The first generation protease inhibitors has been the mainstay of hepatitis C treatment for the last couple of years, showing marked improvement in sustained virological response, but also increased side effects. Infection has emerged as a common complication of telaprevir and boceprevir in combination with peginterferon and ribavirin, usually caused by common pathogens. We present the case of a 65 years old man who developed a Mycobacterium abscessus pulmonary infection during treatment with telaprevir, peginterferon and ribavirin. The patient was successfully treated with amikacin, imipenem and chlarithro-mycin. The present case is relevant for increasing awareness for recognition of opportunistic infections and particularly nontuberculous mycobacterial infections in patients receiving triple therapy for chronic hepatitis C, especially in cirrhotic subjects who develop significant lymphopenia.
Hepatitis C is a chronic infection affecting more than 185 million people in the world, with a raising incidence.1,2 Treatment of chronic hepatitis C is rapidly evolving. In 2011, the first generation of protease inhibitors were approved in the United States and rapidly the triple therapy (peginterferon, ribavirin and a protease inhibitor) was adopted as the standard of care.3–7 The addition of telaprevir or boceprevir to the previous dual therapy meant a very significant increase in the sustained virological response (SVR), but it also was associated with new side effects, some of them severe. These side effects include increased anemia, decompensation of cirrhosis and infections. New alternatives of treatment of hepatitis C infection are being developed, with interferon-free options that are showing excellent safety and tolerability.8,9
We report the case of an HCV infected cirrhotic patient treated with the triple combination of telaprevir, peginterferon and ribavirin who developed a nontuberculous mycobacterial infection while on therapy.
Case ReportThe patient was a 65 years old Hispanic male with a history of a multiple blood derivatives transfusion at age 11 because of nephritis and anemia. He had no history of neither current nor previous smoking and no history of underlying pulmonary disease or symptoms. Chronic hepatitis C in the cirrhotic stage was diagnosed in April 2012 after he presented with hematemesis due to large esophageal varices and antral ulcers. Esophageal varices were eradicated with band ligation and prophylactic propranolol was started. HCV genotype was 1b and viral load was 2,770,00 IU/mL. An abdominal ultrasound and a CT scan showed signs of cirrhosis, splenomegaly (17 cm) and no focal liver lesions. In March 2013 the patient was evaluated for starting antiviral treatment. His hemoglobin was 14.6 g/dL, leucocyte count 3300 cells/μL, absolute neutrophil count (ANC) 1800 cells/μL, absolute lymphocyte count 1,100 cells/μL and platelet count 67,000 cells/μL. Sedimentation rate was 13 mm/h. Albumin was 3.8 g/dL, total bilirubin 0.93 mg/mL, INR was 1.2, alanine aminotransferase (ALT) 62 U/mL, aspartate aminotransferase (AST) 67 U/mL, gamma glutamyl transpeptidase (GGT) was 102 U/mL and alkaline phosphatase (AP) 156 U/mL. HIV serology was negative. Body weight was 75 kg; there was no ascites or edema at the physical examination. Treatment with peginterferon alfa 2a 180 μg/week sq, ribavirin 1,200 mg/d and telaprevir 750 mg q8h was started on March 15, 2013.
The patient developed anemia and fatigability, with a nadir hemoglobin of 8.6 g/dL which was managed with sequential ribavirin dose reductions down to 400 mg/d. Anal discomfort was a relevant complain during the first 8 weeks of treatment. Nadir ANC was 1200 and nadir ALC was 300 cells/μL. A mild skin rash and pruritus was apparent at week 6 of treatment, involving 4% of the body surface which responded to topical corticosteroid therapy and disappeared after week 12.
Telaprevir was discontinued at week 12, as planned, with good adherence and no interruptions. At that time, the patient developed mild ankle edema and ascites. Albumin had dropped to 2.5 g/dL. Bilirrubin was 1.39 mg/dL and INR 1.3. HCV viral load was undetectable (< 12 IU/mL) at weeks 4 and 12. At that time the decision to maintain treatment was taken together with the patient after a careful explanation of the potential risks. Ciprofloxacin 500 mg/d was added as prophylaxis of infection and ascites was treated with sodium restriction and diuretics. The general condition of the patient improved during the following weeks, with ascites and edema resolution. Ciprofloxacin was discontinued at week 16.
At treatment week 24, albumin was 2.9 g/dL, body weight 67 kg and bilirubin 0.84 mg/dL. ALT was 49, AST 88, GGT 246 and AP 329 U/mL. HCV RNA was again undetectable.
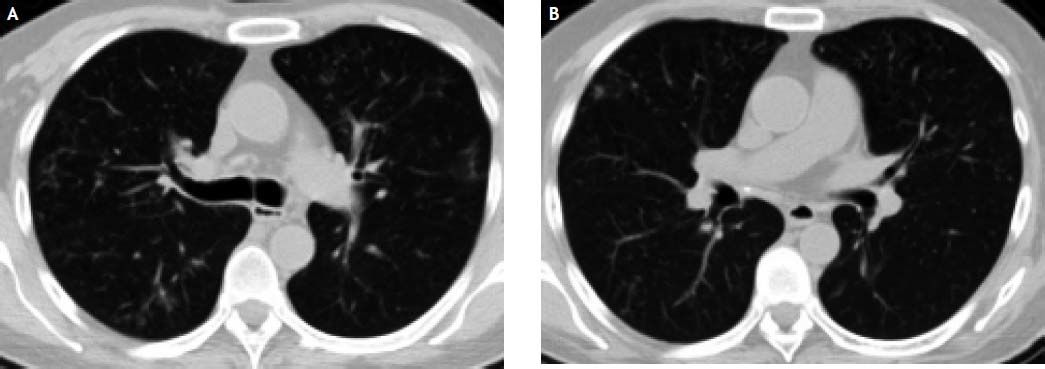
In the following weeks, the patient complained of mild cough with white-yellowish expectoration, which did not respond to antihistamines and salbutamol. A course of antibiotics (levofloxacin 500 mg/d for 10 days) was prescribed with some relief at week 28, but subsequent progressive increase in intensity of cough. Three sputum samples were negative for acid fast smear at week 35. The patient persisted afebrile, but bloody sputum appeared. PA and lateral chest X ray showed upper lobe opacities in both lungs. Subsequent high resolution chest CT scan confirmed multifocal parenchymal lung disease consisting of bilateral areas of small airway inflammation including centrilobular nodules, predominantly in the upper lobes as well as middle lobe, in a pattern consistent with infectious bronchiolitis or active mycobaterial disease (Figure 1).
Peginterferon and ribavirin were interrupted at week 38 and the patient was hospitalized. Hemoglobin was 9.7 g/dL, ALC 500, platelet count 56.000, ANC 1100, ALC 500 cells /μL and sedimentation rate was 124 mm/h. ALT was 43, AST 73, GGT 281 and AP 401 U/mL, bilirubin 1.09 mg/dL, albumin 2.6 g/dL and INR 1.3. HCV viral load was undetectable. Body weight was 63 kg. A bronchioalveolar lavage was performed, showing negative Zielh-Nielsen, fungal and Nocardia stains. Nine days after the lavage, a positive liquid mycobacterial culture for a Mycobacterium sp. was informed. Subsequently it was identified as Mycobacterium chelonae-abscessus by DNA amplification and 16S RNA sequencing. Later it was confirmed as Mycobacterium abscessus by reverse hybridization. Treatment with amikacin 10 mg/kg/d iv for 7 days, imipenem 500 mg q8h iv for 2 weeks and chlarithromycin 500 mg q12h po for 24 weeks was prescribed. The patient was discharged after 2 weeks and was continued on chlarithromycin for 6 months (Figure 1).
The condition of the patient markedly improved over the following weeks, with cough completely disappearing one week after starting treatment. Fatigability slowly improved and the patient started to gain weight, being 70 kg 3 months after stopping HCV antiviral treatment. A follow up CT scan showed significant improvement in the lung lesions. Unfortunately, the patient had a virologic relapse, with an HCV viral load of 122.500 IU/mL 12 weeks after stopping treatment.
DiscussionPeginterferon and ribavirin have been the mainstay of HCV antiviral therapy for more than a decade, but treatment of chronic hepatitis C infection has recently seen a paradigm shift with the approval of direct antiviral agents (DAA). The first generation of these DAA, the protease inhibitors boceprevir and telaprevir, were released in 2011 and despite increasing very significantly response rates and curing infection in approximately 70% of naïve patients, they are associated with new side effects.
Treatment with interferon and ribavirin has been associated with increased risk of infections.10 Identified infections were caused by common bacteria. Decrease of neutrophil count was not associated with a higher risk of infection. The strongest predictors for developing infections during treatment were older age and cirrhosis. A recent study summarizes the infections occurred during the IDEAL trial, the largest hepatitis C study with more than 3,000 patients treated with peginterferon and ribavirin. In more than 1,000 reported episodes of infection, no mycobacterial infections were identified. Interestingly, lymphocyte count decline was associated with the risk of infection, as it was in our patient.11
Nontuberculous mycobacteria are ubiquous free living bacteria that may affect people with or without HIV infection, resulting in pulmonary infection, lymphadenitis, disseminated disease or skin infections.12,13 Pulmonary infection is more frequently secondary to Mycobacterium avium complex (MAC), Mycobacterium kansasii and rapid growing mycobacteria (RGM) species. This last category of mycobacteria usually grows in culture in less than one week after isolation, and comprises three species: M. fortuitum, M. chelonae, and M. abscessus. These infections have been usually described in immunocompromised hosts, especially in patients with underlying T-cell defects. It is remarkable to note that our patient developed significant lymphopenia during peginterferon, ribavirin and telaprevir treatment, with a nadir lymphocyte count of 300 cells/μL. Pulmonary infection by M. abscessus has been associated with esophageal disease,14 chronic pulmonary diseases,15–17 cancer18,19 and rheumatologic diseases.16 The typical imaging findings are similar to those described in MAC infections including small centrilobular nodules (with a tree-in bud pattern on CT scans) and bronchiectasis involving multiple lobes. The upper lobe cavitary form is infrequent (14-16%).20,21 The infection can progress to respiratory insufficiency and ultimately cause death in 14%.20 Treatment generally requires initially combined intravenous therapy during several weeks, followed by oral therapy for months. The susceptibility pattern of isolates of M. abscessus ssp abscessus is generally: Clofazimine (90%), amikacin (90%), imipenem (50%), clarithromycin (100% on initial in vitro susceptibility testing). Response rates, in other settings, were 83% for clinical symptoms and 74% based on CT scan. Persistence of negative cultures for > 12 months occurred in 58%.22
The Compassionate Use of Protease Inhibitors in Viral C Cirrhosis (CUPIC) study, a French multicenter real life experience of treatment of HCV infection reveals very interesting information regarding the safety of triple therapy in cirrhotics.23 This study enrolled 511 patients who had failed to a previous course of peginterferon and ribavirin in 56 sites. All patients were cirrhotic. Treatment (boceprevir or telaprevir) was not randomized. This study was able to show for the first time that this therapy is associated with frequent severe adverse events (50%) and mortality in 11 patients (2.2%). Eight of the 11 patients died from infection. Severe infections in this large real life study occurred in 28 patients (5.5%), and were all common infections (pneumonia, septicaemia, pyelonephritis, skin infections, acute cholecystitis, acute diarrhea, endocarditis and ascites infection). Common bacteria were identified in all cases, with clear predominance of Staphylococcus aureus and Escherichia coli.24 These infections developed relatively early during the course of treatment (at a median time of 14 weeks after starting treatment), and were more common in subjects with serum albumin below 3.5 g/dL and platelet count below 100,000/μL. No mycobacteria or atypical infections were reported.
Tuberculosis infection has been described anecdotally in patients with viral hepatitis undergoing interferon based treatments usually as reactivation of latent cases25 and after liver transplantation in association with interferon, ribavirin and telaprevir treatment in one case.26 Three cases of tuberculosis causing lymphadenopathy during dual peginterferon and ribavirin treatment have been described in Pakistan.27 In Taiwan, among 617 patients treated with peginterferon and ribavirin, 29 patients discontinued treatment for different reasons, one of them because of pulmonary tuberculosis.28 The present case is, to the best of our knowledge, the first report of an atypical mycobacterial infection associated with treatment of chronic hepatitis C with triple therapy with telaprevir.
Interferon has been associated with pulmonary toxicity, with cases of interstitial pneumonitis29,30 and bronchiolitis obliterans-organizing pneumonia.31 Additionally, ribavirin is usually well tolerated but can cause cough and dyspnea.32 Our case broadens the differential diagnosis of pulmonary involvment in patients receiving peginterferon, ribavirin and a protease inhibitor. This differential diagnosis is relevant, given that interferon-associated pneumonitis is usually treated with corticosteroids.33 Clinical diagnosis may not evident, and it only could be established in this case after an invasive procedure, specifically a bronchioalveolar lavage.
In summary, we believe that the present case is relevant for increasing awareness for recognition of opportunistic infections and particularly nontuberculous mycobacterial infections in patients who are receiving triple therapy for chronic hepatitis C, especially in cirrhotic subjects who develop significant lymphopenia.
AcknowledgmentsDr. Soza is partially funded by grant FONDECYT #1130357 from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT).