Mexico is considered a virtually free region of cystic echinococcosis. Almost all case reports within the country involve immigrants or traveling patients. This manuscript presents a Mexican-native human echinococcosis that developed in the setting described below. Review of current evidence suggests that this infection has been underestimated.
Human cystic echinococcosis (HCE) is a well known parasitic disease caused by Echinococcus granulosus that mainly affects the liver. HCE presents an endemic distribution whose effects in Mediterranean Europe, Northern Africa, Asia and South America, are related to significant morbidity and mortality.1 Some areas in Peru have prevalences that reach 5 to 10% of the population.2,3 Although it has been more than sixty years since the first patient with a native echinococcosis was identified in Mexico,4 this is still considered a rare disease given that only ten autochthonous cases have been reported.5-10
Developing HCE requires a very particular setting in which human activity plays the main role. In the parasite domestic cycle, the dog is the definitive host. They harbour the adult tapeworm which produces infective eggs. Dog feces contaminate grass and water that are eaten by sheep, swine or cattle. These mammals develop mature cysts in the liver and lungs (intermediate hosts). In order for the parasite to complete its life cycle, it requires that a human feeds a dog with infected livestock viscera. This allows for the parasite to reenter the canine intestine. Humans are considered accidental hosts, and become infected by their dogs through fecal-oral transmission, that can be carried out either by ingestion of contaminated food or, more frequently, by direct contact between the human and the canine.
This disease usually presents itself as a tender, slow growing, abdominal mass, sometimes associated with fever or epigastric pain. Less than ten percent of patients have jaundice when they seek medical attention.11,12 Diagnosis of human echinococcosis remains highly dependent on imaging techniques (e.g. ultrasound, computed tomography and magnetic resonance) that show space-occupying cysts with different stages of the metacestode infection. Current gold standard for non-invasive diagnosis is serology that detects IgG antibodies against the lipoproteinic antigen B and antigen 5 of Echinococcus granulosus. Unfortunately, it cross-reacts with other taeniid species, that often infect the same risk group of patients.13,14
We present a female patient with a Mexican-acquired disease. This is the eleventh case report in Mexico. No other has presented in this country with obstructive jaundice as the single clinical sign. Patient anonymity is kept, no other ethical issues are involved.
Case ReportA 38-year-old Mexican female with obstructive jaundice was referred to our third-level hospital. We had to evaluate a surgical therapy of a possible main bile duct cyst.
This female completed high school and lived with her family in a small rural town between Mexico City and Toluca (capital city of Mexico City’s neighboring state). In her house, there were poor hygienic habits. Water was acquired from a well and drank without being boiled. House-cultivated vegetables were not washed before meals and there was no sewage system in the town, resulting in ground defecation. Her husband worked as a shepherd. Once a week they killed sheep to cook a stew called “barbacoa” and dogs were feed sheep viscera.
Her chief complain started in June, 2007 with mild whole-body pruritus that persisted throughout one year. In June 2008, pruritus increased and a mild left lower quadrant abdominal pain was perceived. During the final days of June 2008, jaundice, coluria and acholia developed and she sought medical attention. No fever or weight loss had taken place.
In September, 2008, a primary care physician requested laboratory exams and abdominal ultrasonography which showed a total bilirubin level of 2.9 mg/dL, direct bilirubin of 2.5 mg/dL and alkaline phosphatase of 1044 IU/L, as well as a large hepatic cyst next to the main bile duct with minimal bile duct dilation. An abdominal computed tomography showed a large right lobe hepatic cyst that compressed bile ducts and blood vessels. The patient was referred to our hospital.
In October of that year, she was admitted. Jaundice and a 5 cm liver enlargement were evident. A magnetic resonance colangiopancreatography discarded bile duct involvement and showed a 12 cm simple right hepatic lobe cyst without septums.
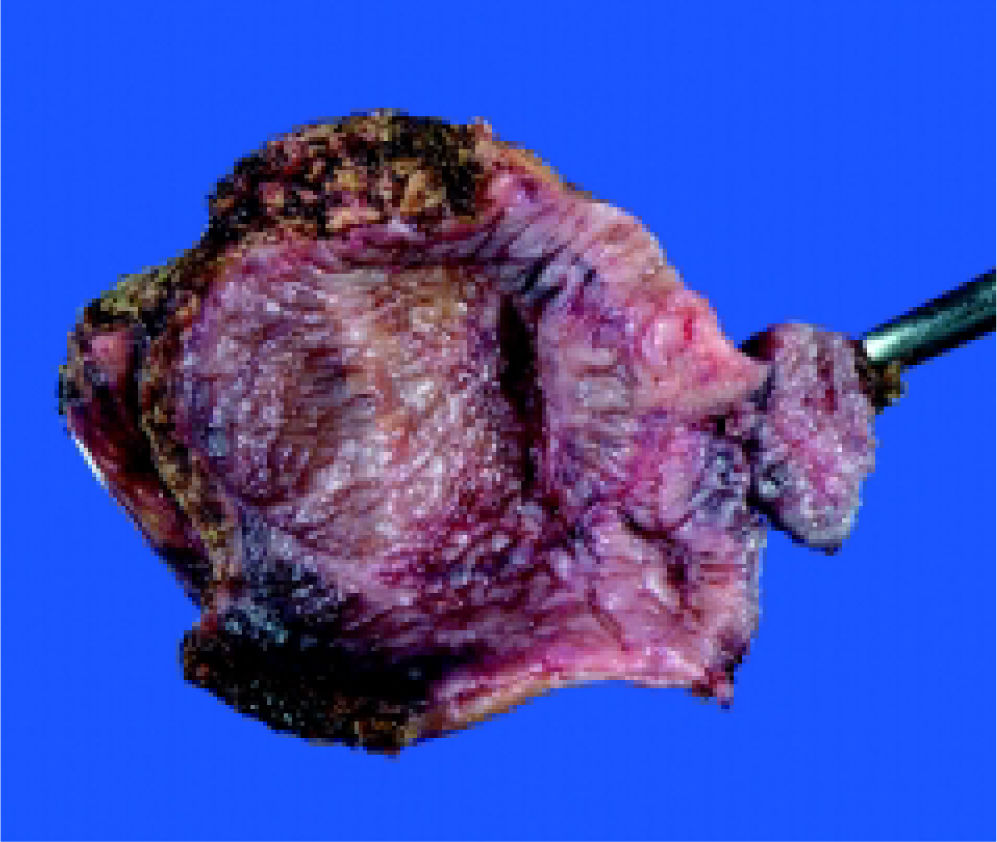
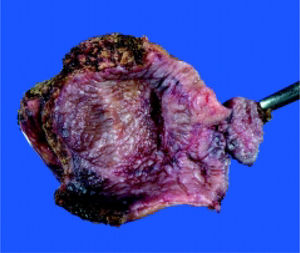
On October 27th, 2008, fenestration of the apparently simple hepatic cyst was performed. During surgery, the cyst was opened and 1,000 cc of a white gelatinous material and two yellow septums were extracted (Figure 1). No daughter cysts were present. Cyst and gallbladder were resected (Figure 2) and no bile leakage was evident during a transsurgical cholecystography. Surgery was completed without any incidents or evidence of anaphylactic shock.
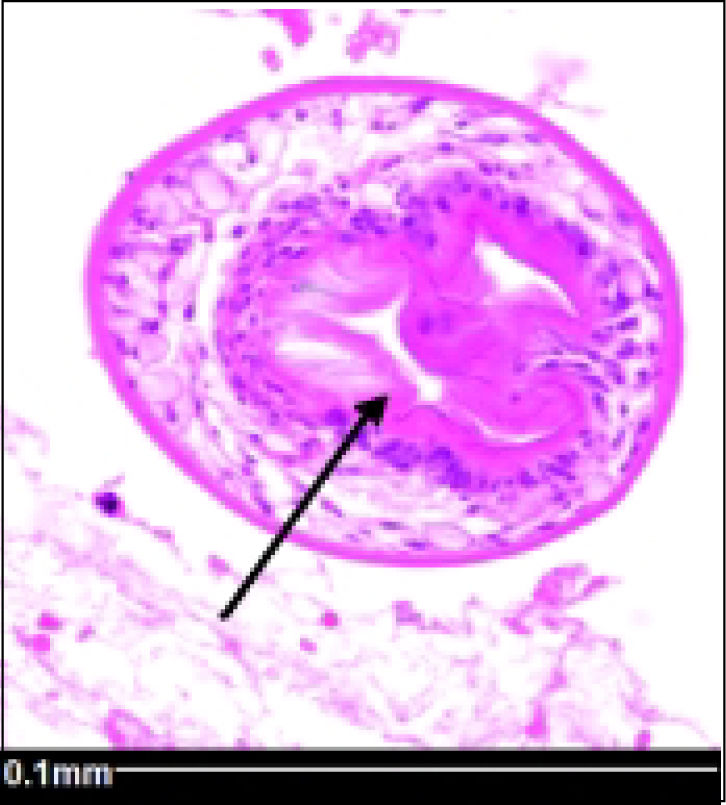
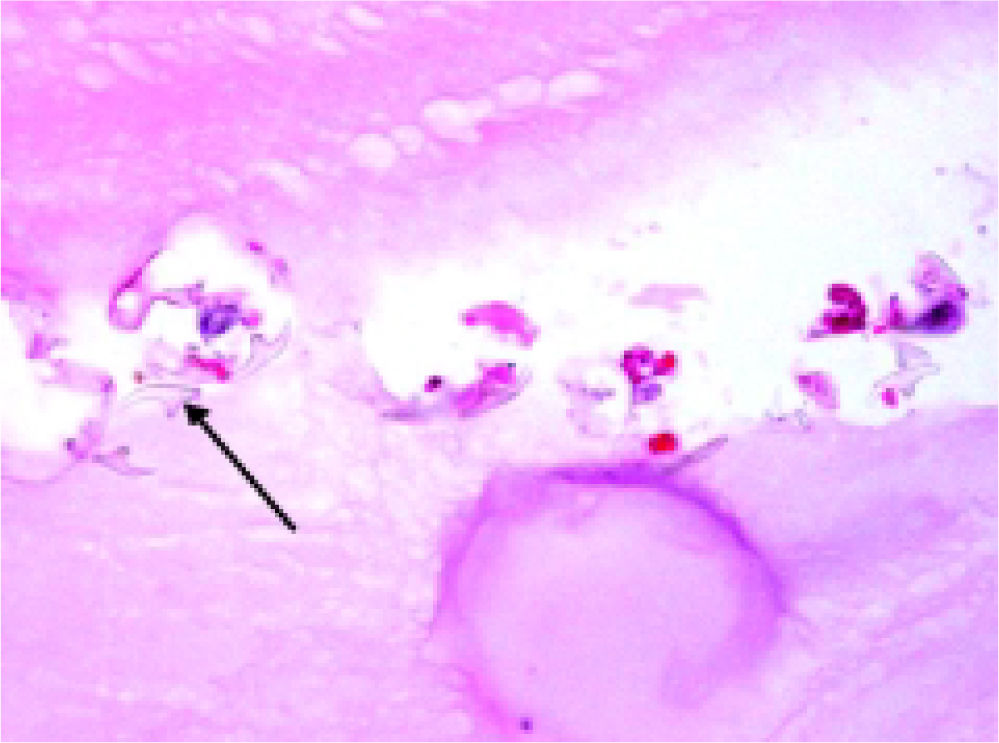
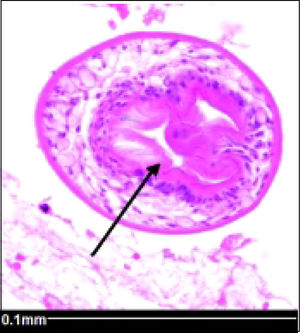
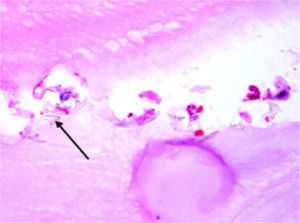
Histopathology reported an irregular hepatic cyst with multiple Echinococcus cysts as well as protoscoleces in the germinal layer (Figure 3 and Figure 4).
Twice a day, during four weeks, the patient received 400 mg albendazol, without complications. His follow up will be at the Infectious Diseases Clinic.
DiscussionHuman echinococcosis studies in Mexico have been published since 1880, but almost all case series involve immigrant or traveling patients.15 This document presents the eleventh case report of a Mexican-acquired human echinococcosis, a virtually free region of this disease. This patient’s living habits show the most common scenario for one to become infected. Until now, this is the only case of obstructive jaundice recorded while seeking medical attention. Neither clinical presentation nor magnetic resonance imaging oriented for this disease and diagnosis was performed until the histopathological report became available.
The patient presented two of the five risk factors exposed by El-Malki, et al.11 for developing deep abdominal complications (preoperative complication and a thick pericyst). Since the diagnosis of HCE was unclear before and during surgery, no preoperative albendazol was administered and the standard “PAIR” procedure (aspiration, infusion of scolicidal agents and reaspiration) was practiced. The operation was performed in a high volume hepatic surgery reference center, ability of surgeons may have contributed to the absence of complications.
A review of current evidence showed that some swine in the State of Morelos were infected by Echinococcus granulosus genotype G1, a serotype that is usually found in sheep and is related to a high level of infectivity in humans.16 Apparently, infected swine and sheep are not exclusive to a specific town, but rather to the central region of Mexico. Isolation of the parasite has been accomplished in several states within Mexico (Morelos, Zacatecas, Oaxaca, and State of Mexico).7,16-19 Two historical epidemiological studies in slaughterhouses during 1990s showed prevalences of 0.27% to 3.1%. The first one limited its research on pigs that are often infected by genotype G7 that is not pathogenic to humans. The second study found 188 out of 2,873 infected pigs and only two out of 3,079 sheep infected. A serological study using passive hemaglutination for risk persons (mainly veterinarians and shepherds) in three states (Guanajuato, Jalisco and Queretaro) showed a 15% prevalence for seropositivity to Echinococcus granulosus,19 but results should be taken with caution given the 9 to 15 percent of the test cross-reactivity with Teania species.13 After one diagnosed patient, a community-based study was performed in the State of Mexico.20 Three out of 401 persons (including index case) were diagnosed by sonography, meaning 0.75% prevalence. Only index case had symptoms and a positive serologic test. During the last five years, five out of the eleven patients with Mexican-acquired echinococcosis were diagnosed.8-10,20 As reports of HCE in Mexico are not reliable, there is a good chance that many cases have been operated without being reported.
In conclusion, although native hydatidosis is a “sporadic disease” in Mexico, as reported by the World Health Organization,21 it cannot be discarded. There is enough evidence to support that some Mexican swine and sheep are infected. A low but still relevant prevalence on a high risk community has been demonstrated. Available information suggests that Mexican-native human echinococcosis is underestimated.