Introduction and alm.Aegle marmelos is an important traditional herbal medicine used in India. The dietary inclusion of the plant has never exposed earlier for its hepatoprotective activity. This study aimed to investigate the modulator efficacy of dietary inclusion of Aegle marmelos against Cisplatin - induced hepatotoxicity in Wistar albino rats.
Material and methods. Animals were divided into five different groups; Group I was given basal diets only, Group II was fed basal diets with Aegle marmelos in 4% concentration, while Group III was fed basal diets co-administered with Cisplatin. Group IV and V were administered diets containing 2% and 4% Aegle marmelos respectively, for 27 days prior to Cisplatin administration. Cisplatin was administered to the rats for 3 days leads to a reduction in the activities of the antioxidant enzymes like lipid peroxidation (LPO) and endogenous antioxidant systems such as reduced superoxide dismutase (SOD), glutathione (GSH) and catalase in liver homogenate caused to produce the impairment of hepatic functions.
Results. The administration of fruit part of Aegle marmelos to Wistar rats showed a significant fall in the elevated Lipid peroxidation, superoxide dismutase, glutathione and catalase concentration, moreover, it diminished the increased serum level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), acid phosphatase (ACP) and bilirubin.
Conclusions. We can conclude that the hepatoprotective activity of Aegle marmeloswas due to its antioxidant effect as evidenced by increasing activity of antioxidant enzymes with enhanced hepatic function and significantly changed the physiological parameters.
The most common source of acute liver injury is the consumption of synthetic drugs. Today, an average patient deals with more co-morbidity, diagnostic and therapeutic procedures with the potential harm to liver functions as compared to 30 years ago. Cisplatin (cis-diamminedichloroplatinum) is an antitumor drug effective against solid tumors in the treatment of cancer. Cisplatin has shown to exert hepatotoxic effects by one or more common pathogenic mechanism. However, Cisplatin is an effective anticancer drug though several in-vitro and in-vivo studies reported the adverse effect of Cisplatin as both hepatotoxic and nephrotoxic drug.1–4 Drug-induced liver injury (DILI) tends to be more common among certain patients and in specific clinical situations. Therefore, successful prevention requires knowledge of pathogenic mechanisms; it may be liver injury, patient-related risk factors, drug-related risk factors and/or preemptive measures coupled with vigilance and early intervention. Ordinarily, preventive measures include alternative hepatotoxic drugs and correcting risk factors possible by adjusting the dosage, monitoring liver function and vital signs during therapy, in addition avoiding hepatotoxicity drug combinations. Hepatotoxicity is a poisonous effect of some substances, consequently, due to both toxic chemicals and medication effect on the liver. Cancer treatment is not deprived of side effects, moreover, this is one of the major problems related to chemotherapy. Chemoprotective agents are still a new ground of study and likely give up agents with superior selectivity for healthy tissue as well as the aptitude to protect a larger number of tissue types. To organize the side effects accompanied with chemotherapy, many drugs have been developed, including Erythropoietics for anemia (e.g. Epogen, Darbepoetin), Antiemetics for nausea and vomiting (e.g. Ondansetron, Aprepitant), and Immunostimulators for neutropenia (e.g. Pegfilgrastim). Failure to provide adequate chemoprotection throughout regimen may mandate removal, rescheduling of treatment (i.e. Dose delay) and lowering the probability of remedial therapy. In addition, constant toxicity can diminish both short- and long-term superiority of life of cancer survivors.5,6
Cisplatin is a platinum co-ordinated complex widely used as a antineoplastic agent for the treatment of metastatic tumors of the testis, metastatic ovarian tumors, lung cancer, advanced bladder cancer and many other solid tumors.7 The cytotoxic action of the drug is due to its ability to bind with the DNA to form Cisplatin-DNA adducts.8 It has been reported that decreased Glutathione level causes more cytotoxicity.9 Glutathione sulphydryl conjugates so formed after glutathione utilization is transported in an adenosine-tri-phosphate (ATP) dependent manner in human erythrocytes, rat hepatocytes and cardiac cells. Inspite of its beneficial antitumor action, dose related nephrotoxicity and neurotoxicity limit its application in clinical oncology.10 The information on Cisplatin-induced liver injury and its mechanism in causing hepatotoxicity is very less. It is known that the drug accumulates in significant amount in hepatic tissue, particularly when injected in high doses.11 Cisplatin is one of the most potent chemotherapy drugs extensively used in cancer treatment.12 The invention of Cisplatin, cis-[PtCl2 (NH3) 2], [Pt (NH3) 2Cl2], or CDDP), was a cornerstone which triggered the curiosity in Platinum (II) - and other metal-containing compounds as potential anticancer drugs. The aim of anticancer therapies is to provoke tumor cell death. Cisplatin used frequently against wide spectrum of human malignancies.13 Platinum compounds are used for the treatment of a variety of cancers, including testis, esophageal, ovary, bladder, neck and head, small and non-small cell cervical, lung, breast, prostate and stomach cancers as well as lymphomas, neuroblastoma, sarcomas, multiple myeloma, melanoma and mesothelioma.
The clinical use of Cisplatin includes treatment of different types of cancer like sarcoma, cancers of blood vessels, muscles, bones and soft tissue. These types of cancers receive enhanced prognosis and therefore became less life threatening now-a-days.14 Clinical achievements of Cisplatin and its derivatives determined a significant attempt to develop other successful metal-based anti-cancer compounds. Oxidative stress can interrupt standard biological functions spaced out from DNA damage. Cisplatin induces formation of reactive oxygen species (ROS) that generate cell death as reported in recent data. Cell death leads to the simultaneous organization of several pathways; however, the specific pathway depends on the cancer cell. The formation of reactive oxygen species depends on the meditation of Cisplatin and the extent of Spotlight.15 The thiol group (-SH) containing molecules maintains intracellular redox homeostasis. Under certain conditions a thiol group could lead to the development of thiyl radicals that in turn can work together with molecular oxygen, therefore generating ROS.16 There are several metals capable to produce ROS in the body. DNA damages occur due to metal-induced free radicals, while in total metals can inhibit the repair of DNA. The two mechanisms result in patching up inhibition of free radical damage are:
- •
Directly by free radical, replacement of zinc in Zn-finger domains or
- •
Indirectly by lowering the intensity of reduced glutathione.
These capacities moderately explain the absurdity that ROS may be carcinogenic, but furthermore use to treat cancer. These diverse effects may occur at different concentrations and time of exposure. The increased concentration of metals more than normal produces toxicity, probably by generating free radicals. Habitually, each cell maintains equilibrium between antioxidants and free radicals. However, ROS might devastate the reduction capacities of the cell. They persuade lipid peroxidation, depletion of the sulphydryl groups, changed DNA damage, calcium homeostasis and signal transduction pathways, it results in aging and/or cancer. In vivo production of ROS due to Cisplatin therapy is responsible for the severe side effects, including hepatotoxicity, nephrotoxicity etc. Conversely, it gets reduced by the addition of antioxidants. However, accepting the expression of pro-apoptotic proteins and anti-apoptosis proteins; their connection to redox system in the cell provides valuable insight into new approaches for the prevention of Cisplatin side effects devoid of affecting its efficiency.10 Platinum is a poisonous compound regardless of its biological activity. So that the patients receiving this agent, experiencing severe side effects that limit its dose. It is critical to manage Cisplatin-induced toxicity for the accomplishment of cancer chemotherapy. Ordinarily, side effects of platinum rehabilitation include general cell damaging effects; more specifically, hepatotoxicity, nephrotoxicity, neurotoxicity and hearing loss.17,18 Cisplatin-related toxicities are dose-dependent, a surplus of dosage might result in momentous morbidity and/or mortality, however, the frequency of Cisplatin overdose remains unknown. Moreover, general guiding principles do not exist in order to manage a Cisplatin overdose to the date. In addition, no definite antidote exists. However, early-phase clinical trials utilizing a high-dose of Cisplatin and case reports in the overdose surroundings have characterized the clinical features associated with Cisplatin overdoses, prominence some therapeutic strategies for deliberation.18
The medicinal plants contain curative properties and therapeutic values due to the presence of various phytochemical compounds. These traditional medicines are assuming greater importance because of its effectiveness, safety, availability and no side effects. The plant Aegle marmelos Linn available in the state of Chhattisgarh contains variety of medicinal value as shown in figure 1. It is a perennial tree, wild in the sub Himalaya tract, central and South India. This plant is commonly called as ‘Bael’ in Hindi, ‘Vilvam’ in Tamil and ‘Bilva’ in Sanskrit. It belongs to the family Rutaceae. It is known by several other names in the different parts of the country and also outside of country.19 The people of India are much known with this plant as it holds Hindu religious value. The physicochemical studies of Bael fruit exhibits rich nutritional value and it is being used for several years ago. Bael pulp is a rich source of glucose, sugar and fiber. Other nutritive elements of Bael areprotein, fat, minerals, fibers, carbohydrates, calcium, phosphate, potassium, iron, vitamins A, vitamin B1, nicotinic acid, riboflavin, and vitamin C.20 The Bael fruit contains several pharmacological activities like antidyspepsia, antidiarrheal and antidysentery. The fruit is also used as a dietary supplements, to cure intermittent fever, mental disease, hypoglycemic effect, anti-fungal, antimicrobial, analgesic, anti-inflammatory, antipyretic, antidyslipidemic, immunomodulatory activities and many more.21–23 The production of oxidative stress occurs during normal metabolic process in the body as well as induced by a variety of environmental and chemical factor, it causes generation of various reactive free radical, in addition to subsequent change in DNA and lipid profile.24 The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity.25–28 The antioxidants perform through two possible mechanisms in the body, (a) by getting oxidized itself, (b) by creating a protective layer around the active constituents of the material. The antioxidant effect of Aegle marmelos is responsible for hepatoprotective activity as reported earlier.29 The fruit part of Aegle marmelos was selected for the study on the basis of literature of the plant; to determine the modulatory effect of dietary inclusion against Cisplatin-induced hepatotoxicity in experimental animals.
Material and MethodsPlant materialsThe fruit part of Aegle marmelos (Figure 1) was collected from the tribal belt of Bilaspur, Chhattisgarh, India during the month of October 2015. The selection of fruit part of the plant was based on the information collected from the tribal people. Initially, the plant was identified by their vernacular names through consultations with the local people. Consequently, the collected plant was recognized and authenticated by Dr. Sunita Garg, Chief Scientist, Raw Material Herbarium and Museum, Delhi (RHMD), CSIR-NISCAIR, New Delhi, India, accordingly, the voucher specimen (AML-1) was deposited in the herbarium in the Columbia Institute of Pharmacy, Tekari, Raipur, Chhattisgarh, India.
Experimental animalsWistar albino rats selected for the study were 91 - 129 g in weight. The experimental animals obtained from animal house of Columbia Institute of Pharmacy, Tekari, Raipur, Chhattisgarh, India were approved by the Institutional Animal Ethical Committee (IAEC Reg no- CIP/ IAEC/2016-17/080). The IAEC is registered under CPC-SEA, New Delhi with the registration number 1321/PO/ ReBi/S/10/CPCSEA; dated 22/10/2014. The experimental animals were kept in the arrangement of 12 h light/dark cycle and temperature maintained at 25 °C with unrestricted access to food and water ad libitum.
Preparation of dietary inclusionThe basal diet was prepared by mixing 50% skimmed milk, 36% corn starch, 10% groundnut oil and premix of 4% mineral and vitamin.
Grouping of animalsThe experimental rats were divided into five different groups, i.e. Group I (basal diet), Group II (basal diet + 4% Aegle marmalos), Group III (basal diet + Cisplatin), Group IV (2% Aegle marmalos + Cisplatin) and Group V (4% Aegle marmalos + Cisplatin), each group containing six animals. Group IV and Group V received 2% and 4% Aegle marmalos respectively for 27 days. Cisplatin was given to Group III, IV and V for 3 days and the experiment terminated after 30 days.29
Analytical proceduresThe rats were subjected to an overnight fasting and decapitated by cervical dislocation for the assessment of antioxidant activities. The blood was rapidly collected in separate EDTA bottles by direct heart puncture and centrifuged at 3000 RPM for 10 min to separate the plasma. Similarly, the livers were isolated, rinsed in cold saline (0.9% NaCl) and then homogenized in phosphate buffer (pH 6.9). The homogenates were centrifuged at 7500 RPM for 10 min to obtain the clear supernatant. The various biochemical assays such as lipid peroxidation (LPO) and endogenous antioxidant systems including reduced superoxide dismutase [SOD, glutathione (GSH) and catalase were determined by using supernatant].30–32 The physiological parameters such as serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), acid phosphatase (ACP) and bilirubin concentration were examined and calculated by using spectrophotometry method.4
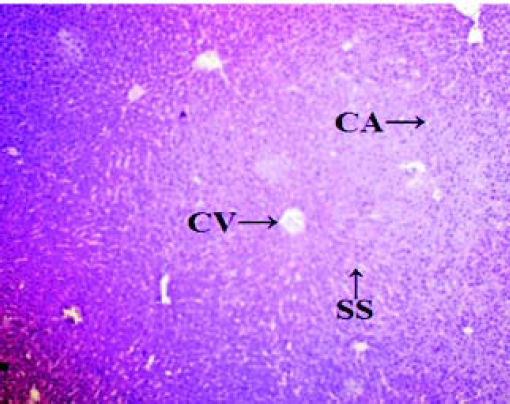
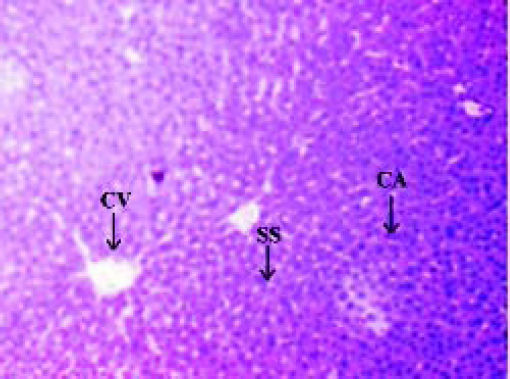
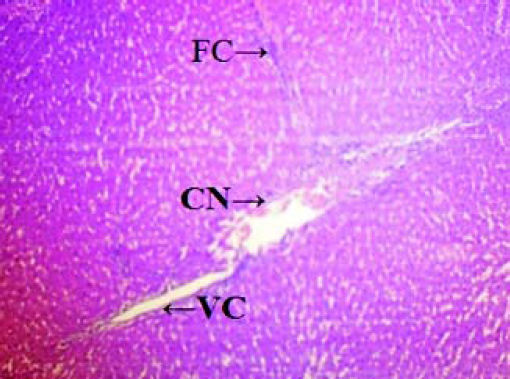
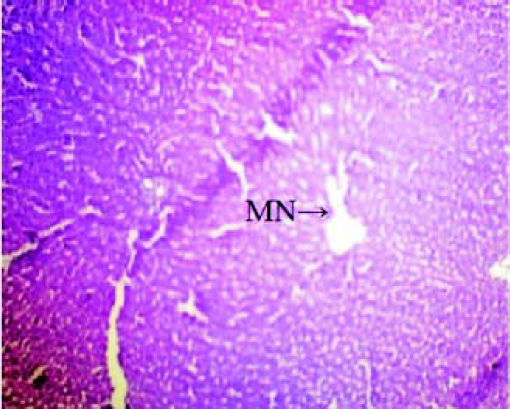
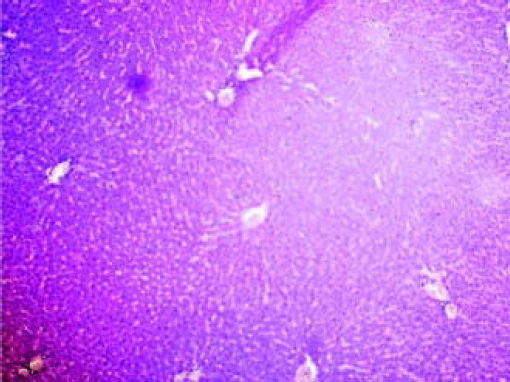
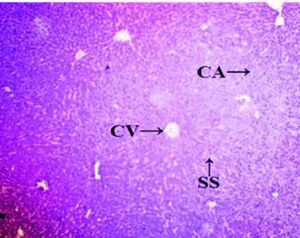
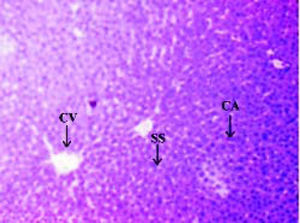
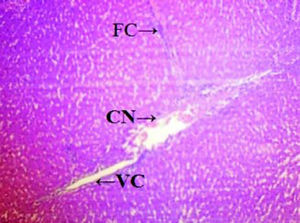
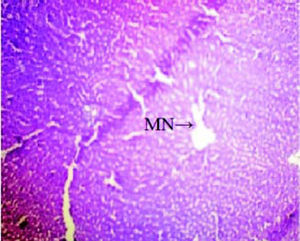
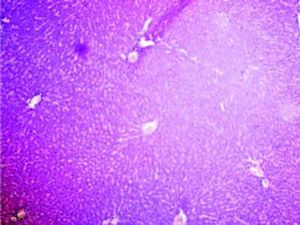
Histopathological analysisThe liver of all experimental animals in each group was dissected out followed by excision and washed with the normal saline. Initially, the materials were fixed at 10% buffered neutral formalin followed by bovine solution and then processed for paraffin embedding using the microtome technique. The sections were taken at 50 μ thicknesses processed in alcohol-xylene series and stained with alum-haematoxylin and eosin. All the sections were examined microscopically for the evaluation of histopathological changes as shown in figures 2-5.
Statistical analysisAll values were expressed as Mean ± SEM. The results were analyzed statistically using one way analysis of variance (ANOVA) followed by Dennett’s test using SPSS software. Values are significantly different at *P < 0.05 when compared with the normal control group.33
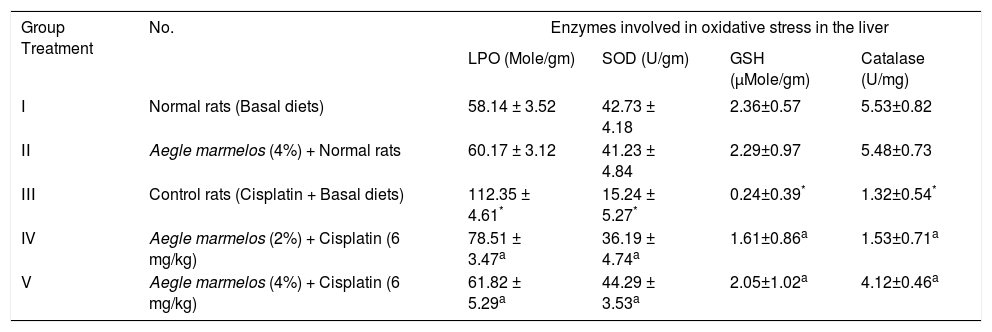
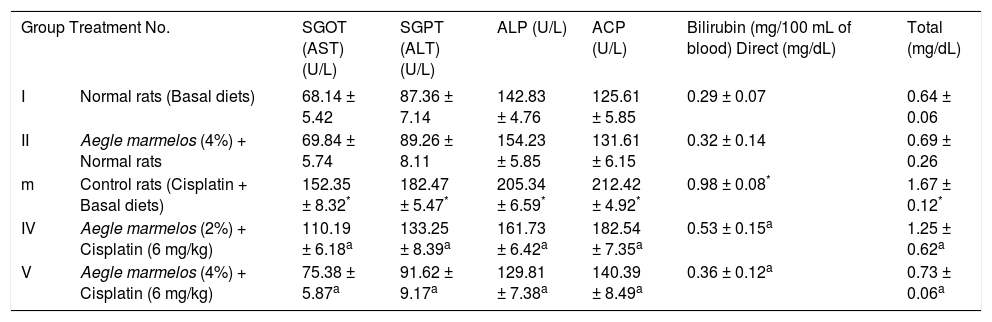
ResultsThe administration of Cisplatin at a dose of 6mg/kg body weight to the rats for 3 days leads to a reduction in the activities of the antioxidant enzymes; lipid peroxidation (LPO) and reduction in the endogenous antioxidant enzymes such as superoxide dismutase (SOD), glutathione (GSH) and catalase in liver homogenate. Cisplatin induced hepatotoxicity was revealed by significant (P < 0.05) elevation of liver damage marker enzymes (super oxide dismutase, catalase and glutathione peroxidase) as shown in table 1. Cisplatin also caused a significant (P < 0.05) alteration in plasma. Conversely, there was a remarkable (P < 0.05) restoration of the antioxidant status coupled with significant (P < 0.05) reduction in the elevated Lipid peroxidation and superoxide dismutase, glutathione and catalase concentration after administration of diets containing Aegle marmelos. The physiological parameters for Cisplatin-induced hepatic damage were determined by serum level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), acid phosphatase (ACP) and bilirubin concentration as shown in table 2. The increased serum level with significant (P < 0.05) alteration was exhibited in groups treated with Cisplatin, which was found to be reduced in groups treated at different concentrations (i.e. 2% and 4%) of Aegle marmelos. However, Aegle marmelos in 4% concentration given to the group II did not affect the Liver functions to both enzymatic and serum levels. The hepatoprotective activity of Aegle marmelos may be due to its antioxidant effect as evidenced by increasing activity of antioxidant enzymes and significantly changed the physiological parameter showing the reduction in increased serum level. Histopathological examination of liver sections of group I and group II showed a normal cellular architecture (CA) with distinct hepatic cells, sinusoidal spaces (SS) and the central vein as shown in figures 2-3 respectively. The liver section of Group III rats exhibited disarrangement of normal hepatocytes with centrilobuler necrosis (CN), vacuolization of the cytoplasm (VC) and fatty changes (FC) (Figure 4). The liver sections of the rats treated with 2% and 4% of Aegle marmelos in group IV (Figure 5) and group V (Figure 6) respectively, revealed signs of protection against Cisplatin intoxication as evident by the presence of normal hepatic cords and absence of necrosis with minimal inflammatory conditions around the central vein.
Effect of diets supplemented with Fruits of Aegle marmelos on oxidative stress induced by Cisplatin in the liver of experimental animals.
| Group Treatment | No. | Enzymes involved in oxidative stress in the liver | |||
|---|---|---|---|---|---|
| LPO (Mole/gm) | SOD (U/gm) | GSH (μMole/gm) | Catalase (U/mg) | ||
| I | Normal rats (Basal diets) | 58.14 ± 3.52 | 42.73 ± 4.18 | 2.36±0.57 | 5.53±0.82 |
| ΙΙ | Aegle marmelos (4%) + Normal rats | 60.17 ± 3.12 | 41.23 ± 4.84 | 2.29±0.97 | 5.48±0.73 |
| ΙΙΙ | Control rats (Cisplatin + Basal diets) | 112.35 ± 4.61* | 15.24 ± 5.27* | 0.24±0.39* | 1.32±0.54* |
| IV | Aegle marmelos (2%) + Cisplatin (6 mg/kg) | 78.51 ± 3.47a | 36.19 ± 4.74a | 1.61±0.86a | 1.53±0.71a |
| V | Aegle marmelos (4%) + Cisplatin (6 mg/kg) | 61.82 ± 5.29a | 44.29 ± 3.53a | 2.05±1.02a | 4.12±0.46a |
Values are expressed as Mean ± SEM, data were statistically evaluated by one way analysis of variance (ANOVA) followed by Dunnett’s test (n = 6) in each group.
Effect of diets supplemented with Aegle marmelos on liver function test for different parameters in animals treated with Cisplatin.
| Group Treatment No. | SGOT (AST) (U/L) | SGPT (ALT) (U/L) | ALP (U/L) | ACP (U/L) | Bilirubin (mg/100 mL of blood) Direct (mg/dL) | Total (mg/dL) | |
|---|---|---|---|---|---|---|---|
| I | Normal rats (Basal diets) | 68.14 ± 5.42 | 87.36 ± 7.14 | 142.83 ± 4.76 | 125.61 ± 5.85 | 0.29 ± 0.07 | 0.64 ± 0.06 |
| II | Aegle marmelos (4%) + Normal rats | 69.84 ± 5.74 | 89.26 ± 8.11 | 154.23 ± 5.85 | 131.61 ± 6.15 | 0.32 ± 0.14 | 0.69 ± 0.26 |
| m | Control rats (Cisplatin + Basal diets) | 152.35 ± 8.32* | 182.47 ± 5.47* | 205.34 ± 6.59* | 212.42 ± 4.92* | 0.98 ± 0.08* | 1.67 ± 0.12* |
| IV | Aegle marmelos (2%) + Cisplatin (6 mg/kg) | 110.19 ± 6.18a | 133.25 ± 8.39a | 161.73 ± 6.42a | 182.54 ± 7.35a | 0.53 ± 0.15a | 1.25 ± 0.62a |
| V | Aegle marmelos (4%) + Cisplatin (6 mg/kg) | 75.38 ± 5.87a | 91.62 ± 9.17a | 129.81 ± 7.38a | 140.39 ± 8.49a | 0.36 ± 0.12a | 0.73 ± 0.06a |
Values are expressed as mean ± SEM, n = 6 in each group.
Chemotherapy is a well-known and effective strategy for the treatment of cancer patients. The treatment involves usage of the chemotherapeutic agents which have limitations due to the several side effects related to these drugs. These limitations are always trying to overcome with the combination of different drugs,which contain higher molecular mechanism and less toxicity, therefore cancer patients show superior survival rates. Cisplatin is the most effective and active cytotoxic chemoprotective agent used in the chemotherapy of cancer with the mechanism of induced mitochondrial dysfunctions and subsequent tissue damages. The fruits of Aegle marmelos are commonly used as an antioxidant to overcome and suppress the hepatotoxicity. The recent studies have shown that dietary inclusion of Aegle marmelos as a chemopreventive agent with antioxidative activity can protect against cisplatin-DILI.34 Cisplatin increases lipid peroxidation and alters the thiol position of the tissue with associated alterations in the enzymatic antioxidants. Cisplatin administration leads to a considerable decrease in the level of glutathione and glutathione reductase, conversely, increases the level of glutathione peroxidase and catalase. The modification of enzymatic antioxidant status with a rise in lipid peroxidation indicates that these enzymes play an important role in combating oxidative stress induced by free radicals.35 The results of the study confirmed that Cisplatin at a dose of 6 mg/kg body weight produced significant (P < 0.05) elevation of liver damage marker enzymes (super-oxidase dismutase, catalase and glutathione peroxidase). However, the antioxidant effect of Aegle marmelosfruits as evidenced by increasing activity of antioxidant enzymes, moreover, significantly changed these physiological parameters. Histological examination of the liver sections revealed that the hepatotoxin intoxication disturbed the normal liver architecture of the rats. However, Aegle marmelos retained the normal cellular architecture in the sections obtained from the Cisplatin-induced hepatotoxic rats as compared to those of the normal control rats. Cisplatin-induced hepatotoxicity can be evaluated by the increased serum level of ALT, AST, ALP, ACP and bilirubin concentration as shown in table 2. The different concentrations of Aegle marmelos (2% and 4%) was employed in the study, it reduced the increased serum level of the Cisplatin-induced hepatotoxic rats. The increased serum level after Cisplatin administration is due to the reaction between nitric oxide and superoxide anion which forms a radical, peroxynitrite. The process was further continued to oxidize the free radicals, it results to damage of cellular structures, moreover,itcauses toxic oxidative damage of lipid components, cell components including proteins, lipids and nucleic acids. Histological studies and determination of different physiological parameters revealed that Aegle marmelos fruit played significant role in the protection of Cisplatin-induced hepatotoxicity. It is to be confirmed that the fruit part of Aegle marmelos possesses protective effect and modulatory effect of dietary inclusion against Cisplatin-induced hepatotoxicity in Wistar rats.
ConclusionIt can be concluded from the study that Aegle marmelos possess hepatoprotective activity due to the improvement in the antioxidant status and oxidative stress level of Cisplatin-induced hepatotoxic rats. Consequently, dietary inclusion of Aegle marmelos may be an economical strategy in the management of acute hepatotoxicity or Cisplatin-induced liver damage. The present work may be helpful for the researchers, physicians and medical scientific societies to overcome Cisplatin related problems. The research findings may be beneficial for the further studies of Aegle marmelos related to its pharmacognostical and pharmacological evaluations.
AcknowledgementAuthors are thankful to the authority of the Columbia Institute of Pharmacy, Tekari, Raipur, Chhattisgarh, India for laboratory facilities and Dr. Sunita Garg, Chief Scientist, Raw Material Herbarium and Museum, Delhi (RHMD), CSIR-NISCAIR, New Delhi, India for identification of the plant.
Conflicts of InterestThere is no conflict of interest in the present work.