Although hyperferritinemia may reflect the inflammatory status of patients with non-alcoholic fatty liver disease (NAFLD), approximately 33% of hyperferritinemia cases reflect real hepatic iron overload.
AimTo evaluate a non-invasive method for assessing mild iron overload in patients with NAFLD using 3T magnetic resonance imaging (MRI) relaxometry, serum hepcidin, and the expression of ferritin subunits.
MethodsThis cross-sectional study assessed patients with biopsy-proven NAFLD. MRI relaxometry was performed using a 3T scanner in all patients, and the results were compared with iron content determined by liver biopsy. Ferritin, hepcidin, and ferritin subunits were assessed and classified according to ferritin levels and to siderosis identified by liver biopsy.
ResultsA total of 67 patients with NAFLD were included in the study. MRI revealed mild iron overload in all patients (sensitivity, 73.5%; specificity, 70%). For mild (grade 1) siderosis, the transverse relaxation rate (R2*) threshold was 58.9 s−1 and the mean value was 72.5 s−1 (SD, 33.9), while for grades 2/3 it was 88.2 s−1 (SD, 31.9) (p < 0.001). The hepcidin threshold for siderosis was > 30.2 ng/mL (sensitivity, 87%; specificity, 82%). Ferritin H and ferritin L subunits were expressed similarly in patients with NAFLD, regardless of siderosis. There were no significant differences in laboratory test results between the groups, including glucose parameters and liver function tests.
ConclusionsMRI relaxometry and serum hepcidin accurately assessed mild iron overload in patients with dysmetabolic iron overload syndrome.
Non-alcoholic fatty liver disease (NAFLD) is a prevalent liver disease commonly associated with obesity, metabolic syndrome, and insulin resistance. Iron overload is present in one-third of patients with NAFLD [1]. Serum ferritin measurement is the most commonly available laboratory test for this condition.
Progressive weight gain increases fat deposition in the liver (simple steatosis), resulting in inflammation and hepatocellular damage (non-alcoholic steatohepatitis) in 30% of cases. Among patients with non-alcoholic steatohepatitis, 15%-25% will develop fibrosis and cirrhosis [2]. Therefore, identifying ways to non-invasively assess risk factors for fibrosis progression, such as iron overload, is essential to disease management.
The association of hyperferritinemia, normal transferrin saturation, mild hepatic iron overload and at least one metabolic disorder (eg, overweight, diabetes, dyslipidemia, hypertension, or NAFLD) is collectively called dysmetabolic iron overload syndrome (DIOS) [3, 4]. Half of patients with DIOS have NAFLD, and 34%-51.5% of patients with NAFLD have DIOS, probably because they share the same risk factors and pathophysiology [5].
Hyperferritinemia is an independent risk factor for histological severity and for poor prognosis [6] that has been associated with overall mortality [7, 8]. However, the significance of hyperferritinemia in NAFLD is controversial. Serum ferritin level reflects iron content, but it is also an acute-phase protein that increases under conditions of low-grade inflammation [9]. The difference between DIOS and dysmetabolic hyperferritinemia is that, in the latter, there is no iron deposition. This differentiation is important, because patients with dysmetabolic hyperferritinemia would not have the aggravating factor of iron and could have another mechanism for inducing insulin resistance [10]. Other components of iron metabolism are less influenced by inflammation than ferritin, such as hepcidin and ferritin subunits.
Hepcidin is responsible for iron balance. This 25-amino acid peptide inhibits iron uptake in the gut, macrophages, and liver by internalizing and degrading ferroportin, the only known cellular iron exporter. When iron is entrapped in cells, blood iron levels decrease. Inappropriately low hepcidin synthesis associated with a lower expression of liver ferroportin has been reported in patients with NAFLD and could be considered part of the iron overload mechanism.
Ferritin consists of varying proportions of 2 subunits, heavy chain (FTH) and light chain (FTL). FTH and FTL expressions depend on the iron needs of each organ [11–14]. FTH accumulates and releases iron faster than FTL, allowing more dynamic iron traffic and acting as an anti-inflammatory protein by reducing iron availability and then reducing reactive oxygen species production [11–15]. On the other hand, FTL can accumulate more iron and retain it more firmly, which is beneficial for iron storage organs, such as the liver and spleen [11–14]. Both subunit types can reduce iron availability and, consequently, reactive oxygen species production. The difference is how fast they perform this task [13]. A recent study suggested that FTH has a pro-inflammatory effect on macrophages, making the FTH a participant in the inflammatory cascade, rather than a consequence of it [16].
The gold-standard method for identifying iron overload is liver biopsy, which is invasive. However, non-invasive methods are preferred. Magnetic resonance imaging (MRI), the most commonly available radiological method, is considered the best non-invasive method for iron measurement and an essential tool for iron overload diagnosis and follow-up [17–19]. However, few MRI studies of patients with DIOS are available [20, 21]. In addition, understanding the blood markers of iron overload would help identify the patients with real iron overload and NAFLD more accurately.
This study aimed to evaluate a non-invasive method for assessing mild iron overload in patients with NAFLD using 3T MRI relaxometry, serum hepcidin, and FTH and FTL expressions.
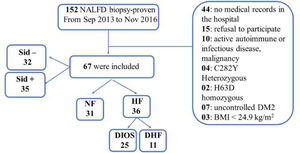
2Patients and methodsFrom September 2013 to November 2016, 152 patients with NAFLD underwent liver biopsy for histological investigation. Of these, 67 with biopsy-proven NAFLD were included in our sample. All procedures were performed at the University of São Paulo Hospital, and all patients provided written informed consent prior to participation. Inclusion criteria were patients aged > 18 years of either sex without acute disease when blood was collected. Exclusion criteria were type 2 diabetes with glycated hemoglobin A1c > 7.5% and causes of liver disease other than NAFLD. In all patients, serum ferritin measurements and MRI were performed within 6 months of liver biopsy.
2.1Clinical evaluationDemographic data (age, sex, race, and medical records) and anthropometric measurements (weight, height, blood pressure, and waist and neck circumference) were collected during the clinical interview. Waist circumference (WC) was measured at the midpoint between the iliac crest and the lowest rib.
We used ATPIII criteria for metabolic syndrome, ie, the presence of at least 3 of the following: WC > 102 cm in men or > 88 cm in women; type 2 diabetes or fasting glucose ≥ 110 mg/dL; HDL < 40 mg/dL in men or < 50 mg/dL in women; triglycerides > 150 mg/dL; and blood pressure ≥ 130/85 mm Hg [22].
2.2Laboratory evaluationBlood samples were collected after a 12-hour fast for liver function assessment, complete blood count, iron status (including ferritin, iron, and transferrin saturation), and metabolic evaluation (glucose, insulin, lipids, and uric acid). Hyperferritinemia was defined as serum ferritin ≥ 200 ng/mL in women and ≥ 300 ng/mL in men [9]. All analyses were performed by the same hospital laboratory.
Serum tumor necrosis factor alpha (TNFα) and interleukin (IL)-6 were measured by ELISA (Quantikine HS ELISA, R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Venous blood was collected after an 8-hour fast for serum hepcidin measurement. A hepcidin-25 assay (DRG Instruments, Marburg, Germany) was used according to the manufacturer's instructions.
2.3Assessment of ferritin subunit expression in venous blood by RNA extractionBlood collection and RNA extraction: Venous blood was collected after an 8-hour fast and stored in PAXgene Blood RNA tubes (QIAGEN, Hilden, Germany) at -4º F until RNA extraction, which was performed according to the manufacturer's instructions. The quantity and quality of RNA samples were assessed by spectrophotometry using the NanoDrop ND 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) with A260/A280 and A260/230 absorbance ratios. The purity and quality gradients were between 2.0 and 2.1. The quantity was satisfactory.
RNA integrity and concentration were analyzed using an RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA) in an Agilent 2100 bioanalyzer (Agilent, Technologies, Santa Clara, CA, USA) according to the manufacturer's instructions. RNA integrity was > 8.0.
RNA reverse transcription was performed using a high-capacity RNA-to-cDNA kit (PN: 4375575) (Applied Biosystems, Waltham, MA, USA) according to the manufacturer's instructions.
FTH and FTL expressions were analyzed using TaqMan polymerase chain reaction FTL (Hs00830226_gH) and FTH1 (Hs01694011_s1) assays according to the manufacturer's instructions. We used 2 endogenous genes as controls: GAPDH (lot 1600800) and β-actin Hs010+0++6-ACTB (lot 1578326), both provided by TaqMan.
The polymerase chain reaction was performed with a Step One Plus Real Time System (Applied Biosystems, Waltham, MA, USA). The gene expression results were analyzed by cycle threshold using the medium value of the 2 endogenous genes as a normalization rate. We used the formula: ∆Ct = Ct target gene – the mean values of endogenous genes [23]. All samples were analyzed twice, and the mean values were considered. The formula 2−∆Ct was applied to calculate expression normalization, after which the formula 2−∆∆Ct was used to calculate the mean value of endogenous controls with normal ferritin.
2.4MRI image analysisThe images were obtained with a 3T scanner (Philips Medical Systems, Amsterdam, Netherlands). We performed a multi-echo gradient echo sequence, with 8 echo times. The time between echoes was 1.2 ms, with an initial echo time of 1.2 ms. The other parameters included: 200 ms repetition time; 20° flip angle; 256×256 matrix; 8 mm thickness (0 mm gap); surface coil; and 1000 Hz/pixel bandwidth. The sequence required 15 s during one breath hold.
The images were analyzed by a radiologist with 13 years of experience in abdominal imaging. Image post-processing was analyzed using specific software (Dive In, MagnePath, Perth, Australia). The software calculated the proton density fat fraction with a magnitude analysis, calculating steatosis values by removing the interference of the iron deposits and the transverse relaxation rate (R2*) without interference from fat deposits in the liver.
2.5Liver biopsyLiver biopsy was used to confirm NAFLD and the iron content measurement. The liver tissue fragments were fixed in buffered formalin (4%) and embedded in paraffin. The slides were stained with hematoxylin-eosin, Mallory's trichrome, and Perls’ staining. NAFLD was classified as steatosis (0-3), lobular inflammation (0-3), ballooning (0-2), or fibrosis (0-4). According to Perls’ staining, iron classification ranged from 0-4.
The liver biopsy slides were evaluated at 2 different time points. After an initial assessment by the pathology team, all slides were subsequently reviewed by the same pathologist. There was good concordance between the analyses as described in Table 1.
All data were entered in Excel and then exported to IBM SPSS Statistics v 19.9 (IBM, Armonk, NY, USA) for statistical analysis. The Kolmogorov-Smirnov test was used for continuous variables, while categorical variables were described as frequency and percentage and were compared with the Q-square test. The Mann-Whitney test was used for continuous variables when the groups were split according to iron status, and the Kruskal-Wallis test was performed when the samples were classified according to ferritin levels and iron overload. The differences were analyzed with a post hoc Tukey's test. Quantitative variables were correlated with the Spearman correlation test. Parametric variables were analyzed with one-way ANOVA and Student's t-test, while non-parametric variables were analyzed with the Kruskal-Wallis test and the Mann-Whitney test.
Ferritin subunit expression was normalized using the mean value of endogenous genes (GAPDH and ACTB). Expression did not adhere to normality. Non-parametric and Wilcoxon tests were performed in JMP (SAS, Cary, NC, USA).
The significance level was set at 5%. A biomedical statistician performed the statistical analysis.
2.7Ethics statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics committee of Hospital das Clínicas da universidade de são Paulo (859.283).
3Results3.1Patient characteristicsOur sample of patients with NAFLD consisted mostly of women (61.1%), with a mean age of 52 years [22-76], mean body mass index of 31.7 kg/m2, and mean WC of 103.7 cm. Hypertension was found in 49% of the participants, and type 2 diabetes in 39%.
The 67 patients were classified in 2 ways (see Figure 1):
- 1)
According to ferritin levels and iron overload status, with patients divided into 3 groups: a normal ferritin group, a dysmetabolic hyperferritinemia (negative siderosis) group, and a DIOS group.
- 2)
According to the presence or absence of iron in the hepatic biopsy regardless of serum ferritin level.
Women predominated (80%) in the normal ferritin group, while men predominated (72%) in the DIOS group. Age, body mass index, and WC were similar in the ferritin and siderosis groups. The median number of metabolic syndrome components was 3 in all groups. Clinical characteristics are detailed in Table 2.
Biochemical characteristics of all groups.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; DHF: dysmetabolic hyperferritinemia; DIOS: dysmetabolic iron overload syndrome; DM: diabetes mellitus; HDL: high-density lipoprotein; HOMA: Homeostatic Model Assessment; IL6: interleukin 6; IR: Insulin Resistance; LDL: low-density lipoprotein; NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III; NF: normal ferritin; Sid-: siderosis negative; Sid+: siderosis positive; TC: total cholesterol; TNFα: tumor necrosis factor alpha; TS: transferrin saturation; WC: waist circumference. 1: chi-square test; 2: Kruskal-Wallis test(*) and Mann-Whitney test(**)–median(IQR); 3: one-way ANOVA (*) and Student's t-test (**). na: not applicable; nc: not calculable.
Ferritin levels correlated with iron histology. The ferritin threshold that identified iron overload was > 180.4 ng/mL in women (sensitivity, 76%; specificity, 64%), and > 350.7 ng/mL in men (sensitivity, 72.7%; specificity, 75%). There were no significant differences in laboratory tests between the groups, including glucose parameters and liver function tests. The biochemical values are detailed in Table 2. Hepcidin levels were higher in the DIOS group than in the other groups and reflected iron content above > 30.2 ng/mL (sensitivity, 87%; specificity, 82%; area under the receiver operating characteristic curve [AUC], 0.896). The values for inflammatory markers, such as IL-6 and TNFα, were homogeneous across the sample, with no significant differences between the groups. Biochemical characteristics are described in Table 2.
Iron deposits were reported both in liver cells and on the reticuloendothelial system in 91% of the biopsies. Except for siderosis, no other histologic characteristic differed between the groups. Histologic characteristics are described in Table 3.
Histological characteristics of all groups.
DHF: dysmetabolic hyperferritinemia; DIOS: dysmetabolic iron overload syndrome; NAS: NAFLD activity score; NF: normal ferritin; Sid-: siderosis negative; Sid+: siderosis positive. p: chi-square test; na: not applicable.
Blood subunit expression was similar in all patients with NAFLD, regardless of iron overload. FTL was positively correlated with metabolic syndrome, WC, FTH, and steatosis grade. Subunit expression did not differ compared to inflammatory markers, hepcidin, and glucose parameters. All correlations are described in Table 4.
Ferritin subunits expression.
FTH: ferritin heavy subunit; FTL: ferritin light subunit, WC: waist circumference; HOMA-IR: Homeostatic Model Assessment Insulin Resistance; TNFα: tumor necrosis factor alpha; NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III; IL-6: interleukin 6. * Statistically significant.
R2* correlated with liver iron content in patients with NAFLD. The MRI results are detailed in Table 5. Ferritin levels also correlated with R2* (Spearman correlation coefficient: 0.651, p < 0.001). The R2* cut-off value that identified iron deposition was 58.9 s−1 (sensitivity, 73.5%; specificity, 70%; AUC, 0.780). The mean R2* was 72.53 s−1 (SD, 33.93) in the grade 1 siderosis group, and 88.22 s−1 (SD, 31.94) in moderate siderosis (grades 2 and 3).
R2* and steatosis percentage.
| mean±SD | NF | DHF | DIOS | p* | Sid- | Sid1 | Sid2/3 | p* |
|---|---|---|---|---|---|---|---|---|
| R2* (s−1) | 54.17±8.7 | 61.41±18.09 | 87.77±32.22 | <0.001 | 54.47±11.91 | 72.53±33.93 | 88.22±31.94 | <0.001 |
| Steatosis (%) | 13.71±7.14 | 17.58±11.15 | 17.28±11.15 | 0.284 | 15.51±8.33 | 14.10±6.47 | 18.09±10.37 | 0.537 |
DHF: dysmetabolic hyperferritinemia; DIOS: dysmetabolic iron overload syndrome; NF: normal ferritin; Sid1: siderosis grade 1; Sid2/3: siderosis grade 2 & 3; Sid-: siderosis negative.
The purpose of this study was to evaluate the main non-invasive methods used to assess iron deposition in patients with NAFLD. Serum hepcidin and MRI relaxometry were the most accurate methods.
Although MRI R2* relaxometry is an established method for assessing iron overload, there is no valid consensus concerning the ideal technical approach [24]. The relationship between liver iron concentration and R2* can be affected by iron characteristics (particle size, distribution, loading factor, and shape), MRI parameters (sequencing, echo time, repetition time, coil type, bandwidth), and histological features [25]. The R2* reference range has not yet been established. Using the above-described 3T MRI settings, we identified mild iron overload in patients with NAFLD when R2* was > 58.9 s−1. In previous studies, the R2* threshold ranged from 70 to 140 s−1 at 1.5T [21, 26-28]. Because few studies have used 3T scanners, we used a conversion formula (R2*3T = [2 x R2*1.5T] – 11±4) to compare our results with previous reports [29]. According to the formula, the threshold obtained in previous studies with a 3T scanner would range from 129 to 269 s−1 (SD, 4). In a study using a 3T scanner in a population that included patients with DIOS, d'Assignies et al. reported that an R2* value of 77 s−1 could detect liver iron concentration (iron deposition) at 32 umol/g (sensitivity, 96%; specificity, 93%), but the influence of steatosis was not considered when calculating R2*, which probably resulted in overestimation [20]. We found a mean R2* of 72.53 s−1 (SD, 33.93) and 88.22 s−1 (SD, 31.94) in mild (grade 1) and moderate (grade 2 and 3) siderosis groups, respectively, which agrees with the validation study by d'Assignies et al. [20].
In addition to the above-mentioned factors, we offer 3 further hypotheses for the varying R2* values among studies: the iron measurement calibration method (biochemical or histologic evaluation), the study population, and the fat correction for R2* calculation. Patients with hematologic disease are the most studied population [30, 31]. These patients have higher iron levels and less liver steatosis than patients with DIOS; when no specific fat correction method is applied, MRI can overestimate iron calculation [25, 32]. Although recent research has suggested that fat correction during iron measurement is not clinically significant, the population on which this argument is based was heterogeneous and included few patients with DIOS [28].
Another interesting result was the correlation between R2* and serum ferritin (correlation coefficient: 0.651; p < 0.001). This most likely occurred because both serum ferritin and R2* were influenced by siderosis, fibrosis, and inflammation [25], although no histological difference was found between the groups. R2* may be a risk factor for worse histologic and metabolic prognosis. Further research is warranted to clarify this issue.
Iron metabolism parameters indicate which patients might have iron overload. In our study, ferritin levels correlated with siderosis, hepcidin, and R2*. There was no linear agreement between ferritin and body iron content, mostly due to the influence of inflammation. This is probably because the ferritin threshold values are difficult to determine. The ferritin threshold could identify iron overload > 180.4 ng/mL in women (sensitivity, 76%; specificity, 64%), and > 350.7 ng/mL in men (sensitivity, 72.7%; specificity, 75%); the median ferritin level in patients with DIOS was 572 ng/mL. The mean ferritin level described in the literature is approximately 500 ng/mL, but it can exceed 1000 ng/mL. One study described a cut-off point of 378 ng/mL, but with no sex distinctions [33–35]; this value agrees with our findings.
There was no correlation between hyperferritinemia, NAFLD activity score (NAS), and fibrosis score. Previous studies have reported a relationship between hyperferritinemia and poor histological characteristics [6]. Ferritin levels were increased in mild (grade 0-1) and moderate disease (grade 2-3) but reduced in advanced fibrosis (grade 4) [35]. A possible explanation for the lack of a relationship between iron and poor NAFLD histological characteristics is that our sample had low-grade siderosis, and the sample size was relatively small. Of the total sample, 77.6% had grade 0 or 1 siderosis (grade 0: n = 32; grade 1: n = 20), and their iron levels were not yet consistent with worse histological prognosis.
We assessed ferritin subunits to distinguish hyperferritinemia due to inflammation from hyperferritinemia due to iron overload. Both subunits were overexpressed in patients with overweight and NAFLD, but neither could identify pathological hepatic iron overload. FTH is expected to be overexpressed in inflammatory conditions, since it is overexpressed in hepatocytes via inflammatory stimuli [12]. This overexpression can only be measured in the hepatocytes, not in blood cells. We decided to use peripheral blood to search for a new non-invasive method to differentiate hyperferritinemia secondary to iron overload from that secondary to low-grade inflammation.
FTL correlated with WC and metabolic syndrome. FTL RNA is overexpressed in the adipose tissue of patients with obesity [36]. FTH might have correlated with steatosis grade because they are both correlated with low-grade inflammation. Both subunits are expressed in patients with overweight and NAFLD, and further information on their behavior might help us understand the significance of hyperferritinemia in patients with NAFLD. We demonstrated that ferritin subunits are expressed in NAFLD according to anthropometric and histologic characteristics. However, this analysis in peripheral blood could not diagnose iron overload in patients with NAFLD. To the best of our knowledge, this is the first study to evaluate ferritin subunit expression in the leucocytes of peripheral blood.
Hepcidin is the central regulator of iron homeostasis [37]. This peptide hormone binds to ferroportin 1, which results in ferroportin 1 internalization and degradation, thus blocking the cellular iron export mechanism [38, 39]. Hepatic iron overload is the main stimulus for hepatic hepcidin production, which explains why these values were higher in patients with DIOS than in the other groups [10, 40]. Adipose tissue can also produce hepcidin [41]. Persons with obesity have shown higher hepcidin levels, without iron overload, than lean controls, probably due to the low-grade inflammation associated with high leptin levels and hemojuvelin gene expression [41]. Rametta et al. argue that patients with DIOS have hepcidin resistance, which plays an important role in preventing more severe iron overload by reducing enterocyte iron absorption, although this process results in iron entrapment in the liver and macrophages [40]. We found a correlation of hepcidin level with hepatic iron content and serum ferritin, but not with metabolic parameters or steatohepatitis grade. Marmur et al. reported similar findings [41]. We found a close relationship between hepatic iron and hepcidin, with serum levels > 30.2 ng/mL in patients with iron overload. Therefore, serum hepcidin could be an important non-invasive method of assessing iron overload.
Because of the cross-sectional design of our study, we could not establish a cause-and-effect relationship among hyperferritinemia, iron overload, and poor metabolic and histologic prognosis. Further longitudinal studies are required to address this issue. We included only patients with NAFLD, which allowed a good analysis of the MRI method in this population.
5ConclusionsMRI relaxometry accurately determined mild iron overload in patients with DIOS, although the influence of steatosis must be considered. Hepcidin correlated with iron overload and was a good non-invasive method of evaluation. Hyperferritinemia reflected iron overload but did not correlate with metabolic syndrome components or with worse NAFLD histological characteristics. There was similar expression of FTH and FTL in patients with NAFLD, regardless of siderosis.
FundingOur research received grant support from the Fundação de Amparo a Pesquisa do Estado de São Paulo (2015/ 1352 4 -3); grant recipient: Cintia Cercato, co-author.
Declaration of interestNone.
Author ContributionsPaula Pessin Fábrega (drafting the manuscript), Claudia Pinto Souza de Oliveira (critical revision of the manuscript for important intellectual content), Hilton Muniz Leão Filho (critical revision of the manuscript for important intellectual content and MRI imaging analysis), Fabiana Roberto Lima (technical support), Aritânia Santos (technical support), Márcio Correa Mancini (study concept and design), Maria Edna de Melo (study concept and design), Flair José Carrilho (study concept and design), Manoel de Souza Rocha (study concept and design), Paul Clark (technical support), Henrique José Pereira Branisso (study concept and design), Cintia Cercato (critical revision of the manuscript for important intellectual content). All authors significantly contributed to the intellectual contents of this manuscript and approved its submission.
The authors would like to thank Dr. Alfredo Halpern (in memoriam) for study design input and Wanida Chua-Anusorn for technical support. They are grateful to the Hospital das Clínicas de São Paulo for providing laboratory analysis services. There appreciation to Scientific Linguagem for their valuable assistance in translating our text into English.