Microlithiasis is the underlying cause in a significant proportion of patients with ‘idiopathic’ acute pancreatitis. The mechanism appears to be a relative deficiency of phosphatidylcholine in bile, with fast and extensive cholesterol crystallization as a result. Diagnosis of microlithiasis by microscopic detection of cholesterol crystals in bile is important and should lead to appropriate therapy (cholecystectomy, endoscopic sphincterotomy or ursodeoxycholic acid maintenance therapy).
Abbreviations:
CCK, cholecystokinin
MDR3, multidrug resistance protein 3
ERCP, endoscopic retrograde cholangiopancreatography
UDCA, ursodeoxycholic acid
Acute pancreatitis is often a severe disease with considerable morbidity and mortality (10-15%).1 Gallstones or alcohol abuse are the most frequent causes. Other well-known causes are hyperlipidemia, hypercalcaemia, hyperparathyroidism, auto-immune disorders, collagen disorders, surgery, trauma, viral infection, end-stage renal failure and drugs. The annual incidence of acute pancreatitis in gallstone patients has been estimated to be 0.31%. According to recent data from the National Information System on Hospital Care, the incidence of acute pancreatitis in the Netherlands has increased by 30% in the period 1985-1995.2 In most patients with acute biliary pancreatitis, gallbladder stones can be detected by transabdominal ultrasonography. Furthermore, gallbladder sludge is a frequent finding during ultrasonography in these patients.3,4 In recent years, several studies have reported that sludge or microlithiasis may also induce acute pancreatitis in the absence of macroscopic stones.5-7
The terms microlithiasis and sludge are often used interchangeably. Nevertheless, in this review we will refer to microlithiasis as the presence of cholesterol crystals in bile, in the absence of macroscopic stones. We define sludge by the presence of low-level echoes that layer in the dependent portion of the gallbladder without acoustic shadowing on ultrasonography.8,9 Sludge consists of cholesterol monohydrate crystals, calcium bilirubinate granules, calcium carbonate salts or small gallstones (< 2 mm), in the gallbladder mostly embedded in strands of mucus.8-10
In 10-40% of patients with acute pancreatitis, no cause can be found after initial diagnostic evaluation (acute idiopathic pancreatitis). More extensive investigations may detect an underlying cause in the majority of these patients. Microlithiasis, sludge, sphincter of Oddi dysfunction, anatomic abnormalities of the pancreas and gene mutations have been identified as the most frequent causes in these patients.5,6,11-16 In this review we will discuss the role of cholesterol microlithiasis as a potential cause of acute ‘idiopathic’ pancreatitis.
Pathogenesis of cholesterol microlithiasisAlthough the pathogenesis of cholesterol microlithiasis is similar to that of cholesterol gallstones, there are distinct differences. Rapid precipitation of crystals from cholesterol supersaturated bile is the key factor in cholesterol microlithiasis. Fracchia et al compared the bile composition of patients with either macroscopic gallbladder stones or with only gallbladder microlithiasis. In bile, aspirated from the duodenum after intravenous infusion of the cholecystokinin (CCK) analogue cerulitide, the biliary lipids were determined. The authors found that patients with gallbladder microlithiasis had significantly lower amounts of phosphatidylcholine in their biles compared to patients with macroscopic gallbladder stones.17 Similarly, Rosmorduc et al recently showed that rapid cholesterol crystallization in hepatic bile was associated with low biliary phosphatidylcholine concentrations.18 This relative phosphatidylcholine deficiency was due to missense mutations in the multidrug resistance protein 3 (MDR3) gene.18 The MDR3 gene encodes for a phosphatidylcholine translocator protein at the canalicular membrane of the hepatocyte, which facilitates the transport of phosphatidylcholine to canalicular bile.19,20 Interestingly, these authors reported in a subsequent study that some cases of acute pancreatitis caused by microlithiasis are associated with point mutations in the MDR3 gene.21 Apparently, a relative phosphatidylcholine deficiency in bile is related to rapid cholesterol crystallization, microlithiasis, and risk of acute pancreatitis.
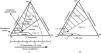
The findings of the above-mentioned studies can be explained by the physical-chemical properties of biliary lipids (i.e. cholesterol, phosphatidylcholine and bile salts) in an aqueous environment, such as bile. Cholesterol is poorly soluble in an aqueous environment, and is solubilized in bile in mixed micelles by bile salts and phosphatidylcholine. In case of cholesterol supersaturation, excess cholesterol may be solubilized in vesicles together with phosphatidylcholine22,23 or may precipitate as solid cholesterol crystals. The studies of Wang & Carey24 have revealed the importance of the relative amounts of bile salts vs phosphatidylcholine in the system for crystallization behavior (Figure. 1A). Based on these data, the equilibrium bile salt-phosphatidylcholine-cholesterol ternary phase diagram (Figure 1A):24 is assumed to contain a bottom one-phase zone (only micelles), a left two-phase (micelles and cholesterol crystals-containing) zone, a central three-phase (micelles, vesicles and cholesterol crystals-containing) zone and a right two-phase (micelles and vesicles-containing) zone. In case of excess phospholipids (high phosphatidylcholine/(bile salts + phosphatidylcholine) molar ratios), solid cholesterol crystals do not occur, and cholesterol is mainly solubilized in vesicular phases. In case of lower amounts of phosphatidylcholine, crystal precipitation proceeds at slow rates (with predominant formation of mature cholesterol monohydrate crystals), and large amounts of cholesterol are solubilized in vesicles together with phosphatidylcholine. In case of excess bile salts (phos-phatidylcholine/(bile salts + phosphatidylcholine) molar ratios <˜0.2), crystals precipitate at fast rates. In addition to mature rhomboid cholesterol monohydrate crystals, various intermediate non-plate-like cholesterol crystals (needles, arcs, tubules, spirals) can be detected by microscopy under these circumstances, probably related to the fast crystallization process.25-27 In contrast to usual cholesterol gallstone patients, bile composition of patients with microlithiasis plots in this left two-phase zone, with fast and extensive crystallization as a result (Figure 1A).
A) Equilibrium taurocholate-phosphatidylcholine-cholesterol phase diagram.24 The components are expressed in mol percent. Depicted are a one-phase (micellar) zone at the bottom, a left two-phase zone (containing micelles and crystals), a central three-phase zone (containing micelles, vesicles and crystals) and a right two-phase zone (containing micelles and vesicles). Hatched areas indicate cholesterol crystal-containing zones. Phosphatidylcholine/(taurocholate + phosphatidylcholine) molar ratios are also given at the bottom axis. Any line connecting the bottom axis with the top of the triangle (100% cholesterol) represents identical phosphatidylcholine/(taurocholate + phosphatidylcholine) molar ratios. For example, for all model biles plotting on the interrupted line, phosphatidylcholine/(taurocholate + phosphatidylcholine) molar ratio is 0.8. In usual cholesterol gallstone patients, bile composition plots in the central three-phase zone with phosphatidylcholine/(bile salts + phos-phatidylcholine) molar ratio ≈ 0.25 (•). Bile of microlithiasis patients plots in the left two-phase zone, since it contains only small amounts of phosphatidylcholine (o). B) Ternary phase diagram with the hydrophilic bile salt tauroursodeoxycholic acid. The right two-phase zone has expanded and shifted to the left with the result that solid cholesterol crystal containing zones (hatched areas) are diminished. Bile composition of UDCA treated patients plots in this zone and crystallization is inhibited (•). PC, phosphatidylcholine.
Hypersecretion of gallbladder mucin is also strongly associated with cholesterol crystallization and microlithiasis.9,28-31 Mucin may enhance cholesterol crystallization by offering low affinity binding sites for phosphatidylcholine and cholesterol.32 On the other hand, mucin may increase bile viscosity leading to the formation of a gel matrix which can entrap cholesterol crystals in the gallbladder.33
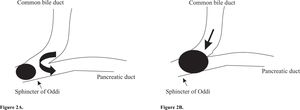
Microlithiasis and acute pancreatitisControversy exists on how microlithiasis may cause acute pancreatitis. Pancreatic duct outflow obstruction appears the primary event in the origin of acute pancreatitis. In a recent study from our group, we found that acute biliary pancreatitis was associated with the presence of both sludge and small stones (≤ 5 mm) in the gallbladder and common bile duct.4 A small gallstone may obstruct the sphincter of Oddi as depicted in figure 2. Microlithiasis may instead cause a functional obstruction at the sphincter of Oddi by inducing papillitis, papillary spasm or papillary stenosis.34-36 The obstruction is followed by reflux of bile, or biliary-pancreatic secretions, into the pancreatic duct at relatively high pressure (common channel hypothesis).37 Cholesterol crystals, and possibly hydrophobic bile salts, may subsequently induce a common pathway of pancreatic duct injury with release of activated pancreatic enzymes into the glandular interstitium, thus triggering the release of cytokines and a bout of acute pancreatitis.38,39 The role of hydrophobic bile salts in the pathogenesis of acute pancreatitis was recently studied by Kim et al. These bile salts impaired Ca2+ signaling in pancreatic acinar cells and activated inflammatory-associated signals (c-Jun amino-terminal kinases and NF-κB) leading to increased cell death.38 Interestingly, these bile salts were shown to be transported into the acinar cells by the transporters Na+-taurocholate co-transporting polypeptide (NTCP) and organic anion transporting polypeptide 1 (OATP1) in the luminal respectively basolateral membranes of the pancreatic cell.38 Previously, the presence of these transporters was thought to be restricted to hepatocytes and kidney cells (OATP1). Under physiologic conditions these transporters may function to clear small amounts of bile salts that reflux into the terminal acini. In case of massive reflux, large amounts of bile salts are present inside the lumen of the terminal acini and within the pancreatic cells. The pancreatic acinar cells’ efflux mechanisms for these bile salts may then be overwhelmed. These circumstances initiate the final cascade, leading to cell death and acute pancreatitis. Pharmacological inhibition of bile salt uptake might prevent development of acute pancreatitis in this situation.
Schematic graph representing the junction of the biliary and pancreatic tree. A) A small gallstone causes obstruction at the sphincter of Oddi. This is followed by reflux of bile containing cholesterol crystals into the pancreatic duct, inducing acute pancreatitis. B) A larger gallstone obstructs both the common bile duct and pancreatic duct. No reflux occurs into the pancreatic duct, but there is obstructive jaundice.
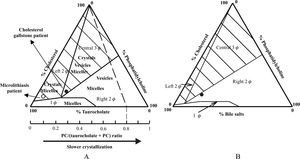
Cholesterol microlithiasis is defined by the presence of typical plate-like rectangular cholesterol monohydrate crystals or intermediate non-plate-like (presumably anhydrous) crystals such as needles, arcs, tubules or spirals in the bile (Figure 3). The gold standard for the diagnosis cholesterol microlithiasis is polarizing light microscopic examination of bile obtained by endoscopic retrograde cholangiopancreatography (ERCP).40 Polarizing light microscopy of aspirated duodenal contents after intravenous CCK infusion is an acceptable alternative.41 Microscopy of the pellet obtained by centrifugation (10 min. 4000 g) may improve the yield.
A) Microscopy of bile of a usual cholesterol gallstone patient containing an aggregation of typical plate-like rectangular cholesterol monohydrate crystals. The small irregular aggregates are calcium bilirubinate granules. B) Microscopy of bile of a microlithiasis patient containing typical cholesterol monohydrate crystals but also many intermediate non-plate like (presumably anhydrous) crystals such as needles, arcs, tubules and spirals (courtesy of Prof. Dr. Piero Portincasa).
Few studies have reported on the risk of acute pancreatitis in patients harboring cholesterol microlithiasis in their gallbladders. However, an association was found between the presence of gallbladder sludge and acute pancreatitis in two prospective studies, although the patient numbers were relatively small.42,43 On the other hand, several risk factors for the development of microlithiasis or sludge have been identified. Pregnancy, rapid weight loss, gastrectomy and octreotide treatment are associated with the development of sludge.44-47 Critically ill patients, or those on total parenteral nutrition, also exhibit a high risk of developing sludge. The latter form of sludge however, consists mostly of calcium bilirubinate granules.47-49
Treatment and PreventionTreatment of patients in the early phase of acute pancreatitis caused by microlithiasis is not essentially different from that in patients suffering from pancreatitis induced by macroscopic gallstones. Intensive supportive care with special attention to volume status and infection is the primary goal. Appropriate pain management and early enteral refeeding are also very important.50,51 Early ERCP with sphincterotomy is required in patients with evident biliary obstruction or cholangitis, and probably in the early phase of severe pancreatitis.52-54 One should realize that the absence of macroscopic stones in the common bile duct during ERCP does not exclude the presence of microlithiasis. Hence, endoscopic sphincterotomy is justified in these patients, even when no bile duct stones are visualized. Surgery is indicated when infection of pancreatic necrosis is suspected by persistent multi-organ failure or proven by fine needle aspiration (FNA) or (peri-)pancreatic air collections on contrast-enhanced computer tomography (CT).55 Ultrasonographic or CT guided drainage may be an appropriate alternative for surgery in some patients.56,57
Patients who have suffered from acute pancreatitis induced by cholesterol microlithiasis are likely to develop recurrent pancreatitis if left untreated. Cholecystectomy is the treatment of choice in patients with a gallbladder in situ and without contraindications for abdominal surgery. Cholecystectomy decreases recurrence rates by decreasing the residence time of bile in the biliary tree, thus allowing less time for nucleation of cholesterol crystals from supersaturated bile.6 Also, hepatic bile is 4-5 fold less concentrated compared to gallbladder bile, with much slower crystallization as a result.58 For patients at high surgical risk, endoscopic sphincterotomy may be an appropriate alternative.59,60 Sphincterotomy decreases recurrence rates by promoting pancreatic and common bile duct outflow.7 Maintenance therapy with ursodeoxycholic acid (UDCA) is reserved for patients with recurrent pancreatitis despite previous cholecystectomy or endoscopic sphincterotomy, or patients with contraindications to surgical and endoscopic treatment.6,7 These therapeutic consequences point to the importance of considering microlithiasis as a potential underlying cause in patients with ‘idiopathic’ acute pancreatitis. In patients with missense mutations in the MDR3 gene, UDCA also decreases recurrence of biliary symptoms during long-term follow-up.18 The mechanism is decreased cholesterol secretion into bile and prolonged crystal nucleation time during treatment with this hydrophilic bile salt.61,62 UDCA reduces cholesterol crystallization by changing the physical-chemical properties of bile. During UDCA treatment, an expansion of the (vesicles and micelles-containing) right two-phase zone in the ternary phase diagram is induced, with the result that formation of solid crystals is inhibited24(Figure 1B).
AcknowledgementsThe authors would like to thank Hein G. Gooszen MD, PhD for helpful discussion.
This study was supported by the “Dutch Digestive Foundation” (“Maag Lever Darm Stichting”).