C cirrhosis or Child-Pugh class B cirrhosis with bleeding at the time of index endoscopy.1 In their landmark publication, however, the mean age of subjects randomized to TIPS was 52 years and subjects over age 75 were excluded from the study. If age ≥ 70 is truly associated with increased early mortality post-TIPS than it may be inappropriate to apply the lessons of this study to older patients until they are explicitly studied in this setting.
Extending this work will require a new survival model that incorporates age and validating that model in an independent cohort. In addition, additional factors will need to be incorporated to permit greater clarity in risk stratification. As an example, the value of the six-minute walk distance as a predictor of post-liver transplant survival has been reported.21 This test is considered a measure of global physical function and has been applied in elderly subjects to assess global health status. The addition of a functional test such as this with other validated variable, for example, MELD, may be one approach to further refine the risk assessment in this group.
This study was also confined to PTFE-covered stent grafts for which there is evidence of improved graft patency, improved rates of relief from symptoms of portal hypertension and trend to increased survival in comparison to uncovered stents.22 Despite the improvements offered by covered stents, the use of these TIPS stent grafts appeared unable to narrow the differences in survival between the two groups.
It is also critical to think beyond mortality in the older age group who require TIPS. The incidence and prevalence of post-TIPS encephalopathy was not systematically assessed in our database but has been demonstrated with other studies.5 Increased rates of encephalopathy in older patients could contribute to their decline though that relationship was not seen with the data from our site.
Thus, though it is important to recognize that TIPS both remains an important technique for the management of portal hypertension, those who are older may require more caution and counseling about the risks and benefits of TIPS.
Abbreviations- •
MELD: Model for End Stage Liver Disease.
- •
modified CCI: modified Charlson Comorbidity Index.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
NS, MKR and KMK conceived the idea, experimental design and analyzed the data. MD created and maintained the database and assisted in writing the manuscript. MKR, AW and JBH collected data for the study. KMK and MKR wrote the manuscript.
FundingThis publication was made possible by Grant Numbers 1 UL1 RR024992-01, 1 TL1 RR024995-01 and 1 KL2 RR 024994-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.