Edited by: Sonia Roman
Last update: January 2023
More infoNon-alcoholic fatty liver disease (NAFLD) produces high morbidity and mortality rates. Its worldwide prevalence is 25%, but evidence from Latin America (LA) is lacking. We aimed to estimate the prevalence of NAFLD in the adult population of LA. We conducted a systematic review and meta-analysis. Data were collected from OVID, Cochrane Library and LILACS search engines. We used terms related to NAFLD and LA countries. Observational studies in adults who were born and live in LA were included. Two reviewers evaluated the articles, extracted data and assessed the risk of bias. Discrepancies were resolved by consensus or by a third reviewer. A validated tool was used to assess risk of bias. We found and analyzed 19 articles (n=5625). The prevalence in the general and captive population found was 24%. Populations with type 2 diabetes mellitus or obesity had a higher mean prevalence that reached 68%. We concluded that the average prevalence of NAFLD in LA is around 24%. Among high-risk groups, this value increases to 68%. Further studies in the general population using appropriate designs are required for an accurate estimate of the prevalence of NAFLD in LA.
Non-alcoholic fatty liver disease (NAFLD) has been classically based on histological or imaging evidence of fatty liver and the exclusion of alcohol consumption and other liver etiologies as viral hepatitis, hepatotoxic drugs, and others [1]. This disease represents a wide spectrum of liver damage that ranges from simple steatosis, nonalcoholic steatohepatitis (NASH) to liver cirrhosis, liver failure and hepatocarcinoma [2]. This disease has been associated with extrahepatic conditions such as obesity, type 2 diabetes mellitus (T2DM), cardiovascular disease, chronic kidney disease [3]. Even a new definition was proposed in 2020: Metabolic (dysfunction) Associated Fatty Liver Disease (MAFLD), which no longer considers the exclusion of other etiologies but only the presence of a metabolic disease [4,5]. This relation with other conditions has led to a significant rise in morbidity and mortality rates as well as in healthcare and socioeconomic burdens [3,6].
During the last decades, the prevalence of NAFLD has increased globally as a consequence of population aging, modifications in lifestyle, poor dietary habits, and the increasing trends of obesity and T2DM [6,7]. Other factors such as risk genes, low physical activity, and poor access to health care services have been also associated with the development of NAFLD in LA [8,9]. Namely, a genetic polymorphism that is present more frequently in Hispanic populations than in other ethnicities, could be associated with more severe presentations of the disease. [4, 9]
Evidence regarding the prevalence of NAFLD in LA is limited [6]. In 2016, its global prevalence was estimated at 25.2%, with one of the highest rates in South America (30.5%) [10]. However, this estimation was obtained from only three studies that were conducted in Brazil, Chile and Colombia, including a total of 424 patients and no regional search engines nor Spanish articles were included for the analysis [10]. Although some literature reviews support the high prevalence of NAFLD in LA [6, 7], solid quantitative data are still required as these reviews were not performed using a systematic approach. Additionally, further studies involving general populations groups of LA are needed, as the majority have been focused on subgroups affected by specific morbidities [7].
In this context, the aim of this systematic review and meta-analysis was to determine the prevalence of NAFLD in the adult population of LA, in order to provide updated and solid evidence regarding the magnitude of the disease in this high-risk population.
2Material and methods2.1Protocol and registrationWe conducted a systematic review following the PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) guidelines [11]. The protocol for the present study was registered in medRxiv [12].
2.2Eligibility criteriaWe included studies that met the following criteria:
- •
Studies including adults aged ≥18 years who were born and live in LA, either from the general population, captive population or patients from any healthcare facility
- •
Cross-sectional, cohort and population-based studies.
- •
Studies that accurately diagnosed NAFLD by clinically validated methods, such as measuring serum transaminases level, imaging techniques or liver biopsy.
- •
Original studies published between 1990-2020.
We excluded case reports, case series, letters, editorials, narrative reviews, clinical trials and case-control studies. Studies that included participants with any chronic liver disease (i.e., alcoholic liver disease, viral infections or hepatotoxic consumption) were also excluded.
2.3Information sources and search strategiesWe conducted a comprehensive literature search in MEDLINE (Ovid), EMBASE (Ovid), Global Health (Ovid), Cochrane Library and LILACS databases from January 1990 to September 2020. We restricted our search to adult human studies and publications between 1990-2020. We did not use language restrictions. The search terms included a combination of “non-alcoholic fatty liver disease”, “NAFLD”, “MAFLD”, and the names of LA countries (see Appendix 1). The names of LA countries in their original languages were also included.
2.4Study selectionTwo reviewers (YOR, CLVC) independently screened all titles and abstracts of the studies identified in the primary search. Studies that were duplicates were excluded. In a second phase, the full texts of the selected articles were reviewed by two authors (YOR & KAB or CLVC & KAB) to determine their eligibility. Any discrepancy between the two reviewers was resolved by consensus between them or with a third author (CLVC or YOR respectively). We reviewed the selected studies to ensure that the same data was not used in multiple publications. Otherwise, the study with the most relevant data for our research purpose was selected.
2.5Data collectionWe developed a data extraction form and tested it using a random sample of five included studies. Two authors (YOR & KAB or CLVC & KAB) independently extracted the information of the articles. Discrepancies between the two reviewers were resolved by consensus or by discussions with a third author (CLVC or YOR, respectively). The following data were extracted from all studies:
- •
Study details: First author, title, country, publication year and year of data collection. We also examined whether the sample was nationally representative.
- •
Population: sample size, mean age, age range, men proportion, and population type (i.e., general population, captive population or patients). From articles including patients, we registered the underlying disease.
- •
Method for diagnosing NAFLD (i.e., liver enzymes, ultrasound, magnetic resonance, liver biopsy, etc.).
- •
Proportion of adults with NAFLD in the study population. We also estimated 95% confidence intervals (95% CI). If available, proportions of adults with NAFLD were calculated by gender.
We tried to contact the corresponding author of the studies with unclear information for data extraction. If the corresponding author did not answer our communications after two weeks, the article was excluded from our review.
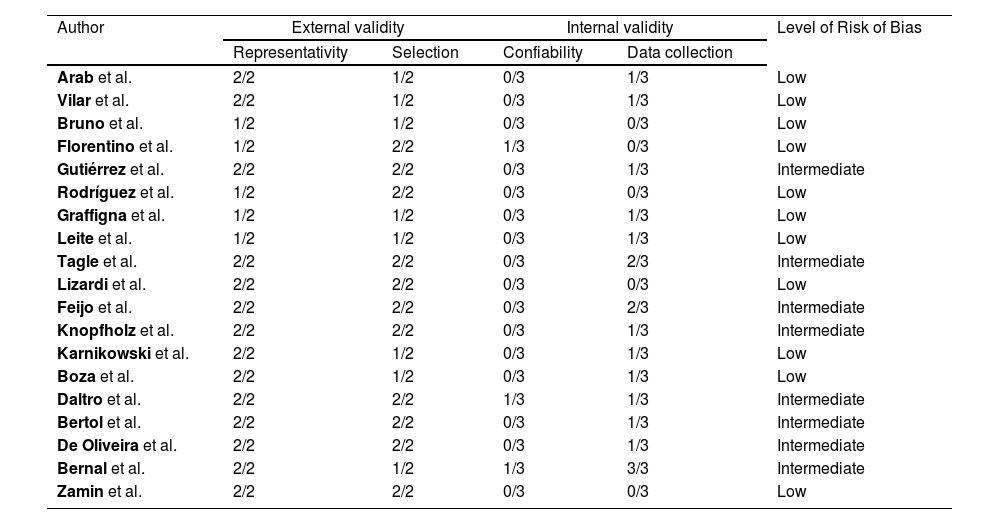
2.6Risk of bias assessmentEach study was independently reviewed by two authors (YOR & KAB or CLVC & KAB) to assess the risk of bias using the risk of bias tool for prevalence studies proposed by Hoy et al. [13]. Each item with a negative evaluation or not evaluable scored 1 point. The risk of bias of each paper was interpreted as low (<4 points), intermediate (5-7 points) or high (≥8 points). All discrepancies were resolved by consensus or discussions with a third author (CLVC or YOR, respectively). See Appendix 2.
2.7Statistical analysisIn a qualitative approach, we present the main characteristics and estimated prevalences of the selected articles in tables. On the other hand, we conducted a quantitative synthesis of prevalence estimates using the random-effects (DerSimonian and Laird) meta-analysis model across the included studies. Subgroup analyses were conducted to estimate the pooled prevalence rate and 95% confidence interval (95% CI) according to the type of population and diagnosis method. Statistical significance was set at p<0.05. The statistical heterogeneity was evaluated using the I2 statistics (I2≥50% indicated significant heterogeneity) [14]. All the analyses were performed using the STATA Statistical Software 16.
2.8Ethical aspectsThis systematic review and meta-analysis was presented to the Office of Regulation and Ethical Assessment of Research (DUICT) of the Universidad Peruana Cayetano Heredia and was exempted from ethical approval as human subjects were not directly studied.
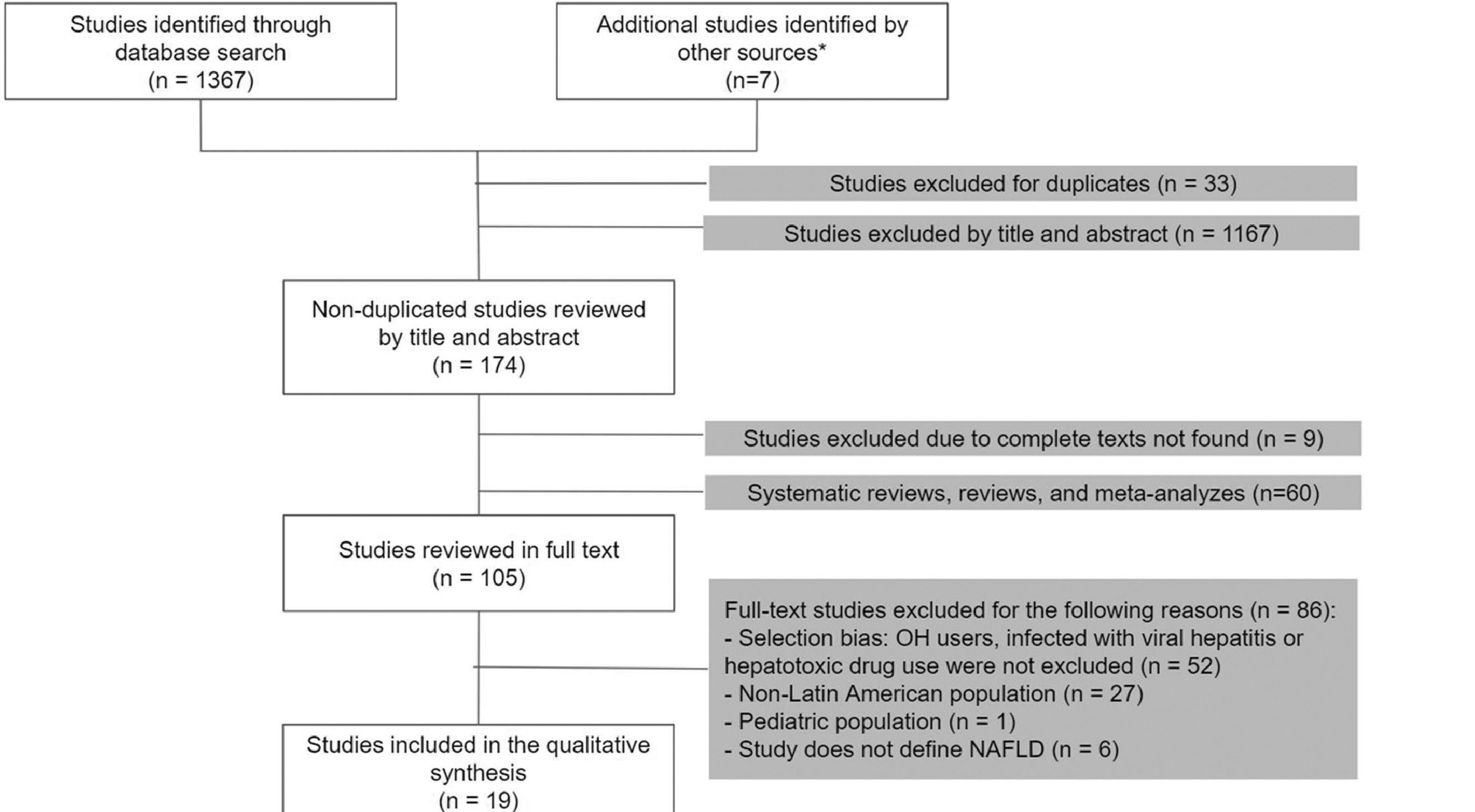
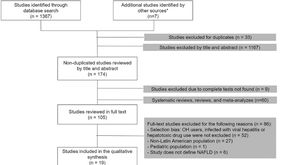
3Results3.1Study selectionOur search was conducted in September 2020. Of a total of 1374 studies, we selected 105 articles for the full-text analysis. Finally, 19 articles were included in our systematic review. The flowchart of the included articles is shown in Fig. 1.
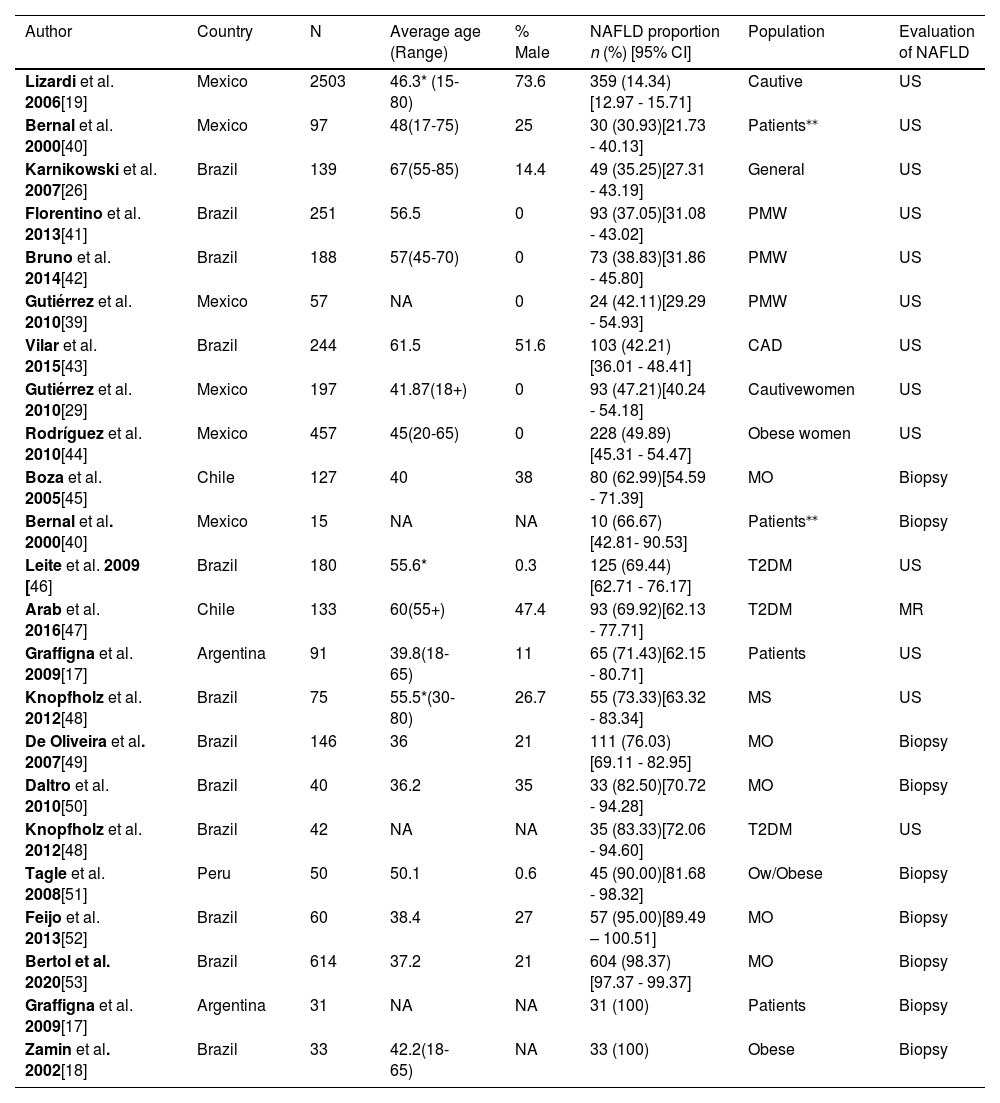
3.2Characteristics of the included studiesWe analyzed 19 articles and all of them were cross-sectional studies. The overall population was 5625. The samples ranged from 40 to 2503 participants, and the age range was between 36 and 67 years old. Most studies were conducted in Brazil (n=11), followed by Mexico (n=4), Chile (n=2), Argentina (n=1) and Peru (n=1). The main objective in most of these studies was to estimate the frequency of NAFLD in high-risk populations. Abdominal ultrasound was the most used diagnosis method (63.2%, n=12), followed by liver biopsy (31.6%, n=6) and magnetic resonance, which was used in only one study (see Table 1).
Overall characteristics of the selected NAFLD studies.
| Author | Country | N | Average age (Range) | % Male | NAFLD proportion n (%) [95% CI] | Population | Evaluation of NAFLD |
|---|---|---|---|---|---|---|---|
| Lizardi et al. 2006[19] | Mexico | 2503 | 46.3* (15-80) | 73.6 | 359 (14.34)[12.97 - 15.71] | Cautive | US |
| Bernal et al. 2000[40] | Mexico | 97 | 48(17-75) | 25 | 30 (30.93)[21.73 - 40.13] | Patients⁎⁎ | US |
| Karnikowski et al. 2007[26] | Brazil | 139 | 67(55-85) | 14.4 | 49 (35.25)[27.31 - 43.19] | General | US |
| Florentino et al. 2013[41] | Brazil | 251 | 56.5 | 0 | 93 (37.05)[31.08 - 43.02] | PMW | US |
| Bruno et al. 2014[42] | Brazil | 188 | 57(45-70) | 0 | 73 (38.83)[31.86 - 45.80] | PMW | US |
| Gutiérrez et al. 2010[39] | Mexico | 57 | NA | 0 | 24 (42.11)[29.29 - 54.93] | PMW | US |
| Vilar et al. 2015[43] | Brazil | 244 | 61.5 | 51.6 | 103 (42.21)[36.01 - 48.41] | CAD | US |
| Gutiérrez et al. 2010[29] | Mexico | 197 | 41.87(18+) | 0 | 93 (47.21)[40.24 - 54.18] | Cautivewomen | US |
| Rodríguez et al. 2010[44] | Mexico | 457 | 45(20-65) | 0 | 228 (49.89)[45.31 - 54.47] | Obese women | US |
| Boza et al. 2005[45] | Chile | 127 | 40 | 38 | 80 (62.99)[54.59 - 71.39] | MO | Biopsy |
| Bernal et al. 2000[40] | Mexico | 15 | NA | NA | 10 (66.67)[42.81- 90.53] | Patients⁎⁎ | Biopsy |
| Leite et al. 2009 [46] | Brazil | 180 | 55.6* | 0.3 | 125 (69.44)[62.71 - 76.17] | T2DM | US |
| Arab et al. 2016[47] | Chile | 133 | 60(55+) | 47.4 | 93 (69.92)[62.13 - 77.71] | T2DM | MR |
| Graffigna et al. 2009[17] | Argentina | 91 | 39.8(18-65) | 11 | 65 (71.43)[62.15 - 80.71] | Patients⧭ | US |
| Knopfholz et al. 2012[48] | Brazil | 75 | 55.5*(30-80) | 26.7 | 55 (73.33)[63.32 - 83.34] | MS | US |
| De Oliveira et al. 2007[49] | Brazil | 146 | 36 | 21 | 111 (76.03)[69.11 - 82.95] | MO | Biopsy |
| Daltro et al. 2010[50] | Brazil | 40 | 36.2 | 35 | 33 (82.50)[70.72 - 94.28] | MO | Biopsy |
| Knopfholz et al. 2012[48] | Brazil | 42 | NA | NA | 35 (83.33)[72.06 - 94.60] | T2DM | US |
| Tagle et al. 2008[51] | Peru | 50 | 50.1 | 0.6 | 45 (90.00)[81.68 - 98.32] | Ow/Obese | Biopsy |
| Feijo et al. 2013[52] | Brazil | 60 | 38.4 | 27 | 57 (95.00)[89.49 – 100.51] | MO | Biopsy |
| Bertol et al. 2020[53] | Brazil | 614 | 37.2 | 21 | 604 (98.37)[97.37 - 99.37] | MO | Biopsy |
| Graffigna et al. 2009[17] | Argentina | 31 | NA | NA | 31 (100) | Patients⧭ | Biopsy |
| Zamin et al. 2002[18] | Brazil | 33 | 42.2(18-65) | NA | 33 (100) | Obese | Biopsy |
Abbreviations:
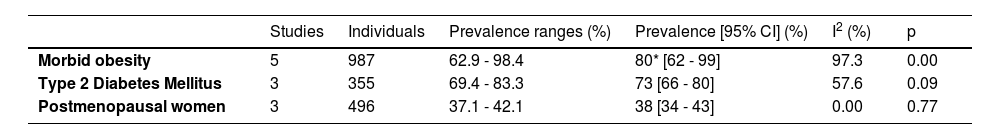
Regarding the type of population, 1 study was conducted in a sample of the general population (n = 139), 2 included captive populations (n = 2700) and 16 were conducted with patients (n = 2948). Among the studies including patients, there were three groups of patients in which at least 2 articles explored a disease in common, 5 (n = 987) measured NAFLD in patients with morbid obesity, 3 (n = 355) in diabetic patients, and 3 (n = 496) in postmenopausal women.
3.3Prevalence of NAFLDThe prevalence of NAFLD in the 19 selected studies ranged between 14.3 to 100%. In the meta-analysis, the overall prevalence was 59% [CI: 38 - 80%], with I2 = 99.81% and p < 0.01. Due to its high heterogeneity, this finding is considered to be statistically non-significant.
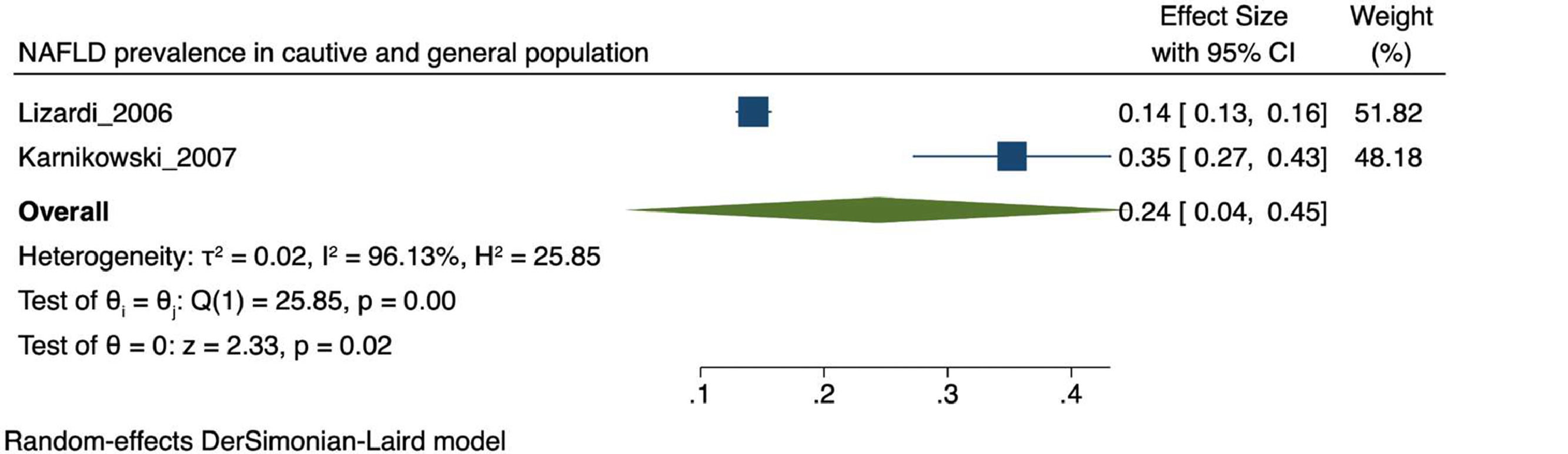
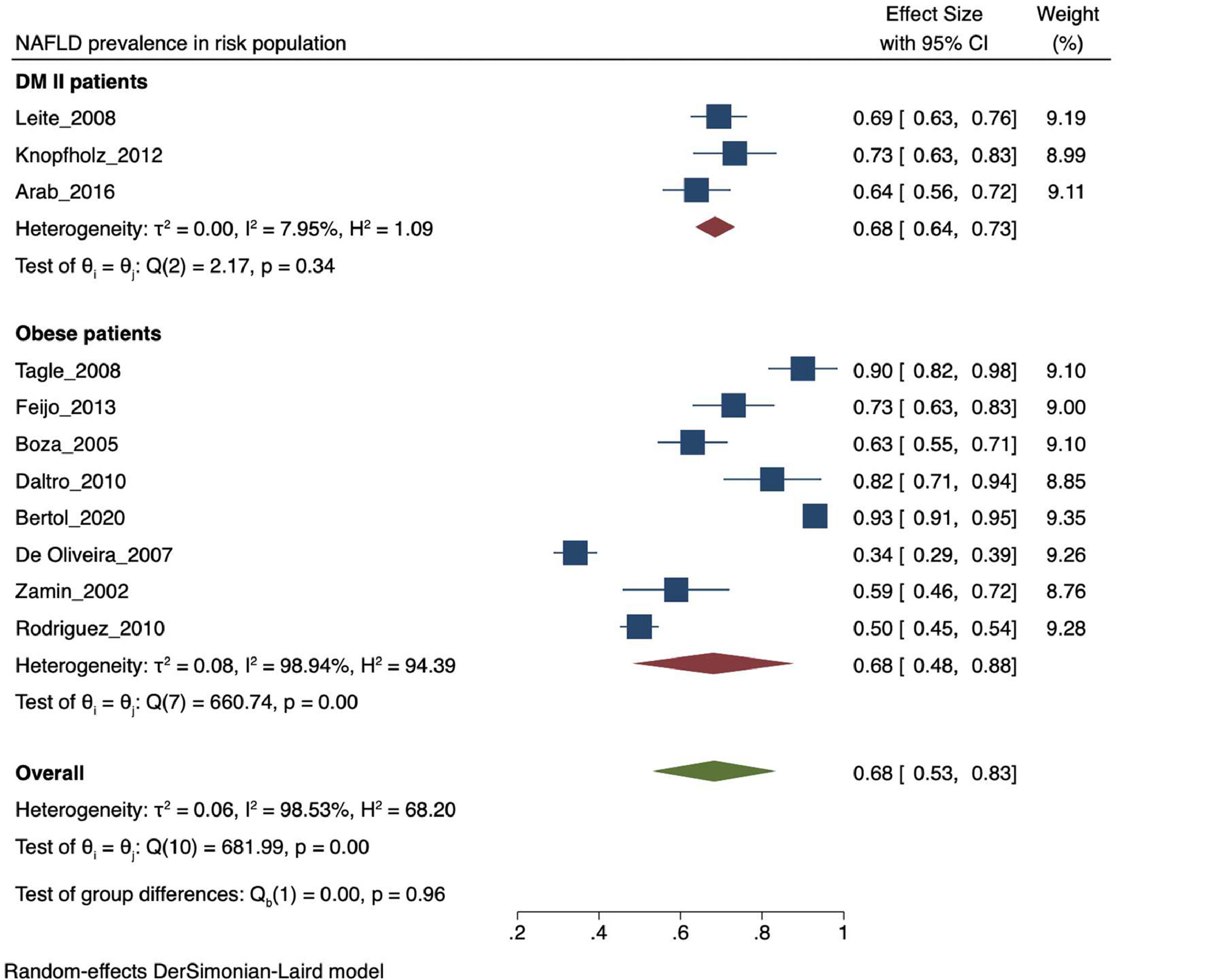
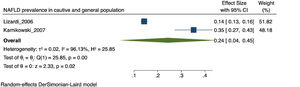
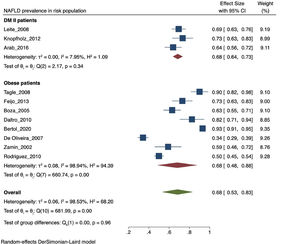
The prevalence of NAFLD among the general and captive populations was 24% [4 - 45%] (see Fig. 2). The high-risk populations (i.e., patients with type 2 diabetes mellitus or obesity) showed a significantly higher mean prevalence of 68% [53 - 83%] (see Fig. 3). Additionally, postmenopausal women had a prevalence of 38% [34 - 43%]. The results by subgroup of patients are detailed in Table 3. NAFLD prevalence also varied depending on the diagnostic method used, as is detailed in Table 2.
NAFLD prevalence in risk population. NAFLD: Non-alcoholic fatty liver disease. LA: Latin-America. MAFLD: Metabolic (dysfunction) associated fatty liver disease. T2DM: Type-2 Diabetes Mellitus. PCOS: Polycystic ovarian syndrome. BMI: Body mass index. PRISMA: Preferred Reporting Items for Systematic review and Meta-Analyses. DUICT: Office of Regulation and Ethical Assessment of Research (in Spanish: Dirección Universitaria de Investigación, Ciencia y Tecnología).
Prevalence of NAFLD according to diagnostic method.
Prevalence of NAFLD according to population characteristics.
| Studies | Individuals | Prevalence ranges (%) | Prevalence [95% CI] (%) | I2 (%) | p | |
|---|---|---|---|---|---|---|
| Morbid obesity | 5 | 987 | 62.9 - 98.4 | 80* [62 - 99] | 97.3 | 0.00 |
| Type 2 Diabetes Mellitus | 3 | 355 | 69.4 - 83.3 | 73 [66 - 80] | 57.6 | 0.09 |
| Postmenopausal women | 3 | 496 | 37.1 - 42.1 | 38 [34 - 43] | 0.00 | 0.77 |
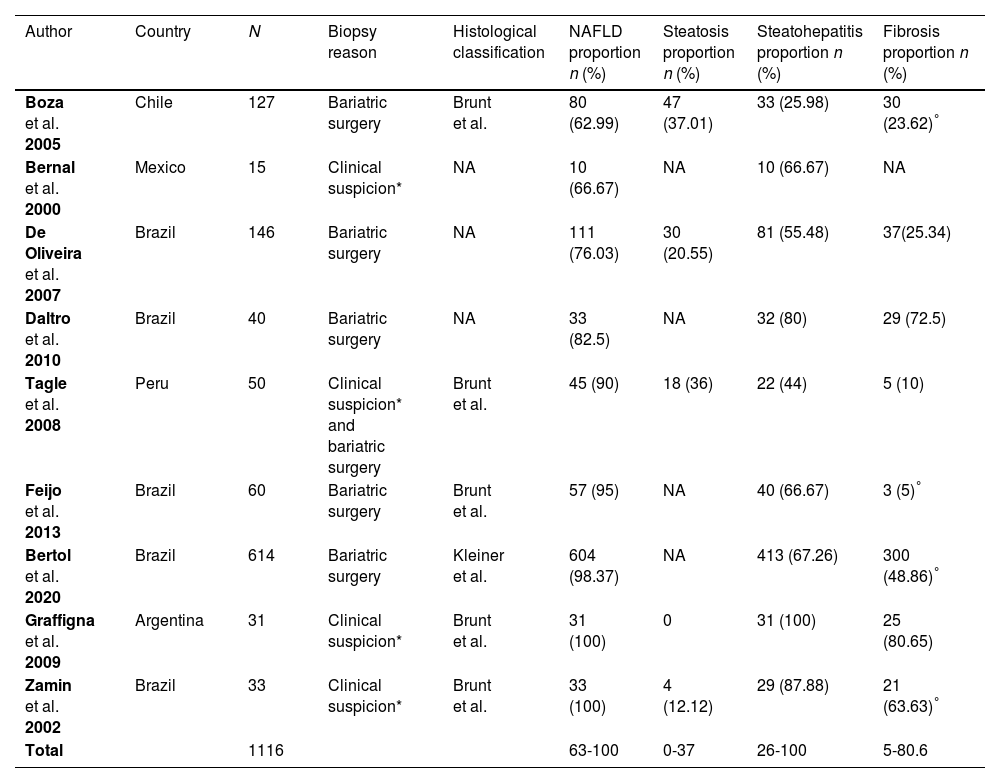
Nine articles used liver biopsy for diagnosing NAFLD and assessing the stages. Of these, five studies used the histological classification of Brunt et al. [15], one used the classification of Kleiner et al. [16], and the rest of them did not specify the classification used. The proportions of adults with steatosis, steatohepatitis and fibrosis are shown in Table 4.
Studies with patients undergoing liver biopsy.
| Author | Country | N | Biopsy reason | Histological classification | NAFLD proportion n (%) | Steatosis proportion n (%) | Steatohepatitis proportion n (%) | Fibrosis proportion n (%) |
|---|---|---|---|---|---|---|---|---|
| Boza et al. 2005 | Chile | 127 | Bariatric surgery | Brunt et al. | 80 (62.99) | 47 (37.01) | 33 (25.98) | 30 (23.62)° |
| Bernal et al. 2000 | Mexico | 15 | Clinical suspicion* | NA | 10 (66.67) | NA | 10 (66.67) | NA |
| De Oliveira et al. 2007 | Brazil | 146 | Bariatric surgery | NA | 111 (76.03) | 30 (20.55) | 81 (55.48) | 37(25.34) |
| Daltro et al. 2010 | Brazil | 40 | Bariatric surgery | NA | 33 (82.5) | NA | 32 (80) | 29 (72.5) |
| Tagle et al. 2008 | Peru | 50 | Clinical suspicion* and bariatric surgery | Brunt et al. | 45 (90) | 18 (36) | 22 (44) | 5 (10) |
| Feijo et al. 2013 | Brazil | 60 | Bariatric surgery | Brunt et al. | 57 (95) | NA | 40 (66.67) | 3 (5)° |
| Bertol et al. 2020 | Brazil | 614 | Bariatric surgery | Kleiner et al. | 604 (98.37) | NA | 413 (67.26) | 300 (48.86)° |
| Graffigna et al. 2009 | Argentina | 31 | Clinical suspicion* | Brunt et al. | 31 (100) | 0 | 31 (100) | 25 (80.65) |
| Zamin et al. 2002 | Brazil | 33 | Clinical suspicion* | Brunt et al. | 33 (100) | 4 (12.12) | 29 (87.88) | 21 (63.63)° |
| Total | 1116 | 63-100 | 0-37 | 26-100 | 5-80.6 |
Eleven articles were considered as having a low risk of bias and eight, an intermediate risk (see Appendix 2). External validity was the domain with the highest risk of bias. None of the studies was conducted with a nationally representative sample. Furthermore, most studies did not use randomization strategies for sampling, and only five had a response rate greater than 75%. Regarding internal validity, all studies included an objective operational definition for the diagnosis of NAFLD. However, three did not have adequate reliability of the diagnostic method used. Finally, three studies used more than one data collection method and only one did not specify the period of data collection.
4DiscussionThis systematic review and meta-analysis was carried out to estimate the prevalence of NAFLD among adults from LA. We analyzed nineteen studies including data from 5626 individuals. The largest prevalence (100%, n = 124) was found in patients with high suspicion of NAFLD who underwent liver biopsy [17, 18], whereas the lowest (14.3%, n = 2503) was observed in a captive population attending a preventive occupational check-up who underwent abdominal ultrasound [19]. The heterogeneity of prevalence rates can be attributed to differences in sociodemographic factors, sample size, diagnostic methods, and particular clinical features of the study populations [10, 20].
In our review, according to the diagnostic method, we found that the prevalence of NAFLD ranged from 14.3 to 73.3% among studies using abdominal ultrasound, while those using liver biopsy showed a prevalence between 63 to 100%. Higher prevalence rates are expected when performing liver biopsy, the gold standard for diagnosing NAFLD, because most patients undergoing this procedure have high suspicion of liver disease or are at an advanced stage. This method may not be ideal for detecting patients at early stages because it is expensive, invasive, and has high sampling error and risk of complications [21]. In this review, some studies used liver biopsy to analyze the stages of NAFLD and report the presence of liver fibrosis, which is particularly relevant because of its association with an increased risk of hepatocarcinoma and death [10]. However, the description of the stages was incomplete, and the histological classification of fibrosis was different across studies or was not even specified, which significantly limited our analysis. In contrast, transient elastography is a non-invasive tool, with a sensitivity and specificity of nearly 90% and a negative predictive value about 95% [22], which makes it a suitable method for screening hepatic steatosis [23]. This tool is reliable for detecting fibrosis and can be a good alternative for liver biopsy in patients who cannot undergo invasive procedures. Nevertheless, it is expensive and not commonly available in low-income countries.
In LA, other tools such as liver ultrasound and liver enzymes may be more accessible. Liver ultrasound is a widely used tool because of its low cost, non-invasive nature and safety, with a sensitivity ranging between 53 to 100% and a specificity of 77% to 98% for detecting NAFLD [24]. In our study, it was the most used diagnostic method followed by liver biopsy. However, ultrasound has some limitations such as operator-dependency, reduced sensitivity with body mass index, and limited ability to detect mild steatosis [24]. Other diagnostic tools, such as liver enzymes, are not reliable markers of NAFLD, as they may be normal in up to 80% of NAFLD patients [25]. Furthermore, decreased levels of amino transferase may be seen in patients with advanced liver disease. [25]
Regarding the type of population, only one study was conducted in the general population. It showed a prevalence of 35.3% (n=139) in middle-aged individuals from Brazil [26]. This estimation is lower than the prevalence found in patients, which may be explained by the lower frequency of risk factors for NAFLD in the general population. There were other articles that evaluated NAFLD in the general population: Younossi et al. found a prevalence of 30.45% in a revision of a few articles from South America [6]. Riquelme found 23.4% of the general population from Chile [27] and Rivera found 59.9% in Guatemala [28]. However, they did not exclude other etiologies of liver disease, therefore, they were not included in our review.
Two studies were conducted in captive populations showing prevalence rates of 14% and 47%, respectively [19, 29]. Unlike the first, the latter was carried out only in women and its higher prevalence may be explained by the inclusion of postmenopausal women and patients with Polycystic Ovary Syndrome (PCOS). Consistently, several studies have found a higher NAFLD prevalence in women with PCOS. [30] In addition, obesity, insulin resistance, and hyperandrogenism are suspected to play important roles in the pathophysiology of this association. [30]. Postmenopausal women had a NAFLD prevalence between 37.1 to 42% in our review. Higher rates are expected in this population because of hormonal mechanisms [31]. To be more precise, the drop in estrogen is associated with an alteration in the metabolism of lipids in the liver [31]. Also at menopause, BMI-adjusted waist circumference and intra-abdominal fat levels increase. [31]
In fact, studies have found less physical activity in women from early ages, which is associated with various psychosocial factors such as less family support, self-efficacy and motivation and increased risk of metabolic diseases and cancer. [32] More research is required on the subject in LA, but we can expect a similar or even worse reality in this region due to the lower Gender Gap Index reported in LA (72.1%) than in other countries (73.1% in Australia, 76% in North America, and 77% in Western Europe) [33]. It is very important to fill this research gap because an early detection and intervention with lifestyle changes in this potentially vulnerable population would allow to attenuate steatosis and steatohepatitis and even achieve complete resolution without progression to fibrosis. [34, 35]
Both T2DM and obesity have been recognized as significant risk factors for developing NAFLD [4, 5]. In our study, the prevalence of NAFLD ranged from 62.9 to 98.4% in morbidly obese patients, consistent with international data reporting a range between 60 to 95% [36]. This result can be explained by the involvement of visceral adipose tissue in the pathophysiology of NAFLD. In 2005, an article estimated an increase in obesity prevalence for 2030 from 49.3% to 81.9% in LA [37], leading to an expected increase in NAFLD prevalence. On the other hand, NAFLD prevalence ranged between 69.4 to 83.3% in diabetic patients, which may be attributed to the documented synergy between NAFLD and T2DM [38]. Consistently, when analyzing the average prevalence of population with risk factors for NAFLD such as obesity and T2DM, it is higher than the value of the general and captive population.
A strength of this systematic review was the use of search terms in the original languages of LA countries and the distinction of Latin Americans from Hispanic individuals, who are not necessarily from LA, to avoid selection bias. We decided to add the term MAFLD in the search for articles, because we consider it to be a more precise concept, but we did not find any article that used the term, probably due to the little diffusion of the concept in Latin America [4]. Thus, we ensured the reliability of the diagnosis of NAFLD by verifying the old definition in all the included articles. Naturally, the disadvantage of using this negative definition is that it does not consider NAFLD patients who may have more than one liver disease. Additionally, a great limitation in our review was the lack of national level studies and the high heterogeneity found, which limited our statistical analysis.
NAFLD research faces several challenges in Latin American countries, which is reflected by the lack of nationally representative databases and low publication rates in scientific journals [24]. Some factors contributing to this problem are income disparities, limited funding opportunities for research activities and health system fragmentation. This reveals an opportunity for intervention at the political level, such as the systematization of health systems in order to share the same data between different health service providers. Such intervention would allow a better understanding of the increasing burden of metabolic diseases including NAFLD. Furthermore, considering the advantage of sharing the same language, Latin American countries could form research alliances between public health systems.
In addition, the lack of knowledge and awareness of NAFLD among healthcare professionals may underestimate the prevalence and associated risks of the disease [10, 24, 39]. Given that primary care physicians are at the front line of seeing patients with NAFLD, targeted educational programs should be offered to them to increase the awareness of the disease and reduce knowledge gaps between medical specialties [39]. These programs should be focused on the identification of associated risk factors for NAFLD, because sophisticated diagnostic tools are not always available in LA. For instance, screening programs to detect cardiovascular diseases and nutritional interventions should be strengthened in primary healthcare services. Furthermore, diagnosed patients could be directed to a multidisciplinary pathway, due to its metabolic nature and relationship with other pathologies.
5ConclusionsAccording to the analysis of clinical studies, we conclude that the average prevalence of NAFLD is around 24%. Higher prevalence rates were found in risk groups such as obese patients (80%), diabetic patients (73%) and postmenopausal women (38%), like results found worldwide. Further studies in the general population and appropriate designs considering new definitions are required for an accurate estimate of the prevalence of NAFLD in LA.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None.
Risk of bias: Low (score ≤ 4), intermediate (score 5 - 7), high (score ≥ 8).