Background. The gene for patatin-like phospholipase domain containing 3 (PNPLA3) is associated with non-alcoholic fatty liver disease (NAFLD) development. We previously found that Mexican indigenous population had the highest frequency reported of the PNPLA3 148M risk allele. Further, we observed a relationship between M148M genotype with elevated ALT levels in individuals with normal weight, overweight and obese. We sought to investigate whether PNPLA3 polymorphism is associated with NAFLD development in Mexicans.
Material and methods. We enrolled 189 Mexican patients with NAFLD and 201 healthy controls. Anthropometric, metabolic, and biochemical variables were measured, and rs738409 (Ile148Met substitution) polymorphism was genotyped by sequencing.
Results. Logistic regression analysis, using a recessive model, suggested that PNPLA3 polymorphism in Mexican population is significantly associated (OR = 1.711, 95% CI: 1.014-2.886; P = 0.044) with NAFLD.
Conclusions: The PNPLA3 gene is associated with NAFLD in Mexican population. More studies are required to explain the high prevalence of PNPLA3 polymorphism in Mexican-Americans, Mexican-Indians, and Mexican-Mestizos.
Non-alcoholic fatty liver disease (NAFLD) is defined as the presence of simple steatosis (SS) with no evidence of hepatocellular injury which can progress to non-alcoholic steatohepatitis (NASH). NASH is characterized histologically by the presence of hepatic steatosis, lobular inflammation, cytological ballooning, and, in some cases progressive fibrosis. Finally, NASH can lead to cirrhosis or hepatocellular carcinoma (HCC). These diseases are strong research areas due to their worldwide increment in prevalence. Several studies have reported that NAFLD prevalence in developed countries is about 20-30% and 2.7-12.2% for NASH.1
The evidence has demonstrated that the development of hepatic steatosis is consequence of dysfunction of several metabolic pathways. It seems that the increase of circulating fatty acid pool is a major determinant factor for fatty liver pathogenesis. Nowadays, it has been increasingly recognized the role of certain transcription factors activation, adipokines action, hepatic fat oxidation derangements and very-low-density lipoprotein secretion. However, the environmental and genetic factors have shown an important role in the NASH progress, cirrhosis and HCC.2 In fact, the first genome-wide association study (GWAS) on NAFLD has demonstrated that the non-synonymous variant I148M (rs738409 C/G), located in human PNPLA3 gene (also known as adiponutrin), is associated with fatty liver. In addition, this is the strongest ever report for a common variant modifying the genetic susceptibility of NAFLD (5.3% of the total variance).3,4
GWAS study by Dallas Heart Study,5 was conducted in a U.S.-based population and published in 2008. This study identified a highly significant association of increased hepatic triacylglycerol (TAG) levels with PNP-LA3 (rs738409) variation. The investigators found ethnic differences in susceptibility to hepatocellular TAG accumulation. In this connection, Rs738409 has been more commonly observed in Hispanic individuals (0.49), less in those of European ancestry (0.23), and the least in African-Americans (0.17).5 In another study conducted in a U.S.-based population, the investigators analyzed data from 4,804 adults (1,825 Non-Hispanic White; 1,442 Non-Hispanic Black; 1,537 Mexican American).6 The aim of this study was found an association between genetic variants of PNPLA3, GCKR, PPP1R3B, LYPLAL1, NCAN and these ethnicities. They observed that PNPLA3 variants were more frequent in Mexican Americans (effect allele frequency [EAF]:0.54) compared with Non-Hispanic Whites and Non-Hispanic Blacks (EAF: 0.25 and 0.14, respectively). In Mexico, the group of Torre, et al. diagnosed 211 patients with different progression of the disease (SS, NASH and fibrosis spectrum), observing the G risk allele in 89% of individuals (CC: 23 [10.5%[, GC: 73 [34.7%], GG 115[54.7%]) and an overall allele frequency of 77%. Further, they reported that the allele frequency was 71% for NASH, 80% for SS and 73% for fibrosis. Furthermore, GG genotype carriers showed 3.8 times (CI 95%:3.03-4.79) increased risk of steatohepatitis and 2.3 times more (CI95%:1.77-3.23) risk of having liver fibrosis (CC).7 We hypothesized that Mexican patients with hepatic steatosis are more likely to harbor PNPLA3 polymorphism compared to controls.
Material and MethodsWe recruited 390 patients at the University Hospital in Mexico City to participate in the present study. The sample size included 189 Mexican patients with NAFLD and 201 healthy controls. The hepatic steatosis was diagnosed by abdominal ultrasound and anthropometric, metabolic, and biochemical variables were measured in both groups. The PNPLA3/I148M variant (rs738409) was genotyped using TaqMan assays. Genotype frequencies of rs738409 were compared by logistic regression analysis using recessive and dominant models adjusting for age, gender, and body mass index (BMI). Liver PNPLA3 mRNA levels were assessed by Polymerase Chain Reaction (PCR) in 11 biopsies.
The local research ethics approved this study.
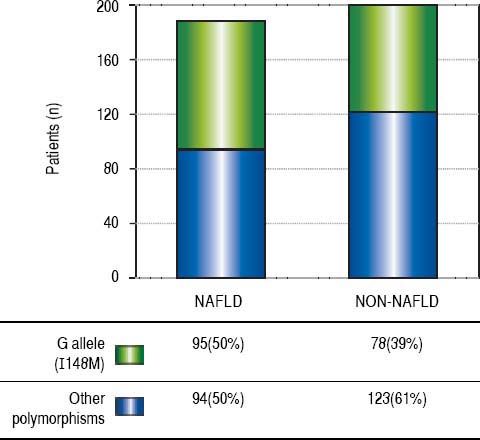
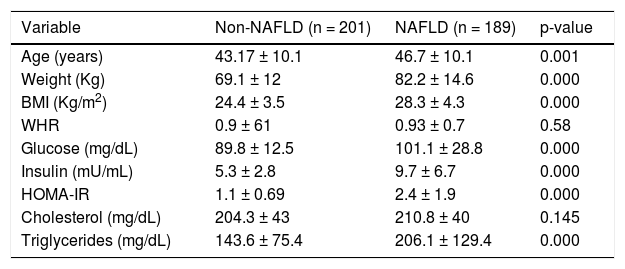
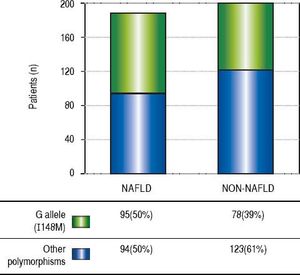
ResultsThe demographic and biochemical test results, of patients with and without NAFLD, at the time of diagnosis are listed in Table 1. In general, most of NAFLD patients presented specific characteristics of metabolic syndrome. On the other hand, the cholesterol and waist-hip ratio (WHR) had not significant differences. G allele (I148M) was found in 50% of NAFLD patients compared with healthy subjects (39%) (Figure 1). The significant association was observed by recessive model (OR = 1.711, 95% CI: 1.014-2.886; P = 0.044). We observed a significantly higher levels of aspartate aminotransferase and alanine transaminase (ALT) in I148M homozygote NAFLD patients and controls (P = 0.002 and 0.036, respectively).
Demographic, clinical and biochemical characteristics.
| Variable | Non-NAFLD (n = 201) | NAFLD (n = 189) | p-value |
|---|---|---|---|
| Age (years) | 43.17 ± 10.1 | 46.7 ± 10.1 | 0.001 |
| Weight (Kg) | 69.1 ± 12 | 82.2 ± 14.6 | 0.000 |
| BMI (Kg/m2) | 24.4 ± 3.5 | 28.3 ± 4.3 | 0.000 |
| WHR | 0.9 ± 61 | 0.93 ± 0.7 | 0.58 |
| Glucose (mg/dL) | 89.8 ± 12.5 | 101.1 ± 28.8 | 0.000 |
| Insulin (mU/mL) | 5.3 ± 2.8 | 9.7 ± 6.7 | 0.000 |
| HOMA-IR | 1.1 ± 0.69 | 2.4 ± 1.9 | 0.000 |
| Cholesterol (mg/dL) | 204.3 ± 43 | 210.8 ± 40 | 0.145 |
| Triglycerides (mg/dL) | 143.6 ± 75.4 | 206.1 ± 129.4 | 0.000 |
Database expressed in mean ± DE. NAFLD: Non-alcoholic Fatty Liver Disease. BMI: Body Mass Index. WHR: Waist-Hip Ratio. HOMA-IR: Homeostatic Model assessment-Resistance Insulin. Values p < 0.05 were significant.
We found that I148M variant of PNLPA3 is more frequent in Mexican patients with NAFLD than in those without it. Two previous studies by our group support these findings. The first, by Larrieta-Carrasco, et al.,8 was conducted in indigenous Mexican and Mestizo individuals, observing an impressive association between the genetic variant and high levels of ALT. Their findings showed a frequency about 18.7% of elevated ALT levels in indigenous population, being modified by BMI. Therefore, they observed raised ALT levels above 14.4% in normal weight, 19.9% in overweight, and 24.5% in obese individuals. The I148M genotype was significantly associated with elevated ALT levels in indigenous individuals (OR = 3.15, 95% CI: 1.91-5.20; P = 7.1 × 10-6), and this association was confirmed in Mexican-Mestizos (OR = 2.24, 95% CI: 1.50-3.33; P = 8.1 × 10-5).4 The second one, by León-Mimila, et al.,9 assessed 130 participants aged 1859 years with severe obesity (BMI ≥ 40 kg/m2) that underwent bariatric surgery. The aim of their study was associated the genetic variants with genetic risk score for elevated liver fat content and NASH in morbidly obese Mexicans. To make this possible, they genotyped its population for six single-nucleotide polymorphisms (SNPs): rs738409 (PNPLA3), rs12137855 (LYPLAL1), rs780094 and rs1260326 (GCKR), rs2228603 (nCAN) and rs4240624 (PPP1R3B). Consequently, they found after adjusting for age, sex and admixture that PNPLA3, LYPLAL1, GCKR were significantly associated to higher hepatic fat content (PNPLA3, LYPLAL1, GCKR: p < 0.05). Surprisingly, our population had similar results according to PNPLA3 “G” allele (P = 0.011).
Similarly, Xu R, et al. meta-analysis assessed a relationship between the PNPLA3 rs738409 polymorphism and susceptibility to develop NAFLD, including its subtypes SS and NASH. They included twenty-three case-control studies of several countries which involve 6071 NAFLD patients and 10,366 controls. Their findings showed a significant association between the rs738409 polymorphism and NAFLD risk in all genetic models (OR: 3.41; 95% CI: 2.57-4.52; p < 0.00001) as well as with NASH risk (OR: 4.44; 95%CI: 3.39-5.82; p < 0.00001). However, they observed that this susceptibility was not influenced by ethnic groups, ages of subjects or the source of controls. Additionally, they demonstrated that the rs738409 polymorphism was only significantly associated with SS risk in the allele contrast model and had no effect in the other genetic models. Therefore, it could be suggested that the rs738409 polymorphism in PNPLA3 gene confers high cross-ethnicity risk to develop NAFLD and NASH. Likewise, Singal, et al. performed a systematic review of 24 studies, with 9,915 patients, to determine the relationship between PN-PLA3 and liver fibrosis severity, HCC risk as well as prognosis among patients with liver disease.11 They observed that cirrhotic patients had an increased risk to develop HCC (OR: 1.40; 95% CI: 1.12-1.75). This risk was higher in patients with NASH or Alcohol-related cirrhosis (OR: 1.67; 95% CI: 1.27-2.21) than in those with other etiologies of cirrhosis (OR: 1.33; 95% CI: 0.96-1.82). Further, they demonstrated an association between PNPLA3 and fibrosis severity (OR 1.32, 95 % CI: 1.20-1.45), with a consistent increased risk across liver disease etiologies.
How can we explain the high prevalence of the PNP-LA3 polymorphism in Mexican-Americans, Mexican-Indians, and Mexican-Mestizos? We believe that the genetic background of Mexicans is important due to Mexican-Americans are a genetically diverse ethnic group with wide ranges of Native American (NA), European, and African ancestry. Also compared with non-Hispanic European Americans, NA and Hispanic Americans have higher BMI, an increased prevalence of obesity, and a higher incidence of type 2 diabetes (T2D). Several studies have suggested that NA genes are involved in susceptibility to metabolic disorders. For example, Norden-Krichmar, et al.12 found that genetic and cultural environmental, rather than socioeconomic factors, contribute to higher BMI in Native American community. It is well known that excessive BMI is linked to major medical disorders such as diabetes and cardiovascular disease. Martinez-Marignac, et al.13 performed a study in Mexico City in which they assessed, by admixture mapping, the genetic risk of type 2 Diabetes (T2D) development in Mexican population. They characterized the admixture proportions in sample of 286 T2D patients and 275 controls to observe a relationship between ancestry and T2D prevalence in this population. They found that average proportion of NA, European and, West African admixture was 65%, 30% and 5%, respectively. However, the difference between T2D patients and controls was not significant. Further, T2D prevalence showed an association with ancestry, being increased in NA admixture proportion (OR: 1.6; CI 95%: 0.6-4.3). Consequently, it can be assumed that NAFLD may precede the development of T2D.
On the other hand, Garner, et al. studied the frequencies of NAFLD susceptibility SNPs in non-Hispanic white and Hispanic population who attended a clinic in Albuquerque, New Mexico.14 The investigators found that chronic disease indicators were associated with specific alleles in NAFLD susceptibility SNPs but were not the same between non-Hispanic white and Hispanic populations.15 However, they observed that the major allele of PNPLA3 (rs738409) was more frequent in non-Hispanic whites.
In terms of therapeutic targets for NAFLD, the feeding and genetic factors have been recognized as promising treatments. Particularly, herbal extracts or flavonoids consumption, such as quercetin, in individuals with non-favourable genotype GG of PNPLA3. Rojas, et al. performed a study in which elucidated, in an in-vitro model, the role of quercetin and other water soluble-extracts on decreasing lipid accumulation in hepatocytes.15 They demonstrated that quercetin and other natural extracts can reduce the intracellular lipid accumulation and triglycerides levels by the down-regulation of SREBP-1c, PPARγ and ACAT1 expression levels as well as by the increment of PPARα expression. At present, there are not approved therapies for NASH patients due to inconsistent results in clinical trials. However, the treatment suggested by Rojas, et al. could be novel to modify, from lipids metabolism, the natural history of the disease. In fact, in the last issue of Journal of Hepatology, Konerman, et al.16 carried on a review comparing the endpoints and outcomes of main trials. Interestingly, they showed the pioglitazone as the drug with the best results for NASH resolution, hepatic steatosis and fibrosis improvement.
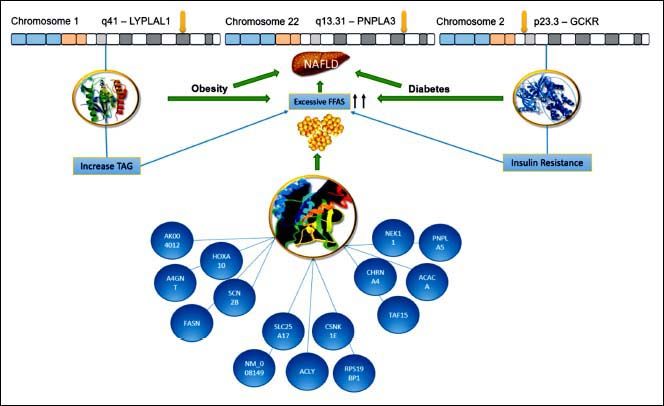
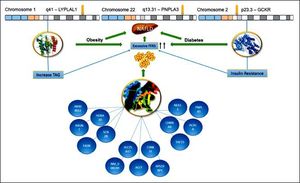
ConclusionPNPLA3 has been the main gene for the development of NAFLD in Mexican population. We suggested (Figure 2) that the enormous interaction among diverse genes, particularly the variants of LYPLAL1 and GCKR and their linkage with several others, could help us to understand the development and progression of the disease. We know that all of these genetic variants are related to the increase of TAG and resistance of insulin, leading to obesity or diabetes. This is of particular importance for Mexican population due to Mexico has the highest prevalence rates of dyslipidemia, obesity and diabetes.17,18 In this regard, Mexico has an overall obesity prevalence of 72.5%, being the second most obese country worldwide.19,20 Further, diabetes is the first cause of death for Mexicans, having a prevalence of 10.4%.21,22 Consequently, the overview is alarming, especially, if we keep in mind that SNP PNPLA3 is very frequent in Mexican population. For this reason, the implementation of health policies is urgent in order to diminish the risk of NAFLD development in these vulnerable inhabitants.
Hypothesized Mexican network showing interaction between the PNPLA3, LYPLAL1 and GCKR. All of them were involved in NAFLD development. The lines represent transcriptional regulation between genes. PNPLA3 gene is located in chromosome 22 and encodes a protein closely related to triglyceride lipase, the main TAG hydrolase in adipose tissue. The 148 M mutation determines a critical aminoacid substitution near the catalytic domain, decreasing the access substrates and PNPLA3 enzimatic activity towards lipids. Additionally, the genetic variant of GCKR, rs780094, reduces the ability to inhibit glucokinase in response of fructose-6-phosphate. It is important to recall that this gene is a glucokinase regulator, thereby, its inhibition results in insulin resistance. Finally, LYPLAL1 has demonstrated a role in consecutive steps of triglyceride breakdown. The combined effects of thes e polyphomisms have been proposed to explain an important role of NAFLD development. LYPLAL1: Lysophospholipase like 1. PNPLA3: patatin-like phospholipase domain containing 3. GCKR: Glucokinase regulatory protein. TAG: Triacylglycerol. FFAs: Free Fatty Acids. NAFLD: Nonalcoholic Fatty Liver Disease.
- •
ALT: Alanine Transaminase.
- •
BMI: Body Mass Index.
- •
NA: Native American.
- •
NAFLD: Non-Alcoholic Fatty liver Disease.
- •
NASH: Non - Alcoholic Steatohepatitis.
- •
PCR: Polymerase Chain Reaction.
- •
PNPLA3: patatin-like phospholipase domain containing 3.
- •
SNPs: Single-Nucleotide Polymorphisms.
- •
T2D: Type 2 diabetes.
- •
TAG: Triacylglycerol.
- •
WHR: Waist-Hip Ratio.
This work was supported by Medica Sur Clinic and Foundation.
Conflicts of InterestThe authors declare no competing professional or financial interests. This study followed the Declaration of Helsinki on medical protocol and the informed consent was obtained from all individual participants.