Background: Insulin resistance plays an important role in the pathogenesis of NAFLD. Pharmacological treatment of patients with NAFLD is still evolving. Insulin sensitizing drugs like metformin may be effective in these patients. Twenty five adult patients with NAFLD who did not achieve normalization of alanine transaminases (ALT) after 6 months of lifestyle interventions and UDCA were treated with metformin 500mg tid for 6 months. Insulin resistance was determined by HOMA-IR. Liver function tests were done monthly and patients were defined having no response, partial response or complete biochemical response depending on the change in ALT. Results were compared with 25 patients with NAFLD from the same cohort treated only with lifestyle interventions (disease controls). Results: Thirteen (52%) patients had class III (n = 5) or class IV (n = 8) disease amounting to histological NASH. Of these 13 patients none had severe inflammation and none had stage 4 fibrosis (cirrhosis). All 25 patients with NAFLD had insulin resistance in comparison to healthy controls. In comparison to disease controls (127.5 ± 41.8 vs. 118 ± 21.6 p = NS), all patients treated with metformin had partial biochemical response (mean ALT 122.2 ± 26.8 vs 74.3 ± 4.2 p < 0.01) and 14 (56%) of them achieved complete normalization of ALT. Conclusions: Metformin is effective to achieve biochemical response in patients with NAFLD who do not respond to lifestyle interventions and UDCA.
Abbreviations
Nonalcoholic fatty liver disease - NAFLD
nonalcoholic steatohepatitis - NASH
insulin resistance -IR
ursodeoxycholic acid - UDCA
alanine transaminase - ALT
hepatitis B surface antigen -HBsAg
anti hepatitis C virus antbodies - anti HCV
anti-nuclear antibody - ANA
anti-smooth muscle antibody - ASMA
anti-liver kidney microsomal antibody - LKM
anti-mitochondrial antibody - AMA
body mass index -BMI
world health organization – WHO
fasting plasma glucose - FPG
high density lipoprotein –HDL
low density lipoprotein - LDL
triglycerides - TG
tumor necrosis factor alpha - TNF-a
homeostasis model assessment for insulin resistance - HOMA-IR
IntroductionNonalcoholic fatty liver disease (NAFLD) includes patients with simple steatosis and nonalcoholic steatohepatitis (NASH), which can even progress to cirrhosis and hepatocellular carcinoma.1,2 Pharmacological treatment of patients with NAFLD is still evolving and since insulin resistance (IR) plays a key role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD), insulin sensitizing drugs like metformin may have role in the treatment of patients with NAFLD.3-9
Objective of this study was to determine the role of metformin in achieving biochemical response in patients with nonalcoholic fatty liver disease who did not respond to lifestyle interventions and ursodeoxycholic acid (UDCA).
Patients and methodsPatientsIn a prospective analysis (April 2001 – March 2007), 25 patients with NAFLD who did not have complete biochemical response after 6 months of lifestyle interventions and UDCA were included in the study after an informed consent. The project had the approval of institute’s ethical committee. Life style interventions included moderate sustained exercise like brisk walking, jogging, swimming, cycling etc at least for 30-45 minutes per day, low fat and low calorie diet and slow weight reduction (10% of base line in 6 months, not more than 1.6 Kg/week) in those with overweight and obesity. UDCA was given in a dosage of 300 mg twice daily. Inclusion criteria were adult (> 16 yrs.) nonalcoholic individuals (total abstinence or intake less than 20 g/day, confirmed by two family members) with raised serum alanine aminotransferase (ALT) (> 1.5 x ULN x at least 6 months), ultrasound showing hyperechoic liver or other features of steatosis, negative viral markers (HBsAg, anti HCV - 3rd generation), negative autoimmune markers [anti-nuclear antibody (ANA), anti-smooth muscle antibody (ASMA), anti-liver kidney microsomal antibody (LKM), anti-mitochondrial antibody (AMA)], normal serum ceruloplasmin & absent Keyser Fleisher rings on slit lamp examination, normal iron parameters and a liver biopsy consistent with NAFLD. Pregnant females and patients with diabetes mellitus were excluded from the study.
ControlsTwenty five patients with NAFLD from the same cohort with comparable age, gender, BMI, waist, baseline ALT, insulin resistance and metabolic syndrome were included as disease controls. These patients were also non responder to lifestyle interventions and UDCA and were continued on only lifestyle interventions without metformin
AnthropometryAll patients underwent a detailed physical examination including anthropometry. Overweight (BMI ≥ 23 but < 25 kg/m2), obesity (BMI ≥ 25 kg/m2) and abnormal waist circumference [> 90 cm (males), > 80 cm (females)] were defined as per the Asia Pacific criteria.10,11
BiochemistryDiabetes mellitus was defined as per the WHO criteria with fasting plasma glucose (FPG) ≥ 126 mg/dL or plasma glucose ≥ 200 mg/dL in a symptomatic patient or a 2-hour plasma glucose on glucose tolerance test ≥ 200 mg/ dL12 and patients qualifying these criteria were excluded from the study. Lipid profile was taken as abnormal when serum cholesterol was > 200 mg/dL, serum high-density lipoprotein (HDL) < 40 mg/dL in males & < 50 mg/dL in females, serum low-density lipoprotein (LDL) > 130 mg/ dL and serum triglycerides were > 150 mg/dL.13
In addition to measurement of fasting serum insulin levels (radioimmunoassay), fasting C-peptide (ELISA) and serum TNF- α (ELISA) levels were also determined in these patients.
Metabolic syndromeMetabolic syndrome was defined by the presence of at least ≥ 3 out of five modified adult treatment panel III criteria including modified abnormal waist as per the Asia Pacific criteria, FPG >110 mg/dL, hypertension (blood pressure ≥ 130/85 mmHg or on anti-hypertensive drugs), serum triglycerides > 150 mg/dL, and serum high density lipoprotein (HDL) < 40 mg/dL (males) & < 50 mg/dL (females).13
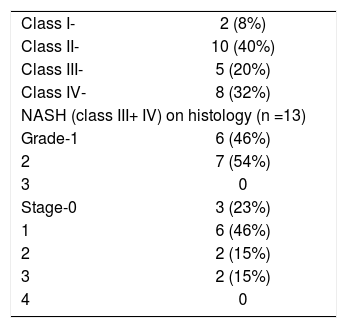
HistopathologyHistologically patients were classified into four classes as per Matteoni et al (Class – 1 = Simple steatosis, Class – 2 = Steatosis + lobular inflammation, Class – 3 = + Ballooned hepatocytes, Class – 4 = + Mallory hyaline or fibrosis) and those patients with class 3 or 4 were defined as having NASH.14 Further patients with NASH were graded and staged according to Brunt et al.15
Insulin resistanceInsulin resistance was determined by homeostasis model assessment for insulin resistance (HOMA-IR) calculated as the product of fasting insulin (μU/L) and fasting plasma glucose (mmol/L) divided by 22.5. An absolute value of HOMA-IR > 1.64 was taken as abnormal.3,16
Treatment and follow upPatients were treated with tablet metformin 500 mg/tid for 6 months in addition to the life style interventions. Disease controls were continued on only lifestyle interventions without metformin. Liver function tests were done monthly and patients were defined as having no response, partial response or complete biochemical response depending on the change in ALT.
Statistical analysisAll data is expressed in mean ± SD until otherwise specified. Students‘t’ test, chi square test and Mann-Whitney U test were used to determine the differences between different groups. A p value of < 0.05 was taken as significant.
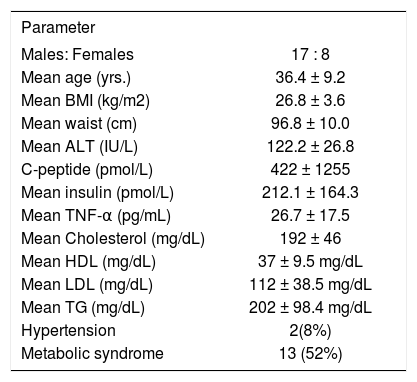
ResultsPatientsThere were 25 patients (males 17 mean age 36.4 ± 9.2 years). Other clinical and biochemical details including metabolic syndrome are shown in Table I.
Baseline characteristics of 25 patients with NAFLD.
| Parameter | |
|---|---|
| Males: Females | 17 : 8 |
| Mean age (yrs.) | 36.4 ± 9.2 |
| Mean BMI (kg/m2) | 26.8 ± 3.6 |
| Mean waist (cm) | 96.8 ± 10.0 |
| Mean ALT (IU/L) | 122.2 ± 26.8 |
| C-peptide (pmol/L) | 422 ± 1255 |
| Mean insulin (pmol/L) | 212.1 ± 164.3 |
| Mean TNF-α (pg/mL) | 26.7 ± 17.5 |
| Mean Cholesterol (mg/dL) | 192 ± 46 |
| Mean HDL (mg/dL) | 37 ± 9.5 mg/dL |
| Mean LDL (mg/dL) | 112 ± 38.5 mg/dL |
| Mean TG (mg/dL) | 202 ± 98.4 mg/dL |
| Hypertension | 2(8%) |
| Metabolic syndrome | 13 (52%) |
BMI – Body mass index ALT – Alanine aminotransferase TNF – Tumor necrosis factor HDL – High-density lipoprotein LDL – Low-density lipoprotein TG – Triglycerides
Details of the histopathology are shown in Table II. Thirteen (52%) patients had class III (n = 5) or class IV (n = 8) disease amounting to histological NASH. Of these 13 patients none had severe inflammation and none had stage 4 fibrosis (cirrhosis).
Insulin resistancePatients with NAFLD had significantly higher HOMA-IR in comparison to the historical healthy controls. (Mean HOMA – IR = 7.6 ± 2.9 vs 1.4 ± 1.2) (p = < 0.01).
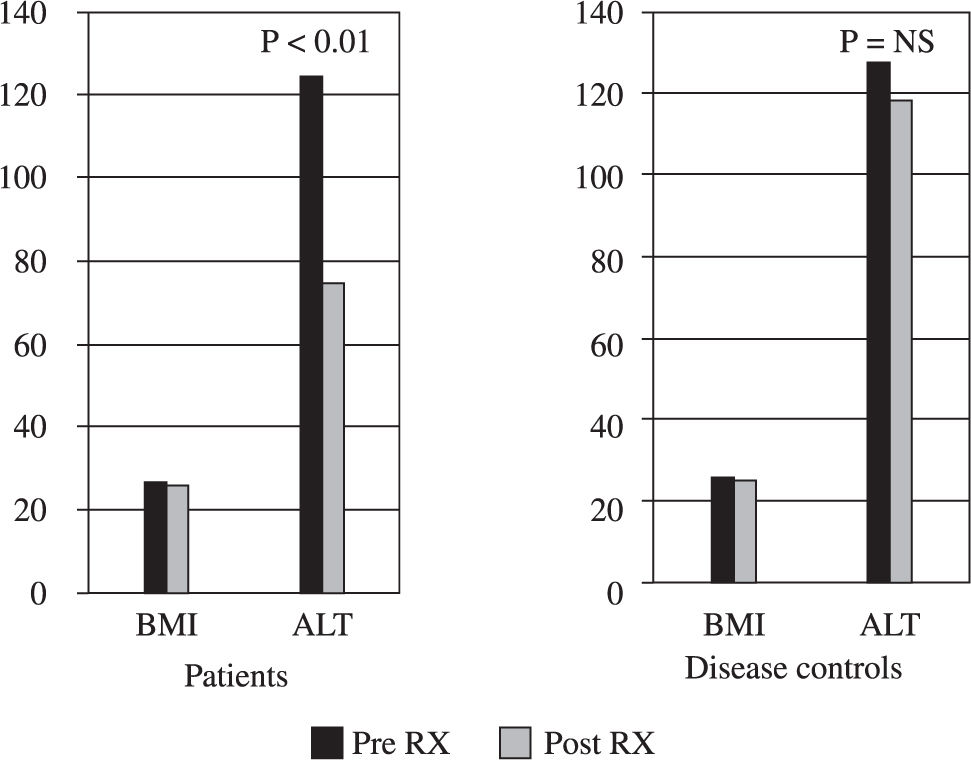
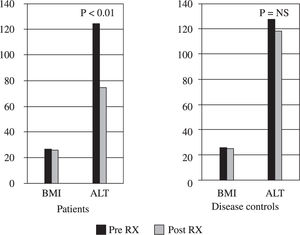
TreatmentThough BMI did not change significantly in both the groups, in comparison to disease controls (127.5 ± 41.8 vs 118 ± 21.6 p = NS), all patients treated with metformin had partial biochemical response (mean ALT 122.2 ± 26.8 vs 74.3 ± 4.2 p < 0.01) and 14 (56%) of them achieved complete normalization of ALT and continue to do so on follow up for 3 months (Figure 1). No patient experienced any side effect and there were no dropouts.
DiscussionInsulin resistance, which is responsible for increased lipolysis from adipose tissue and hepatic steatosis, is very common in patients with NAFLD.3,17 Increased fatty acid oxidation, oxidative stress and cytokines may lead on to steatohepatitis and its consequences in some of these patients.18 All our patients with NAFLD had IR as demonstrated by significantly higher HOMA-IR in comparison to healthy controls. High C-peptide to insulin ratio (Table I) in our patients with NAFLD is indicative of primary insulin resistance in these patients rather than the hyperinsulinemia occurring due to decreased hepatic extraction of insulin due to any liver disease.1
First line of treatment in patients with NAFLD is always weight reduction by life style interventions and control of other risk factors. Pharmacological treatment for NAFLD is still evolving and there is no single agent, which is effective in achieving the complete response.19 Earlier studies did show UDCA to be an effective mode of treatment in these patients20 but a recent randomized placebo controlled trial showed that UDCA is no better than placebo in the treatment of patients with NASH.21 The options in patients like ours who do not respond to above measures are limited. Since insulin resistance is the major pathogenetic mechanism, insulin-sensitizing drugs have a role in the treatment.22-24 Thiazolidinediones group of drugs including troglitazone, rosiglitazone and pioglitazone have been used in the treatment of NAFLD. Troglitazone has been withdrawn from the market due to its potential hepatotoxicity, which has been described even with rosiglitazone and pioglitazone.22-24
Metformin through its anti-TNF and insulin sensitizing actions is an effective drug for achieving biochemical response in patients with NAFLD, and data on its use both experimental25 and in patients4-9 with NAFLD is evolving. Most of the studies on metformin are open label studies.4,8 One randomized trial using metformin found it to be better than vitamin E and lifestyle interventions in improving the liver biochemistry and histological activity including steatosis, inflammation and fibrosis and none of the patients had any side effects.9 In another study 36 patients with NASH were randomized to lipid and calorie-restricted dietary treatment alone and metformin 850 mg b.d. plus dietary treatment for 6 months. The mean serum alanine/aspartate aminotransferase, insulin and C-peptide levels decreased and the index of insulin resistance improved significantly from baseline in the group given metformin, significantly greater than those in the group given dietary treatment alone. Though not significant, more patients in the metformin group showed improvement in the necro-inflammatory activity, compared with the group given dietary treatment alone.7
Though we did not evaluate them for histological improvement, our patients had significant biochemical response in comparison to patients continued only on lifestyle interventions with normalization of ALT in 14 (56%) of them. We also found it to be safe with no patient experiencing any side effect and there were no dropouts.
Our study has certain drawbacks. Number of patients is small and results may change with large number of patients. We evaluated patients only for biochemical response and since ALT may not have good correlation with histology in patients with NAFLD, improvement in ALT in our patients may not signify histological response.
In conclusion, our study shows that insulin resistance is common in patients with NAFLD.
Metformin is an alternative option to achieve biochemical response in patients with NAFLD who do not respond to lifestyle interventions and UDCA. Results need to be reproduced in larger number of patients in a randomized placebo controlled trial with histological data.