We report the case of a 53-year-old-man who developed human T-cell leukemia virus type-1-associated myelopathy (HAM) after ABO-incompatible liver transplantation for alcoholic liver cirrhosis. The living donor was seropositive for human T-cell leukemia virus type-1 (HTLV-1) and the recipient was seronegative for HTLV-1 before transplantation. After transplantation, the recipient developed steroid-resistant acute cellular rejection, which was successfully treated using anti-thymocyte globulin, and he was eventually discharged. He underwent spinal surgery twice after the transplantation for the treatment of cervical spondylosis that had been present for a period of 9 months before the transplantation. The surgery improved his gait impairment temporarily. However, his gait impairment progressed, and magnetic resonance imaging revealed multiple sites of myelopathy. He was diagnosed with HAM 16 months after the transplantation. Pulse steroid therapy (1000mg) was administered over a period of 3 days, and his limb paresis improved. Presently, steroid therapy is being continued, with a plan to eventually taper the dose, and he is being carefully followed up at our institution. Our case suggests that liver transplantation involving an HTLV-1-positive living donor carries the risk of virus transmission and short-term development of HAM after transplantation.
Human T-cell leukemia virus type-1 (HTLV-1) is a retro-virus endemic to southwest Japan, West Africa, the Caribbean, South America, and New Guinea [1,2]. Although most carriers of this virus remain asymptomatic during their life-time, a small number of carriers develop HTLV-1-associated diseases, such as adult T-cell leukemia/lymphoma (ATL) [3,4], and HTLV-1-associated myelopathy (HAM) [5]. In Japan, approximately 5% and 0.25% of carriers develop ATL and HAM, respectively [6]. Activated T cells and exaggerated cytotoxic T-lymphocytes that respond to HTLV-1 appear to play a role in the pathogenesis of HAM [7]. Accordingly, strategies involving immunosuppressive agents, such as cyclosporine, tacrolimus [8], interferon [9,10], and steroids [11], have been proposed, but these strategies are not yet fully established. Moreover, the reasons for the development of HAM in only a small proportion of HTLV-1 carriers and the key mechanisms that underlie this development remain unknown.
With regard to liver transplantation, one case of donor-transmitted HAM (HTLV-1-positive donor to negative recipient) has been reported following deceased-donor liver transplantation (DDLT) [12]. On the other hand, several cases of HAM or ATL have been reported after living-donor liver transplantation (LDLT) in HTLV-1-positive recipients who received a graft from an HTLV-1-positive or negative donor [10,12–15]. Although the risks of HTLV-1 transmission and HTLV-1-associated disease development remain unclear, a large viral load and an immunosuppressive condition appear to be associated with the development of ATL and HAM after organ transplantation [12,16]. Although HTLV-1 transmission has been described for renal transplantation, the risk of transmission from an HTLV-1-positive living donor to an HTLV-1-negative recipient is not well known for liver transplantation [17]. Here, we present a case of donor-transmitted HAM as an HTLV-1-associated disease that developed after ABO-incompatible liver transplantation. To our knowledge, this is the first such case in the literature.
2Case reportThe patient received a detailed explanation and provided informed consent to publish the case details. The patient was a 53-year-old man, with decompensated alcoholic liver cirrhosis, who was referred to our hospital for LDLT. His wife was the candidate donor. A physical examination revealed jaundice, gynecomastia, lower limb edema, and limb paresis, which resulted from severe cervical spondylosis that had developed 9 months previously. He required a wheelchair for mobility. His laboratory findings on admission were as follows: total bilirubin, 4.0mg/dL; direct bilirubin, 0.8mg/dL; aspartate aminotransferase, 40IU/L; alanine aminotransferase, 32IU/L; albumin, 2.7g/dL; prothrombin time, 56%; prothrombin time/international normalized ratio, 1.41; and creatinine, 0.71mg/dL. Additionally, his Model for End-Stage Liver Disease score was 16.
His wife was born in southwest Japan, and laboratory testing revealed a high HTLV-1 antibody titer of 2048, using the particle agglutination (PA) method. On the other hand, he was seronegative for HTLV-1 antibodies prior to LDLT, and both he and his wife did not present with any symptoms of HTLV-1-associated diseases prior to LDLT. Considering his poor survival prognosis, LDLT was the only option, and his wife was the only available donor. He was informed about the risk of HTLV-1-associated diseases after transplantation, and he provided consent to proceed with the ABO-incompatible liver transplantation (recipient: B Rh [+], donor: A Rh [+]).
He was administered 500mg/body of rituximab (anti-CD20 antibody) 12 days prior to LDLT, because of the blood-type incompatibility. He underwent LDLT with a right lobe graft from his wife, using standard surgical procedures according to our institutional protocol, which has been described elsewhere [18,19]. We performed a splenectomy with catheter insertion into the portal vein for infusion therapy, according to our protocol for ABO-incompatible liver transplantation [20]. The weight of the liver graft was 670g, and the graft/recipient weight ratio was 0.82. The post-transplantation immunosuppressive therapy included tacrolimus (initial target trough level, 12–15ng/mL), steroids (80mg), and mizoribine (200mg; mizoribine is occasionally used instead of mycophenolate mofetil). The dose of tacrolimus and steroids were gradually tapered. Additionally, postoperatively, steroids, prostaglandin El, and gabexate mesylate were administered via the portal vein catheter for 2 weeks in order to overcome issues with ABO-incompatible liver transplantation, as described previously [21,22].
His liver function deteriorated on postoperative day 14, and a liver biopsy confirmed acute cellular rejection (ACR). Mizoribine was switched to mycophenolate mofetil (500mg), and pulse steroid therapy (1000mg) was added, with gradual tapering over a 3-day period. However, his liver function further deteriorated, with increases in the levels of aspartate aminotransferase (181IU/L), alanine aminotransferase (405IU/L), gamma-glutamyltransferase (260IU/L), and total bilirubin (6.5mg/dL). He was diagnosed with steroid-resistant ACR. Thus, anti-thymocyte globulin (ATG; 1.5mg/kg) was administered intravenously for 5 consecutive days, starting on postoperative day 19. After treatment, his liver function promptly improved, and the effectiveness of the ATG treatment for ACR was confirmed on liver biopsy. He was eventually discharged on postoperative day 90, and steroid therapy was maintained on an out-patient basis for 6 months after LDLT. At 6 months after LDLT, he underwent magnetic resonance imaging (MRI) of the spine, which revealed a high intensity intramedullary lesion at C3-4 that was compatible with cervical spondylosis (Fig. 1A). Thus, he was treated with C3-4 laminectomy. His lower limb paresis improved after surgery, and he regained the ability to walk independently. However, he reported recurrence of lower limb weakness at 6 months after laminectomy. He underwent anterior spinal fusion 8 months after laminectomy; however, he showed a rapid increase in lower limb paresis at 2 months after the surgery, requiring readmission.
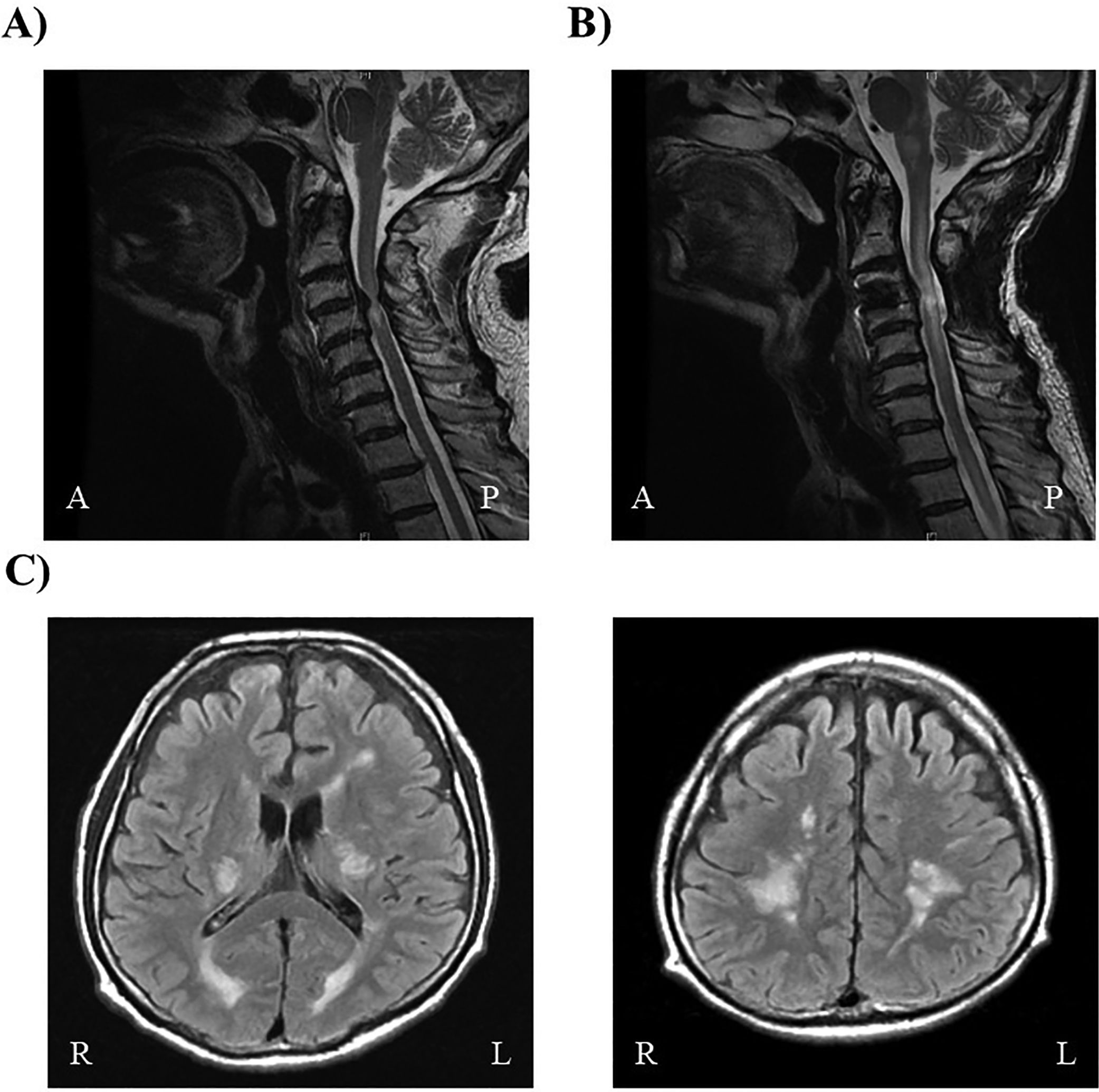
(A) Spinal magnetic resonance imaging performed before the first surgery for cervical spondylosis. An intramedullary limited lesion with high intensity is visible in the mid-portion of the cervical spinal cord (C3–4) on T2-weighted imaging. (B) Spinal magnetic resonance imaging performed after the second surgery for cervical spondylosis. An intramedullary lesion with high intensity is visible, extending into the mid-portion of the cervical spinal cord (C2-6) and brainstem on T2-weighted imaging. (C) Brain magnetic resonance imaging. Multiple high intensity lesions are visible within the internal capsule and deep white matter on both sides on T2-weighted imaging.
On readmission, he presented with spastic paraplegia and numbness below the neck. MRI revealed an extension of the original high-intensity lesion from C2 to 6 in T2-weighted images (T2WIs; Fig. 1B). Brain MRI revealed high-intensity lesions in the internal capsule and deep white matter on both sides (Fig. 1C), and these lesions showed point and linear enhancement effects in T2WIs. Laboratory assessments revealed a high HTLV-1 antibody titer (>256 [PA]), with a high titer in the cerebrospinal fluid (CSF; >32 [PA]), and a high neopterin level (30pmol/mL; reference: <5pmol/mL). Additionally, human herpes virus-6 (HHV-6) was detected in the CSF. Other autoantibodies related to myelitis were serologically negative. MRI findings were not typical of HHV-6-associated myelitis but were compatible with HAM, and the clinical manifestations fulfilled the diagnostic criteria for HAM [23]. Immediately after confirming the diagnosis of HAM at 16 months after LDLT, pulse steroid therapy (1000mg) was administered over a period of 3 days, and his limb paresis improved. Presently, steroid therapy is being continued, with a plan to eventually taper the dose and he is being carefully followed up at our institution.
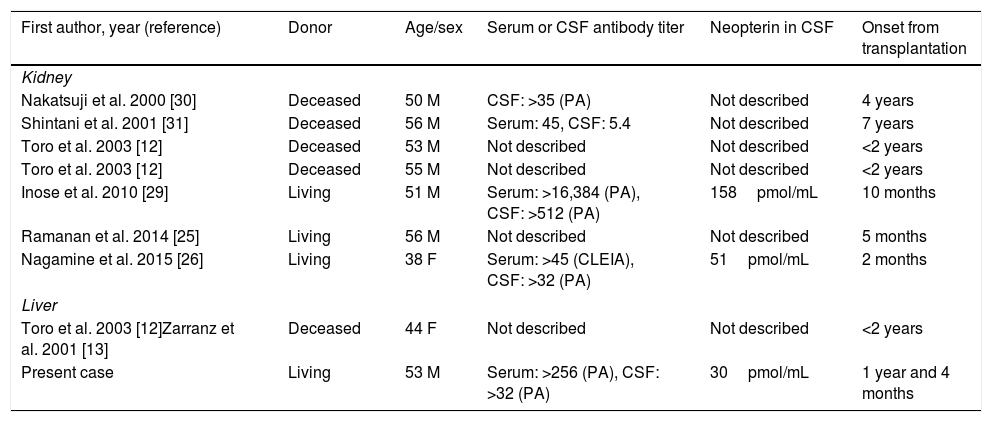
3DiscussionThe rate of HAM development in HTLV-1 carriers has been reported to be 0.25% in a non-transplantation setting [6], and the incidence rate of HAM has been reported to be 2.3% (2/88cases) among HTLV-1-associated recipients in a transplantation setting (HTLV-1 positivity was noted in both recipients and one of the donors before LDLT) [17]. The occurrence of ATL has been reported to be higher than that of HAM after liver transplantation in HTLV-1-positive recipients [17], and the 5-year incidence rate has been reported to be 9.2%, which is higher than the rate of 3–5% reported in HTLV-1-positive individuals who do not undergo liver transplantation [6]. Immunosuppression appears to play a key role in the high incidences of ATL and HAM among HTLV-1-positive individuals after transplantation [16]. In our case, LDLT involved an HTLV-1-positive donor and an HTLV-1-negative recipient. Table 1 summarizes the previous reports of HAM development associated with a high likelihood of transmission from an HTLV-1-positive donor after renal or liver transplantation. To the best of our knowledge, there is only one reported case of donor-transmitted HAM after DDLT [12], and there is no reported case of donor-transmitted HTLV-1-associated disease after LDLT. We believe that our case is important and informative, as it indicates the risk of donor-transmitted HTLV-1-associated disease after LDLT.
Published cases of HTLV-1-associated myelopathy (HAM) development possibly related to transmission from an HTLV-1 donor after renal or liver transplantation.
| First author, year (reference) | Donor | Age/sex | Serum or CSF antibody titer | Neopterin in CSF | Onset from transplantation |
|---|---|---|---|---|---|
| Kidney | |||||
| Nakatsuji et al. 2000 [30] | Deceased | 50 M | CSF: >35 (PA) | Not described | 4 years |
| Shintani et al. 2001 [31] | Deceased | 56 M | Serum: 45, CSF: 5.4 | Not described | 7 years |
| Toro et al. 2003 [12] | Deceased | 53 M | Not described | Not described | <2 years |
| Toro et al. 2003 [12] | Deceased | 55 M | Not described | Not described | <2 years |
| Inose et al. 2010 [29] | Living | 51 M | Serum: >16,384 (PA), CSF: >512 (PA) | 158pmol/mL | 10 months |
| Ramanan et al. 2014 [25] | Living | 56 M | Not described | Not described | 5 months |
| Nagamine et al. 2015 [26] | Living | 38 F | Serum: >45 (CLEIA), CSF: >32 (PA) | 51pmol/mL | 2 months |
| Liver | |||||
| Toro et al. 2003 [12]Zarranz et al. 2001 [13] | Deceased | 44 F | Not described | Not described | <2 years |
| Present case | Living | 53 M | Serum: >256 (PA), CSF: >32 (PA) | 30pmol/mL | 1 year and 4 months |
CSF: cerebrospinal fluid, PA: particle agglutination, CLEIA: chemiluminescent enzyme immunoassay.
ATL and HAM develop in individuals with HTLV-1 infection after a latency period that can range from years to decades [24]. However, previous studies have reported that the latency period for HAM development is shorter after solid organ transplantation (2 months to 7 years for renal transplantation and 15–46 months for liver transplantation) [16,17,25,26]. In our case, the interval between LDLT and HAM development was only 16 months. Kida et al. reported increased levels of neopterin in the CSF of patients with rapidly progressive HAM [27]. Additionally, Sato et al. reported that the levels of neopterin in the CSF were strongly correlated with the rate of HAM progression [28]. Similarly, increased levels of neopterin were noted in our case. Toro et al. reported particularly high virulence with a large viral load and an immunosuppressed state after transplantation, which may contribute to the unusually rapid development of HAM [12]. The latency period of HAM development in our case was compatible with the period reported previously, and HAM occurred within a short period after transplantation [12,13,25,26,29–31].
In the present case, considering that ABO-incompatible liver transplantation with a HTLV-1-seropositive donor was planned, the recipient received strong immunosuppressive treatment involving a calcineurin inhibitor, steroids, and an antimetabolite; received steroids, prostaglandin El, and gabexate mesylate via portal infusion; and underwent splenectomy. These precautions are not usually necessary in ABO-identical and ABO-compatible recipients. Although our protocol for ABO-incompatible liver transplantation is designed to overcome issues associated with the immune system, strong immunosuppression may increase the risk of rare infections, as in our patient. This increase in the risk of rare infections has been reported previously [21]. Furthermore, it is notable that our patient developed steroid-resistant ACR with ATG infusion, which was needed to overcome refractory ACR. The induction of a strong immunosuppressive condition can explain the rapid development of HAM after LDLT in our case. However, HHV-6 was also detected in the CSF. Although neurological involvement of HHV-6 could not be fully excluded, the hyper-intensity of the spinal cord lesion observed on MRI was not typical of HHV-6 myelitis reported previously after transplantation [32,33]. Moreover, gastric symptoms have been reported to be important symptoms of HHV-6 infection after transplantation [34]. However, there was no involvement of the digestive tract in our case. Therefore, we speculate that the myelitis in our patient was associated with HAM rather than HHV-6.
Cases of living donor-transmitted HAM have been reported following renal transplantation [25,26,29]. In December 2014, the Japanese Ministry of Health, Labor and Welfare publically reported cases of recipients of living donor renal transplants who were HTLV-1 negative prior to transplantation but showed HTLV-1 infection after receiving a renal graft from an HTLV-1-positive donor. Furthermore, these recipients developed HAM more rapidly and at a higher rate than reported previously [26]. In these recipients, symptoms progressed and included serious impairments in gait within several years after HAM development. As HTLV-1 infection is relatively rare in eastern and northern Japan, transplantation centers in these areas had not cautiously considered the donor and recipient HTLV-1 status prior to performing living-donor renal transplantation until this announcement. The Japan Society for Transplantation recommended serology tests for all donors and recipients prior to living-donor renal transplantation in order to reduce the risk of unintentional transmission of HTLV-1 through the transplantation. The guidelines further recommended that living-donor renal transplantation with an HTLV-1-positive donor or an HTLV-1-positive recipient should be performed only after highlighting the increased risk of HAM development and obtaining informed consent from both the recipient and donor. The Society further recommended the implementation of careful follow-up for the early identification of any HTLV-1-associated disease in an HTLV-1-positive recipient. A large Japanese surveillance study on LDLT reported that among six HTLV-1-negative recipients who received grafts from HTLV-1-positive donors (including one case from our institution in 2005), no case of ATL or HAM development was identified at the time of publication [17]. The article further described nine ABO-incompatible cases without HTLV-1 transmission [17].
In conclusion, we believe that a liver transplant from an HTLV-1-positive living donor carries the risk of virus transmission and HAM development. Therefore, careful preoperative assessment of the HTLV-1 status of both the recipient and donor is recommended, and HTLV-1-positive LDLT should be performed only after obtaining appropriate informed consent. Further studies are warranted to clarify the factors associated with HAM development after LDLT.AbbreviationsHTLV-1 human T-cell leukemia virus type 1 adult T-cell leukemia/lymphoma human T-cell leukemia virus type 1 associated myelopathy deceased-donor liver transplantation living-donor liver transplantation anti-thymocyte globulin particle agglutination acute cellular rejection magnetic resonance imaging T2-weighted images cerebrospinal fluid human herpes virus-6
All authors declare that they received no funding for this report.