Background. The prevalence of occult HBV, defined by the presence of HBV DNA in individuals with antibodies to HBV core antigen and with absence of HBV surface antigen, but its clinical significance and virological features in HIV-infected patients is still unclear.
Aim. To investigate the prevalence, clinical significance and molecular characterization of occult hepatitis B virus infection in ART-Naive HIV-positive individuals.
Material and methods. Among the 1077 HIV-infected patients with different risk factors for HIV infection, 297 were HBsAg-ve ART-naive, of them 112 was randomly selected for the study. HBV DNA was tested by in-house PCR and quantified by qPCR. Molecular characterization was performed by sequencing the envelope and overlapping polymerase genes.
Results. We found the prevalence of occult HBV to be 10.7% among a randomly selected group of HBsAg-ve/antiHBc+ve HIV-infected patients. Overall 33.9% (38 of 112) of the patients were antiHBc positive indicating exposure to HBV infection. HBV DNA was detected in 12/38 (31.5%) antiHBc positive samples and 50% of them had CD4 T cell count < 200 cells/mm3. HCV coinfection was low (2.7%). No surrogate marker for OBI could be identified. Presence of antiHBs antibodies did not rule out OBI. Liver biopsy in six cases showed varying stages of chronic hepatitis. Several mutations were detected but not the common immune escape mutant G145R.
Conclusion. In conclusion the prevalence of OBI was significantly high among HIV coinfected patients, which highlights the importance of HBV DNA testing in these patients and indicates need for further prospective studies in larger cohorts to assess its clinical significance.
Hepatitis B virus (HBV) is a major health problem with approximately 400 million chronically infected people worldwide, and an estimated more than 40 million chronic carriers in India.1 These chronically infected individuals are not only at increased risk of developing liver cirrhosis and hepatocellular carcinoma but also serve as a potential reservoir of infection. The major structural protein of virus envelop, hepatitis B surface antigen (HBsAg); is universally considered as a diagnostic marker of HBV infection. The absence of HBsAg in the serum and presence of antibodies to core antigen (antiHBc) and viral surface antigen (anti-HBs) usually indicates resolved infection. Occult HBV has been defined as chronic low-level HBV replication in the absence of detectable HBsAg.2 Individuals with occult HBV infection (OBI) usually have serological evidence of previous exposure to the virus, mainly positive antiHBc, although the absence of serological evidence of previous HBV infection has been described in a few cases.2,3
HIV co-infection has been reported to modify the natural history of HBV with potential consequences on morbidity and mortality.4 Data on OBI in ART untreated HIV patients is limited from a vast country like India; where prevalence of HBV mono-infection, mode of transmission, viral genotype and mutational pattern varies considerably in different parts of the country. Our previous study from eastern India showed high prevalence of OBI among blood donors, but no study has been carried out on HIV co infected patients from this part of the country. This is particularly important as, exposure to HBV is common among HIV infected cases because of shared routes of transmission.5 Notably, there is considerable variation in prevalence of HIV/HBV co infection according to geographic region and exposure risk.6
Successful implementation of ART leads to immune reconstitution that can potentially result in immune mediated liver injury in the setting of HBV coinfection. Some studies have reported an association between OBI and elevation of transaminases7,8 therefore identification of OBI is of importance.
Most of our patients are screened for HBV co-infection by ELISA for HBsAg which is cheap, widely available and often the screening test of choice in developing countries with high HIV prevalence. In this study we have analyzed the prevalence and associated biochemical, virological and molecular features of OBI among ART-naïve HIV patients; with a view to identify surrogate markers of occult infection.
Material and MethodsPatients groupThe Apex Referral Centre for HIV/AIDS at Medical College, Kolkata, cares for a large number of HIV infected cases every year coming from different regions of eastern India. Of 1077 HIV-infected patients enrolled in the Apex Referral Centre for HIV/AIDS at Medical College, Kolkata, between August 2006 and October 2006, we identified 297 HBsAg-ve ART-naïve subjects. Among them 112 subjects were selected for the study by simple random method. Informed consent was obtained from the patients, and the Institutional ethical committee approved the study protocol. The sera were stored at-80 °C until analysis.
Serological markersAll samples were tested for HBsAg, anti-HBs, anti-HBc, anti-HCV and anti-HIV using commercial ELISA kits (Biomerieux, Boxtel, Netherlands). All anti-HBc positive samples were retested for HBsAg as well as for anti-HBc and only repeat positive samples were included in the study.
DNA extraction and amplificationDNA was extracted from serum as described previously.9 HBV DNA was detected using in-house nested polymerase chain reactions (PCR) targeting the DNA sequences encoding the surface regions by the method described earlier.10,11 Instructions to prevent cross contamination were followed strictly12 and the results were considered valid only when they were repeatedly found positive in duplicates.
HBV DNA sequencingThe amplified HBV DNA was sequenced from both directions by using ABI Prism 3130 xl automated DNA sequencer (Applied Biosystems, Foster City, CA, USA). The sequence aligning and editing was performed using BioEdit version 7.0.5.3.13 Mega 3.1 was used for genotyping and phylogenetic analysis.14
Quantification of HBV-DNAHBV-DNA was quantified by real-time PCR assay as previously described.15
Quantification of HIV-RNAHIV-RNA was quantified by using commercially available kit Amplicor HIV-1 Monitor Test Version 1.5 (Roche diagnostic corporation, Indianapolis, USA)16 with lower detection limit of 50 RNA copies/mL.
Statistical analysisMedian age and all the numerical data were analyzed using the student’s t-test. The chi-square test and the Fisher’s exact test were used to compare categorical data. For the purpose of our study, a p-value ≤ 0.05 was considered statistically significant.
ResultsAll newly diagnosed HIV patients attending the HIV apex clinic at Medical College Kolkata were enrolled for the study over a period of one year. The demographic, biochemical and virological parameters of the study group are summarized in table 1. The median age was 30 (range, 17-48) years. Majority (60%) had multiple sexual partners, 27% (30 of 112) of the subjects had a history of concomitant alcohol use. HCV coinfection was low among the study group (only 3 of 112, 2.7%).
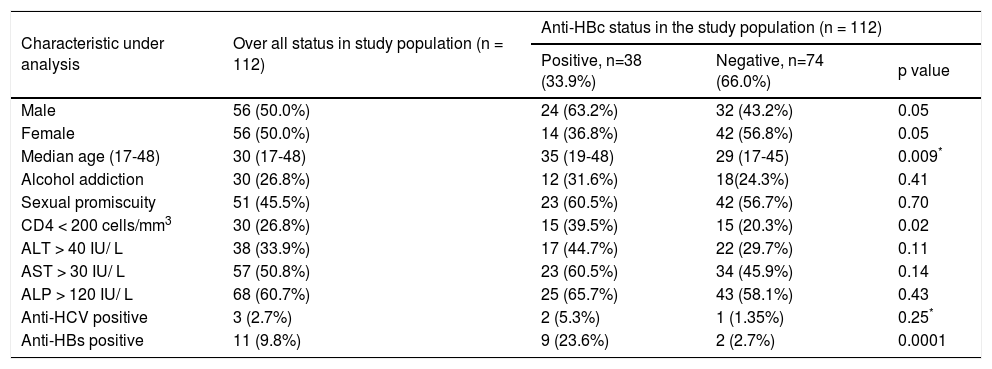
Evaluation of overall demographic, biochemical and virological parameters of the study population.
| Characteristic under analysis | Over all status in study population (n = 112) | Anti-HBc status in the study population (n = 112) | ||
|---|---|---|---|---|
| Positive, n=38 (33.9%) | Negative, n=74 (66.0%) | p value | ||
| Male | 56 (50.0%) | 24 (63.2%) | 32 (43.2%) | 0.05 |
| Female | 56 (50.0%) | 14 (36.8%) | 42 (56.8%) | 0.05 |
| Median age (17-48) | 30 (17-48) | 35 (19-48) | 29 (17-45) | 0.009* |
| Alcohol addiction | 30 (26.8%) | 12 (31.6%) | 18(24.3%) | 0.41 |
| Sexual promiscuity | 51 (45.5%) | 23 (60.5%) | 42 (56.7%) | 0.70 |
| CD4 < 200 cells/mm3 | 30 (26.8%) | 15 (39.5%) | 15 (20.3%) | 0.02 |
| ALT > 40 IU/ L | 38 (33.9%) | 17 (44.7%) | 22 (29.7%) | 0.11 |
| AST > 30 IU/ L | 57 (50.8%) | 23 (60.5%) | 34 (45.9%) | 0.14 |
| ALP > 120 IU/ L | 68 (60.7%) | 25 (65.7%) | 43 (58.1%) | 0.43 |
| Anti-HCV positive | 3 (2.7%) | 2 (5.3%) | 1 (1.35%) | 0.25* |
| Anti-HBs positive | 11 (9.8%) | 9 (23.6%) | 2 (2.7%) | 0.0001 |
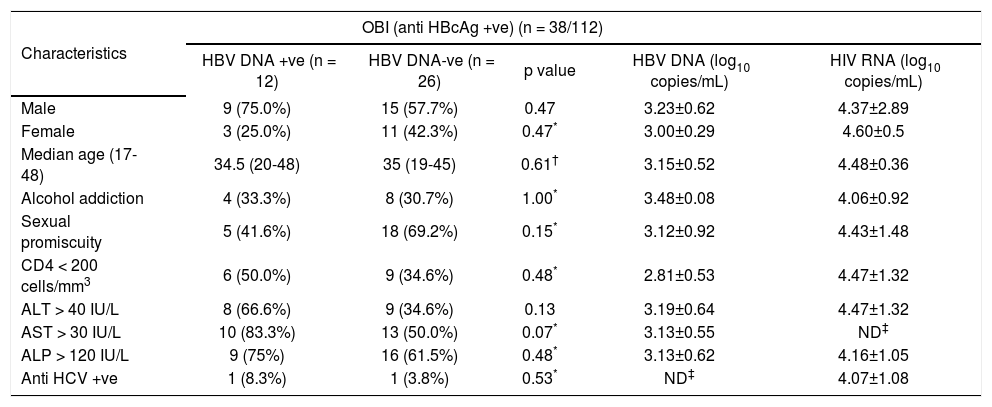
Overall 33.9% (38 of 112) of patients were reactive for antiHBc indicating prior exposure to HBV infection and of these 9 (23.6%) were antiHBs positive. Analysis of HBV DNA by PCR among antiHBc positive patients, revealed that 31.5% (12 of 38) were positive for HBV DNA and majority of them were male (75%, 9 of 12) (Table 2). Thus, in the total study population 10.7% (12 of 112) was identified with OBI. Among 38 antiHBc positive patients, overall HBV DNA load and HIV RNA load was 3.15 ± 0.52 and 4.09 ± 1.08 respectively. The HIV RNA load among the HBV DNA positive and HBV DNA negative cases was 4.51 ± 1.21 and 3.86 ± 0.98 respectively and the difference was not statistically significant (p = 0.25). Comparing the samples with CD4 count greater than and lesser than 200 cells/mm3, HBV DNA load was 2.81 ± 0.53 and 3.42 ± 0.35 (p = 0.1) respectively. CD4 count was below 200 for 50% of patients with OBI. Serum levels of AST and ALT were higher among patients with OBI in comparison to antiHBc +ve/HBV DNA negative individuals, but the difference failed to reach statistical significance (p = 0.13 and p = 0.07 respectively). The comparison of different demographic, biochemical and virological factors between HBV DNA positive and negative cases was illustrated in table 3.
Evaluation of overall demographic, biochemical and virological parameters of anti-HBc positive samples.
| Characteristics | OBI (anti HBcAg +ve) (n = 38/112) | ||||
|---|---|---|---|---|---|
| HBV DNA +ve (n = 12) | HBV DNA-ve (n = 26) | p value | HBV DNA (log10 copies/mL) | HIV RNA (log10 copies/mL) | |
| Male | 9 (75.0%) | 15 (57.7%) | 0.47 | 3.23±0.62 | 4.37±2.89 |
| Female | 3 (25.0%) | 11 (42.3%) | 0.47* | 3.00±0.29 | 4.60±0.5 |
| Median age (17-48) | 34.5 (20-48) | 35 (19-45) | 0.61† | 3.15±0.52 | 4.48±0.36 |
| Alcohol addiction | 4 (33.3%) | 8 (30.7%) | 1.00* | 3.48±0.08 | 4.06±0.92 |
| Sexual promiscuity | 5 (41.6%) | 18 (69.2%) | 0.15* | 3.12±0.92 | 4.43±1.48 |
| CD4 < 200 cells/mm3 | 6 (50.0%) | 9 (34.6%) | 0.48* | 2.81±0.53 | 4.47±1.32 |
| ALT > 40 IU/L | 8 (66.6%) | 9 (34.6%) | 0.13 | 3.19±0.64 | 4.47±1.32 |
| AST > 30 IU/L | 10 (83.3%) | 13 (50.0%) | 0.07* | 3.13±0.55 | ND‡ |
| ALP > 120 IU/L | 9 (75%) | 16 (61.5%) | 0.48* | 3.13±0.62 | 4.16±1.05 |
| Anti HCV +ve | 1 (8.3%) | 1 (3.8%) | 0.53* | ND‡ | 4.07±1.08 |
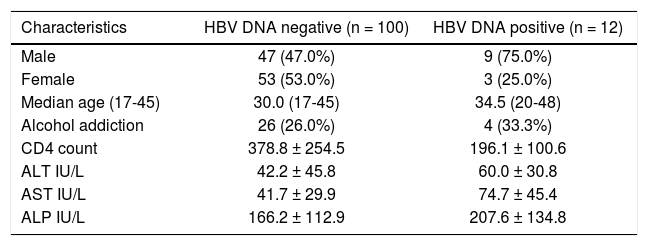
Comparison of different demographic, biochemical and virological factors between HBV DNA positive and HBV DNA negative.
| Characteristics | HBV DNA negative (n = 100) | HBV DNA positive (n = 12) |
|---|---|---|
| Male | 47 (47.0%) | 9 (75.0%) |
| Female | 53 (53.0%) | 3 (25.0%) |
| Median age (17-45) | 30.0 (17-45) | 34.5 (20-48) |
| Alcohol addiction | 26 (26.0%) | 4 (33.3%) |
| CD4 count | 378.8 ± 254.5 | 196.1 ± 100.6 |
| ALT IU/L | 42.2 ± 45.8 | 60.0 ± 30.8 |
| AST IU/L | 41.7 ± 29.9 | 74.7 ± 45.4 |
| ALP IU/L | 166.2 ± 112.9 | 207.6 ± 134.8 |
PCR amplicon derived from five occult HBV infected patients were successfully sequenced. Phylogenetic analysis based on S region showed that the three isolates belonged to genotype D (subtype ayw3), one as genotype A (subtype adw2) and the remaining one as genotype C (subtype adr). Sequence analysis for amino acid substitution in the surface gene region (aa 68-168) was performed. None of them had G145R mutation indicating the absence of commonly found immune escape mutant among the OBI cases.
Liver biopsy was performed in six patients with OBI and showed varying stages of chronic hepatitis and significant fibrosis in all (HAI score 9.7 ± 2.7; range 5-15) even in those with a normal transaminases and no radiological evidence of chronic liver disease. The stage and grade of chronic hepatitis did not correlate to the HBV or HIV viral load in these patients. Among these six patients, only one was anti-HBs positive.
DiscussionThe present study represents a comprehensive cross-sectional analysis of prevalence of OBI in an ART-naive HIV-positive cohort comprising various risk groups. Most previous studies looking at the clinical effects of OBI in HIV include a large number of patients on anti-HBV drugs as a component of ART. This study describes risk factors associated with OBI, frequency of anti-HBc positivity and its possible value as a serological marker for identifying HIV-infected patients who would benefit from HBV DNA assay.
We found the prevalence of occult HBV to be 10.7% among a randomly selected group of HIV-infected patients. The prevalence of OBI in HIV positive individuals varies worldwide between 0-90%, depending on geographic regions, risk groups and the type of exposure involved.5,17,18,19,20 The present study as well as our previous pilot study on antiHBc positive blood donors showed significant prevalence of OBI in our community.21 In the present study, the prevalence of antiHBc (33.9%; 38 of 112) and OBI (31.5%; 12 of 38) among the ART-naive HIV-positive cohort was higher compared to our previous report on blood donors from the same geographical area which reported 21.3% OBI among the HBsAg negative/antiHBc positive donors.21
Within India HBV and HCV co-infection among HIV infected patients have been reported sporadically from different regions.22,23 Most of the studies aimed at detecting HBV prevalence in the HIV population are based on HBsAg positivity (prevalence 9.9 to 11%),24,25 but report on OBI is limited. Our previous study on intravenous drug users in northeastern India detected a prevalence of 15.9% OBI among the HIV infected injecting drug users.26
Among the OBI cases, the rate of antiHBs was lower (3/12; 25%), which might be due to the fact that HIV infected patients are prone to lose anti-HBs immunity at a higher frequency than the general population.27
Previous reports suggest that lower HBV replication is associated with milder hepatic damage28 but our results differ from them as in our study group hepatic injury was found in spite of low HBV viral loads. Among the subjects with OBI elevated ALT or AST was found among 66.67% (8 of 12) and 83.3% (10 of 12) respectively, though statistically not significant. However, our study is cross-sectional; therefore evaluation of long term clinical significance of OBI should be better addressed by follow up studies.
HIV patients are screened for concomitant chronic hepatitis B using HBsAg ELISA and it is not considered cost-effective to perform HBV DNA testing for all in our resource poor setting. Our study tried to identify possible clinical and serological markers which could guide DNA testing in these patients. OBI is reported to be common among HCV infection but we found its prevalence to be low among our study group (112 patients). However, anti-HCV was not tested among the other HIV positive samples attending the Apex clinic. Furthermore, none of the risk factors were found to be statistically significant markers of OBI and cannot be used as an independent marker for identifying patients who would benefit from HBV DNA estimation. However as one third of the antiHBc positive HBsAg negative patients were positive for HBV DNA (12 of 38), it is recommended that HIV positive patients with HBsAg negative/antiHBc positive pattern should be tested for the presence of HBV DNA irrespective of their antiHBs status.
Nevirapine is commonly included in the first-line ART regimens at most treatment centers in India. A higher frequency of severe hepatotoxicity has been observed among persons co infected with HBV/HCV, receiving a Nevirapine-containing ART regimen compared with other regimens. In our study group one patient with occult HBV infection developed fulminant hepatic flare after initiation of Nevirapine containing HAART, suggesting need for further follow up studies aimed at the effects of potentially hepatotoxic drugs on liver function in patients with occult HBV.
Our study identified only 12 subjects with OBI and all the samples were collected from a single centre, indicating that results might differ in settings with significantly different demographic characteristics. Thus in future multicentric study involving large sample size should be taken up.
ConclusionIn conclusion, the prevalence of OBI in our HIV positive, ART naïve study population was found to be high, irrespective of antiHBs status. Presence of histological evidence of chronic hepatitis in our OBI patients who underwent biopsy highlights the clinical importance of identifying these patients early in the course of HIV treatment. Subsequent multicentric prospective studies aimed at evaluating the long-term clinical implications of OBI in HIV-infected patients are required to find out the clinical significance of OBI in HIV HBV co-infection.
Abbreviations- •
HBV: hepatitis B virus.
- •
HBsAg: hepatitis B surface antigen.
- •
OBI: occult hepatitis B virus infection.
- •
Anti-HBc: antibodies to hepatitis B core antigen.
- •
Anti-HBs: antibodies against HBsAg.
- •
HBeAg: hepatitis B e antigen.
- •
Anti-HBe: antibodies to HBeAg.
- •
ALT: alanine aminotransferase.
- •
PCR: polymerase chain reaction.
- •
HBc: hepatitis B core protein.
- •
ART: antiretroviral therapy ELISA.
- •
ELISA: enzyme linked immunoassay.
- •
NAT: nucleic acid amplification technology.
This research was supported by the Indian Council of Medical Research (ICMR), New Delhi, India. We thank Tapan Chakraborty for excellent technical assistance.