Introduction. Oxymatrine (OMTR) is widely used for the treatment of chronic hepatitis B (CHB) in China. Several recent reports revealed that OMTR together with interferon yielded a higher sustained virological response (SVR) than interferon alone.
Aim. To elucidate this topic using meta-analysis of data from published randomized controlled trials (RCTs).
Material and methods. The Cochrane Central Register of Controlled Trials, Medline, Science Citation Index, EMBASE, China National Knowledge Infrastructure, Wanfang Database and China Biomedical Database were searched to identify RCTs that evaluated SVR to interferon therapies and interferon plus OMTR therapies in CHB patients.
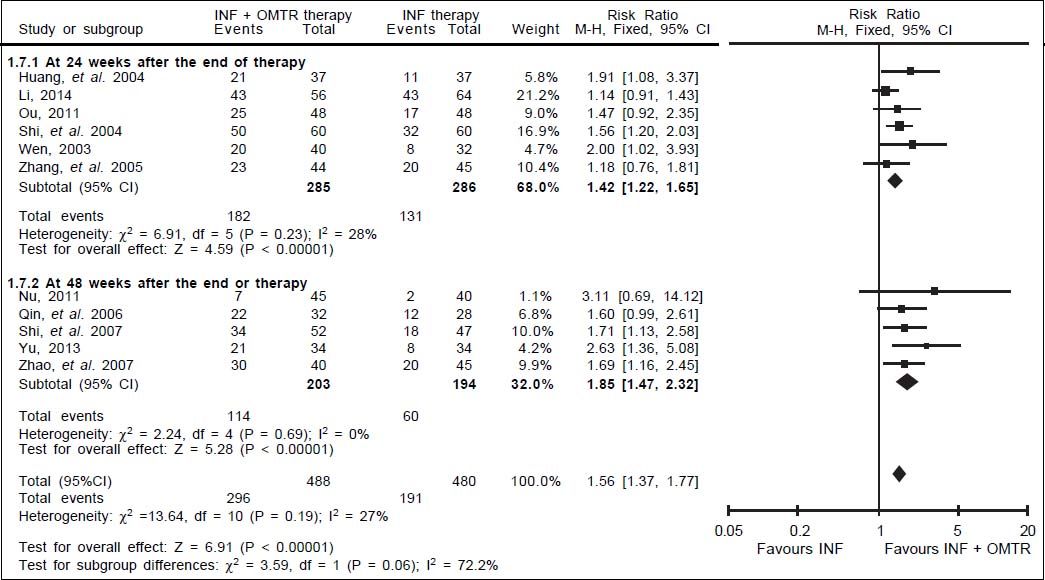
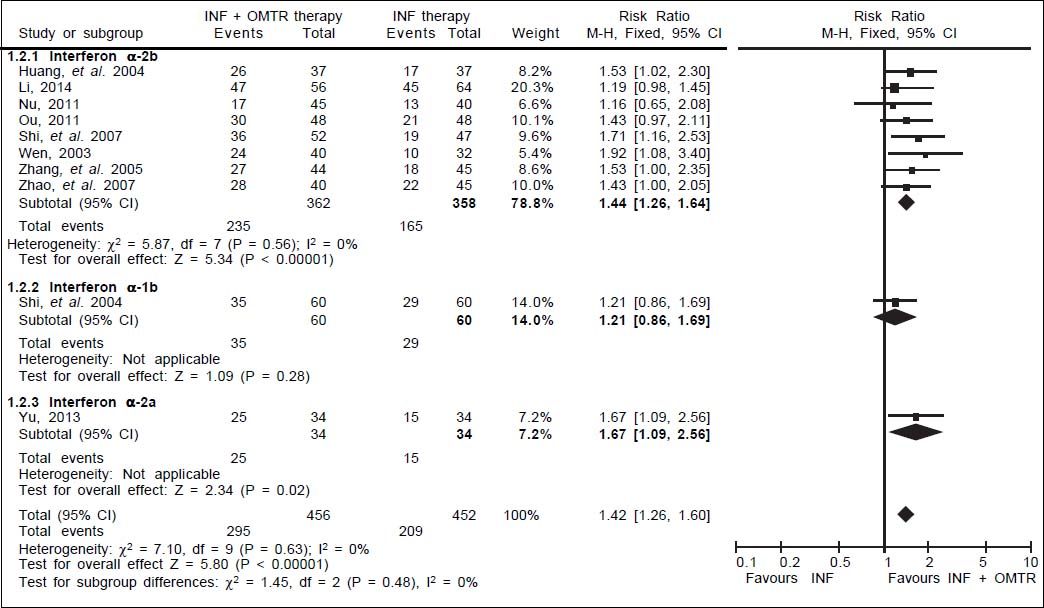
Results. The literature search yielded 238 studies, and 11 RCTs comprising 968 patients matched the selection criteria. Overall, SVR was significantly higher in patients treated with interferon plus OMTR than in patients treated with interferon alone (SVR: 60.7 vs. 39.8%; relative risk: 1.56; 95% confidence interval: 1.37-1.77; p < 0.05). Combined therapy of interferon plus OMTR were also superior to interferon therapies alone in achieving the end-of-treatment viral response, alaninetransaminase normalization, HBeAg loss, and HBeAg seroconversion.
Conclusions. Combined therapy of interferon plus OMTR may yield a higher SVR than interferon therapies. The exact outcome needs to perform rigorously designed, multicenter, and large randomized controlled trials.
Chronic infection of hepatitis B virus (HBV) poses serious public health problems because of the high prevalence rates and adverse long-term clinical outcomes, including premature deaths from hepatic decompensation, cirrhosis, and hepatocellular carcinoma (HCC).1 Approximately 350 million people around the world are chronically infected with HBV. The majority of countries in Asia has low-income economies and is at high endemicity of HBV infection.2 It is estimated in China that there are 120 million chronically infected carriers; up to 12 million people suffer from chronic hepatitis B (CHB), and about 300,000 people die each year.3 The goal of therapy for chronic hepatitis B (CHB) is to improve quality of life and survival by preventing progression of the disease to cirrhosis, decompensated cirrhosis, end-stage liver disease, HCC and death. This goal can be achieved if HBV replication can be suppressed in a sustaining manner.1 According to European Association for the Study of the Liver Clinical Practice Guidelines in 2012, a more realistic and desirable end point of CHB therapy is the induction of sustained or maintained virological remission. But the treatment rate among diagnosed patients with CHB in Asia is low (4 vs. 20% in USA, 17-28% in Europe and 8% in Japan) due to high cost.2 However treatment of chronic HBV infection is a complex task, a more important and most critical challenge is the high cost of medical care and antiviral drugs in Asia.2 Although new generations of anti-HBV drugs are available, interferon is still widely used in Asia due to the high cost of new antiviral drugs. It is necessary to deeply exploring various new strategies including appropriate combination therapy to achieve optimal curative effect for CHB patients.
Oxymatrine (OMTR) (MW: 264.31), a kind of alkaloid extracted from a Chinese herb Sophora alopecuraides L., had also been found to be capable of inhibiting of HBV and relieving hepatic fibrosis.4–7 It has been approved for the treatment of hepatitis B by China Food and Drug Administration, and is listed as one of the recommended anti-HBV agents in the Guideline for Prevention and Treatment of CHB jointlyproposed by the Chinese Society of Hepatology and the Chinese Society of Infectious Diseases. Interferon plus OMTR therapies are considered to have better incremental cost-effectiveness ratio and thus are a common treatment plan for CHB in China.8 Recent reports revealed that combination of OMTR with Interferon raise SVR than Interferon monotherapy. However, convincing evidence of Interferon plus OMTR therapies is still needed. In addition, these studies, published in Chinese, cannot be accessed by non-Chinese speaking scientists.
The aim of this study was to elucidate this topic using meta-analysis of data from published randomized controlled trials (RCTs). The results should provide some useful information for clinical treatment and future research of CHB.
Material and MethodsEligibility criteriaThe inclusion criteria were the following:
- •
Clinical diagnosis must meet the diagnostic criteria for CHB (Chinese Commission of Infectious and Parasitic Diseases, Viral Hepatitis Prevention and Treatment Programs).
- •
The included RCT studies were designed to compare the therapeutic effects of interferon therapies with interferon plus OMTR therapies in CHB patients; patients co-infected with hepatic cellular cancer and/or other viral infection (HAV, HCV, HDV, HEV) were excluded.
- •
Patients were treated for at least 24 weeks, and
- •
The publications could be written in any language. Reports of duplicated studies were excluded by examining the author list, parent institution, sample size and results.
The primary outcome was SVR, and other measures including the end-of-treatment viral response (ETVR), alanine transaminase (ALT) normalization, HBeAg loss, HBeAg seroconversion and occurrence of adverse events. The SVR was defined as the lack of detectable HBV RNA for at least 24 weeks after the end of therapy. ETVR was defined as undetectable HBV RNA at the end of therapy.
Information sources and searchesA search of the literature was conducted for studies that reported the therapeutic effects of interferon with or without OMTR therapies in CHB patients. The Cochrane Central Register of Controlled Trials, Medline, Science Citation Index, EMBASE, China National Knowledge Infrastructure, Wanfang Database and China Biomedical Database were searched to identify RCTs published in the field of antiviral therapy for CHB. The keywords used in literature searches included the following: chronic hepatitis B, hepatitis B virus, HBV, Oxymatrine, interferon, peginterferon, PEGylated interferon, treatment and trial.
Study selection and data collectionTwo authors (Min He and Yu Wu) independently screened titles and abstracts for potential eligibility and the full texts for final eligibility. We extracted the data from the included trials independently for quantitative analyses, and any disagreement was subsequently resolved by discussion. The quantitative data included the sample size; the pretreatment patient characteristics, including the age range and gender; the type of interferon (α-2a, α-2b or 1b); the doses of OMTR; SVRs; ETVRs; ALT normalization; HBeAg loss; HBeAg seroconversion and adverse effects.
Assessment of study qualityTwo authors (Mengmeng Wang and Wenwen Chen) independently assessed the quality of the included studies according to the descriptions provided by the authors of the included trials. Disagreements were resolved by discussion with Jian Jiang. The methodological quality of the trials was assessed by the method of Jadad 5.9 The scores range from one to five, one or two being considered as low quality trials, and three to five as high quality.
Statistical analysisMeta-analysis was performed using fixed effect or random effect methods, depending on the absence or presence of significant heterogeneity. Statistical heterogeneity was assessed using the I2 statistic, and subgroup and sensitivity analyses were used to account for potential sources of heterogeneity. We used the relative risk (RR) of the main dichotomous outcomes as the measure of efficacy. The 95% confidence interval (CI) for the combined RR was also provided. Data analysis was carried out with the use of Review Manager Software 5.3.2 (Cochrane Collaboration, Oxford, United Kingdom).
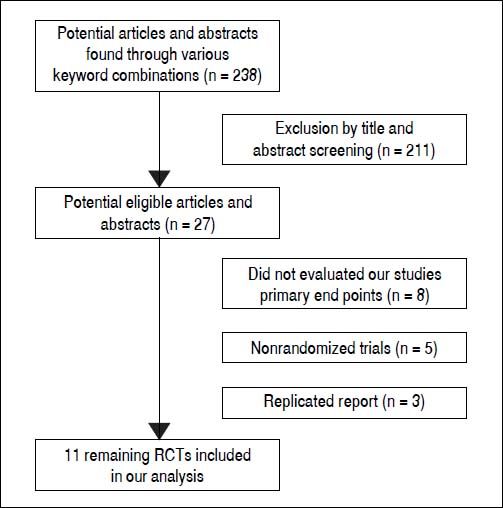
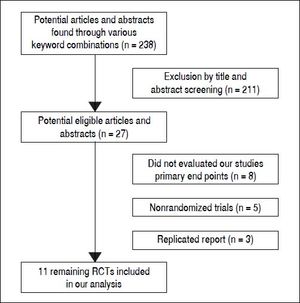
ResultsLiterature searchFigure 1 shows the results of the study screen. The literature search yielded 238 studies, 11 of which matched the selection criteria.10–20 There was unanimous agreement between the two authors regarding the selection of relevant articles (Min He and Yu Wu).
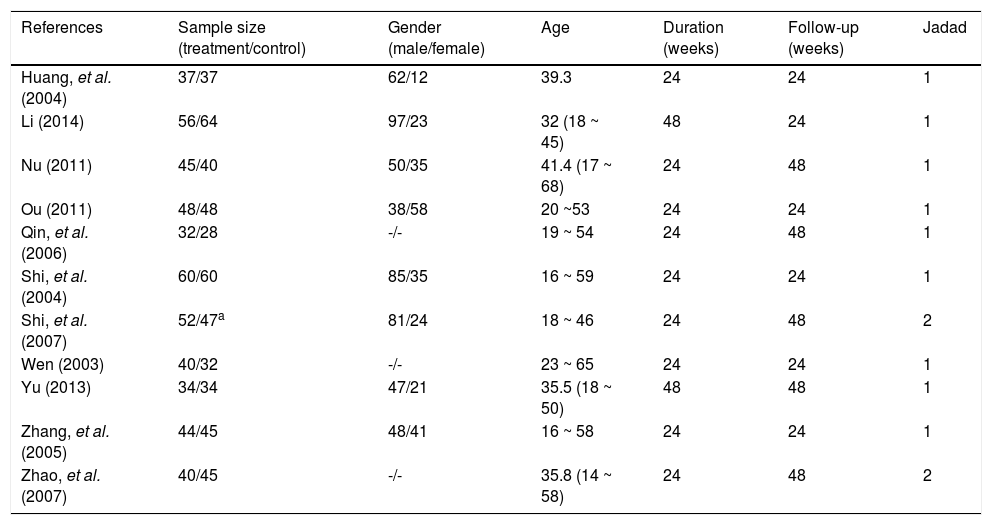
Patient characteristics and study qualityAll RCTs included were published as full-length articles. The patients included in the eleven trials were randomly assigned to accept interferon plus OMTR therapies or interferon therapies alone. Of the 968 patients, 488 patients had therapy with interferon plus OMTR, and 480 patients had therapy with interferon alone. All studies were single-centre trials. The baseline characteristics of the eleven included trials are summarized in tables 1 and 2. Information on the methodological quality was incomplete in the majority of eligible RCTs. The methodological quality of all eligible RCTs was not high.
Characteristics of the trials included in the meta-analysis.
| References | Sample size (treatment/control) | Gender (male/female) | Age | Duration (weeks) | Follow-up (weeks) | Jadad |
|---|---|---|---|---|---|---|
| Huang, et al. (2004) | 37/37 | 62/12 | 39.3 | 24 | 24 | 1 |
| Li (2014) | 56/64 | 97/23 | 32 (18 ~ 45) | 48 | 24 | 1 |
| Nu (2011) | 45/40 | 50/35 | 41.4 (17 ~ 68) | 24 | 48 | 1 |
| Ou (2011) | 48/48 | 38/58 | 20 ~53 | 24 | 24 | 1 |
| Qin, et al. (2006) | 32/28 | -/- | 19 ~ 54 | 24 | 48 | 1 |
| Shi, et al. (2004) | 60/60 | 85/35 | 16 ~ 59 | 24 | 24 | 1 |
| Shi, et al. (2007) | 52/47a | 81/24 | 18 ~ 46 | 24 | 48 | 2 |
| Wen (2003) | 40/32 | -/- | 23 ~ 65 | 24 | 24 | 1 |
| Yu (2013) | 34/34 | 47/21 | 35.5 (18 ~ 50) | 48 | 48 | 1 |
| Zhang, et al. (2005) | 44/45 | 48/41 | 16 ~ 58 | 24 | 24 | 1 |
| Zhao, et al. (2007) | 40/45 | -/- | 35.8 (14 ~ 58) | 24 | 48 | 2 |
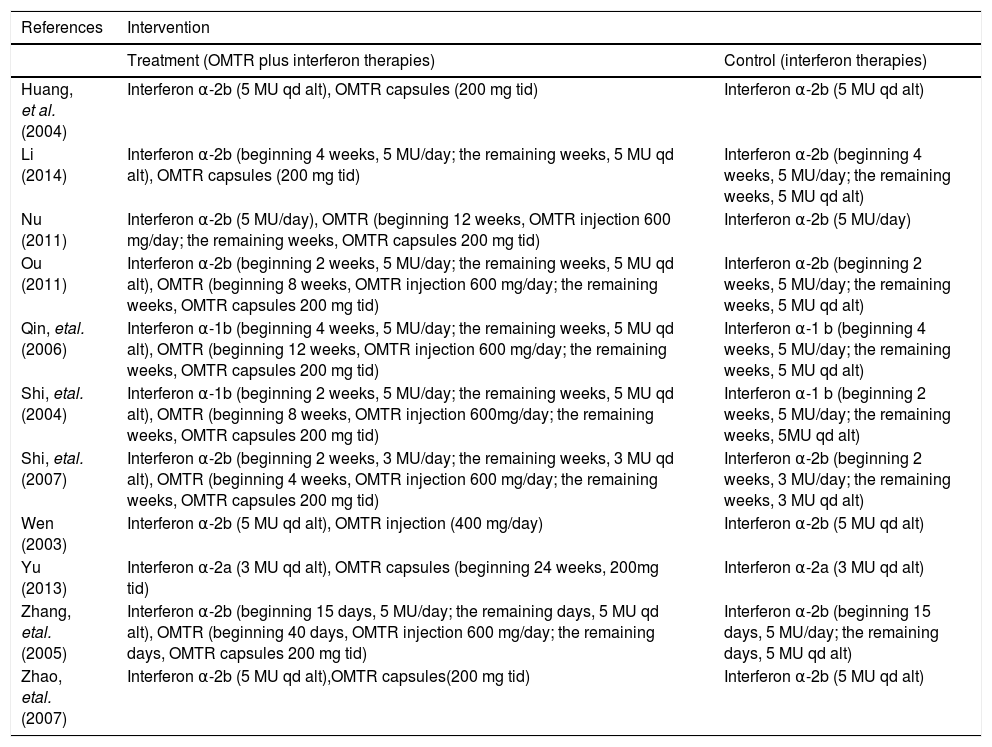
Interventions of the trials included in the meta-analysis.
| References | Intervention | |
|---|---|---|
| Treatment (OMTR plus interferon therapies) | Control (interferon therapies) | |
| Huang, et al. (2004) | Interferon α-2b (5 MU qd alt), OMTR capsules (200 mg tid) | Interferon α-2b (5 MU qd alt) |
| Li (2014) | Interferon α-2b (beginning 4 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt), OMTR capsules (200 mg tid) | Interferon α-2b (beginning 4 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt) |
| Nu (2011) | Interferon α-2b (5 MU/day), OMTR (beginning 12 weeks, OMTR injection 600 mg/day; the remaining weeks, OMTR capsules 200 mg tid) | Interferon α-2b (5 MU/day) |
| Ou (2011) | Interferon α-2b (beginning 2 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt), OMTR (beginning 8 weeks, OMTR injection 600 mg/day; the remaining weeks, OMTR capsules 200 mg tid) | Interferon α-2b (beginning 2 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt) |
| Qin, etal. (2006) | Interferon α-1b (beginning 4 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt), OMTR (beginning 12 weeks, OMTR injection 600 mg/day; the remaining weeks, OMTR capsules 200 mg tid) | Interferon α-1 b (beginning 4 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt) |
| Shi, etal. (2004) | Interferon α-1b (beginning 2 weeks, 5 MU/day; the remaining weeks, 5 MU qd alt), OMTR (beginning 8 weeks, OMTR injection 600mg/day; the remaining weeks, OMTR capsules 200 mg tid) | Interferon α-1 b (beginning 2 weeks, 5 MU/day; the remaining weeks, 5MU qd alt) |
| Shi, etal. (2007) | Interferon α-2b (beginning 2 weeks, 3 MU/day; the remaining weeks, 3 MU qd alt), OMTR (beginning 4 weeks, OMTR injection 600 mg/day; the remaining weeks, OMTR capsules 200 mg tid) | Interferon α-2b (beginning 2 weeks, 3 MU/day; the remaining weeks, 3 MU qd alt) |
| Wen (2003) | Interferon α-2b (5 MU qd alt), OMTR injection (400 mg/day) | Interferon α-2b (5 MU qd alt) |
| Yu (2013) | Interferon α-2a (3 MU qd alt), OMTR capsules (beginning 24 weeks, 200mg tid) | Interferon α-2a (3 MU qd alt) |
| Zhang, etal. (2005) | Interferon α-2b (beginning 15 days, 5 MU/day; the remaining days, 5 MU qd alt), OMTR (beginning 40 days, OMTR injection 600 mg/day; the remaining days, OMTR capsules 200 mg tid) | Interferon α-2b (beginning 15 days, 5 MU/day; the remaining days, 5 MU qd alt) |
| Zhao, etal. (2007) | Interferon α-2b (5 MU qd alt),OMTR capsules(200 mg tid) | Interferon α-2b (5 MU qd alt) |
OMTR: oxymatrine. MU: million unit, qd alt: quaque die alterna. tid: thrice-daily
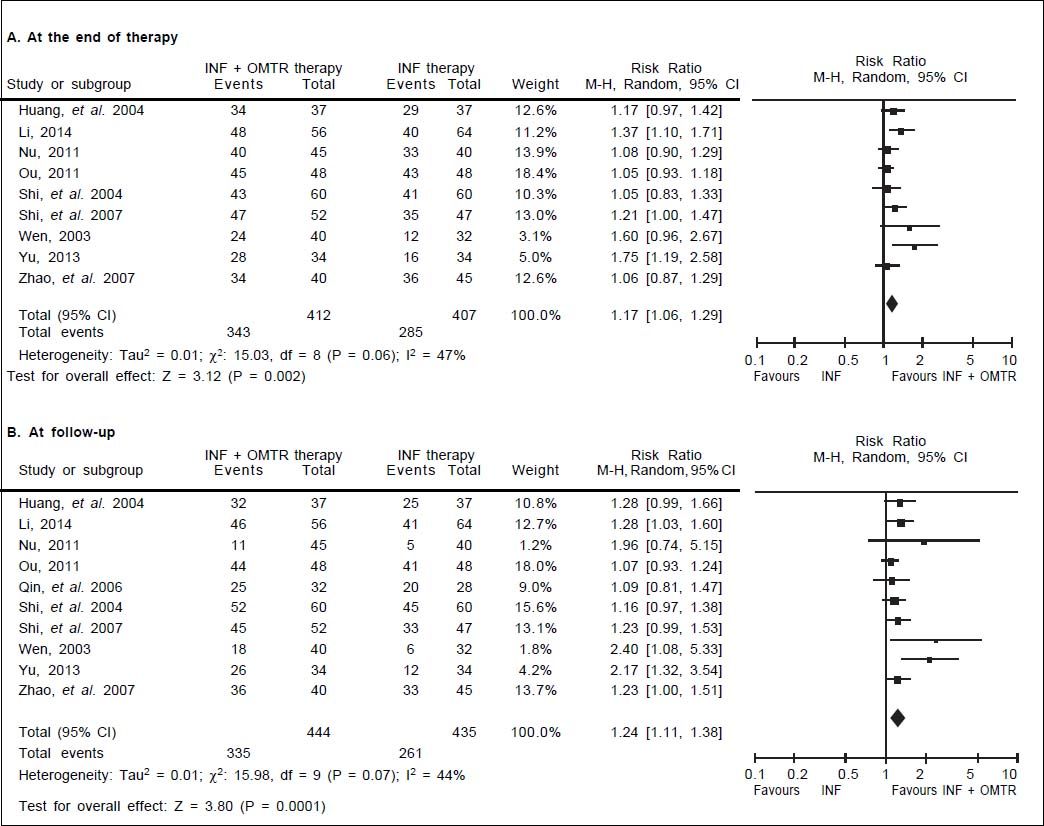
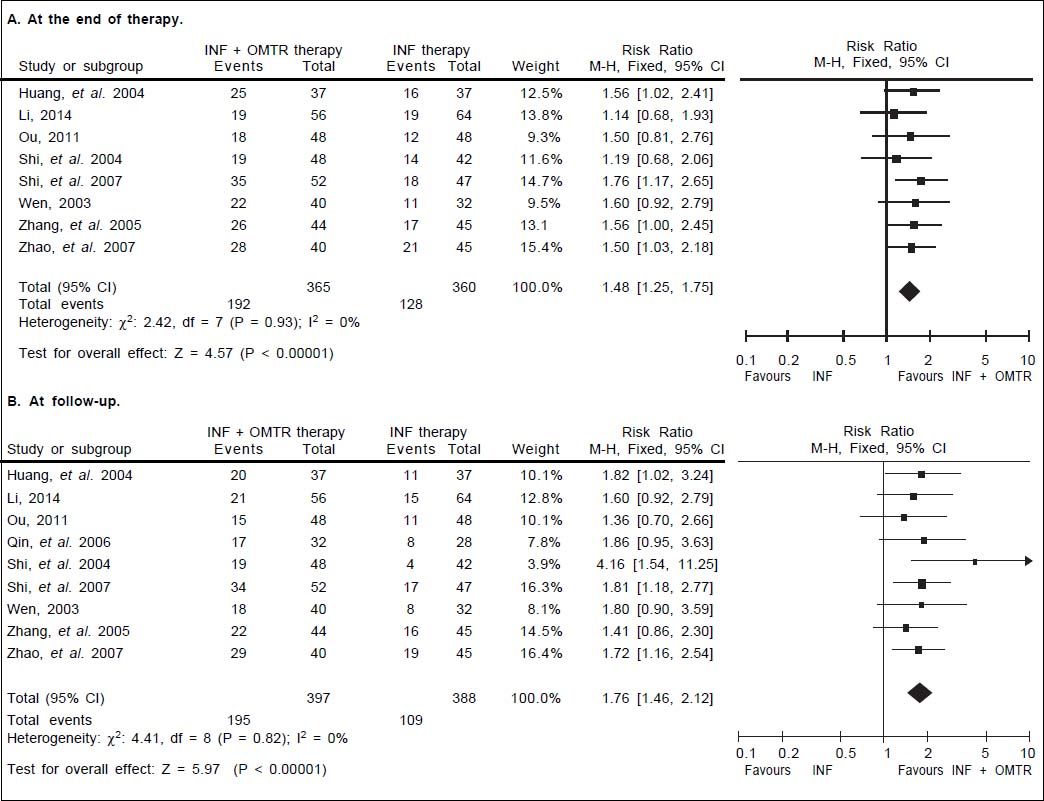
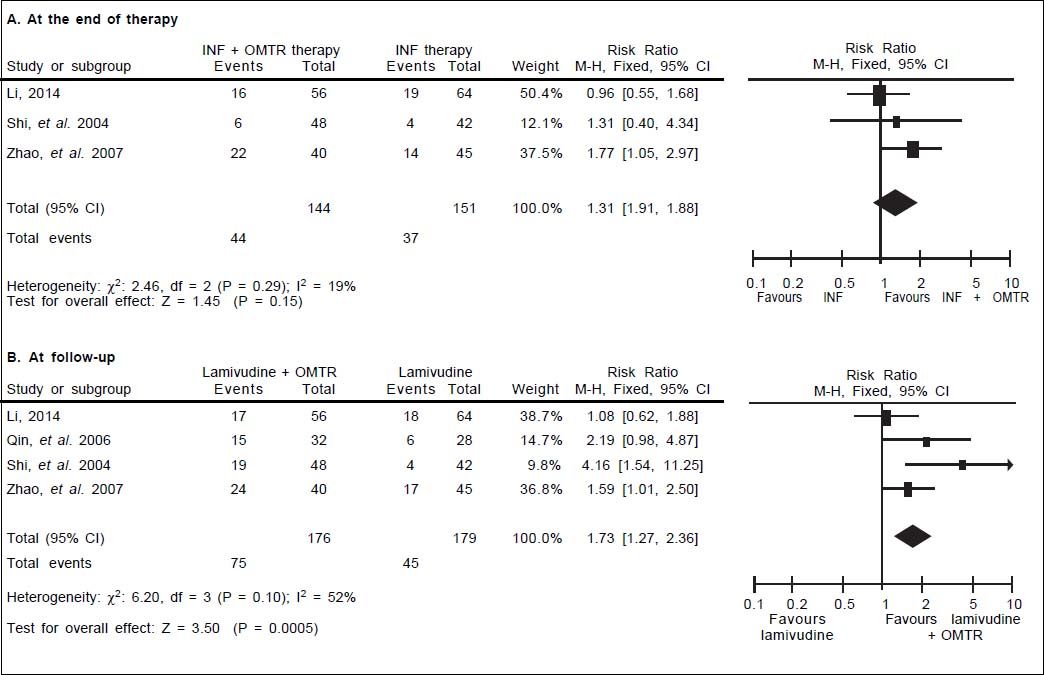
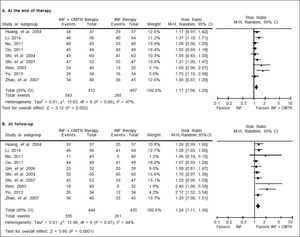
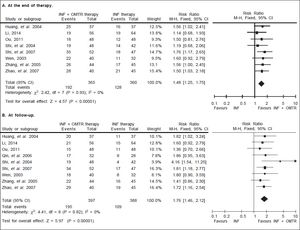
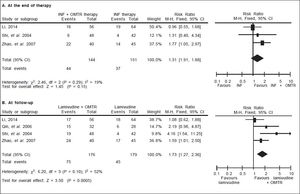
In this study, the combined therapy of interferon plus OMTR were superior to interferon therapies alone. Patients treated with interferon plus OMTR achieved higher SVR and ETVR than patients treated only with interferon (SVR: 60.7% (296/488) vs. 39.8 % (191/480); RR: 1.56; 95% CI: 1.37-1.77; p < 0.05. ETVR: 64.7 %( 295/456) vs. 46.2% (209/452); RR: 1.42; 95% CI: 1.26-1.60; p < 0.05) (Figures 2-3). Patients treated with combined therapy also achieved significantly higher ALT normalization and HBeAg loss at both the end of treatment and follow-up (ALT normalization: at the end of therapy: 83.3% (343/412) vs. 70.0% (285/407); RR: 1.17; 95% CI: 1.061.29; p < 0.05. Follow up: 75.5% (335/444) vs. 60.0% (261/ 435); RR: 1.24; 95% CI: 1.11-1.38; p < 0.05. HBeAg loss: At the end of therapy: 52.6% (192/365) vs. 35.6%(128/360); RR: 1.48; 95% CI: 1.25-1.75; p < 0.05. Follow up: 49.1% (195/397) vs. 28.1% (109/388); RR: 1.76; 95% CI: 1.46-2.12; p < 0.05 (Figures 4-5). HBeAg seroconversion at follow up was also higher in patients treated with combined therapy compared to the patients treated with interferon alone, but at the end of therapy there is no difference (At the end of therapy: 30.6% (44/144) vs. 24.5%(37/151); RR: 1.31; 95% CI: 0.91-1.88; p = 0.15. Follow up: 42.6% (75/176) vs. 25.1% (45/179); RR: 1.73; 95% CI: 1.27-2.36; p < 0.05) (Figure 6). In this meta-analysis for SVR, ETVR, HBeAg loss and HBeAg seroconversion, there was no apparent heterogeneity, but for ALT normalization (Figure 4).
ALT normalization: comparison of interferon plus OMTR therapies and interferon therapies. RR: relative risk. CI: confidence interval; test for heterogeneity: χ2 statistic with its degrees of freedom (d.f.) and p-value; inconsistency among results: I2 test for overall effect; Z statistic with p-value.
HBeAg loss: comparison of interferon plus OMTR therapies and interferon therapies. RR: relative risk. CI: confidence interval; test for heterogeneity: χ2 statistic with its degrees of freedom (d.f.) and p-value; inconsistency among results: I2 test for overall effect; Z statistic with p-value.
HBeAg seroconversion: comparison of interferon plus OMTR therapies and interferon therapies. RR: relative risk. CI: confidence interval; test for heterogeneity: χ2 statistic with its degrees of freedom (d.f.) and p-value; inconsistency among results: I2 test for overal effect; Z statistic with p-value.
Six included trials11,15–17,19,20 reported side effects, and one included trials15 clearly reported treatment discontinuation. Adverse events were also reported in the included trials (including thrombocytopenia, neutropenia, fever, headache, fatigue, depression and nausea). The overall adverse events were no difference in patients treated with interferon plus OMTR than in patients treated with interferon alone, according to the reports of the included trials.
Sensitivity analysesExcluding one of subgroup did not change the pooled estimate. The sensitivity analysis revealed that the RR for the outcome measure remained stable.
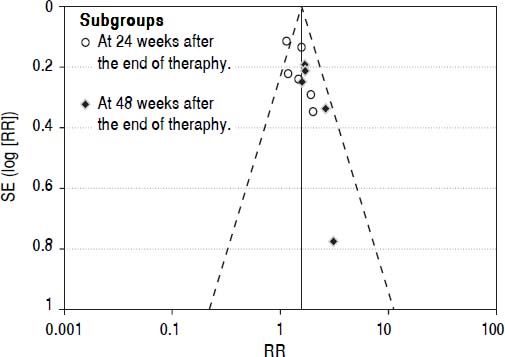
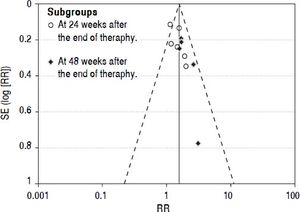
Publication biasWe performed funnel plot analysis for SVRs to explore publication bias. Eleven trials were included for the comparison of interferon plus OMTR and interferon therapies, and ten of these trials included in the meta-analysis lay within the 95% CI line and one trial lay outside the 95% CI line (Figure 7). These results implied the existence of some publication bias.
DiscussionTherapy for CHB must ensure a degree of virological suppression that will then lead to biochemical remission, histological improvement and prevention of complications. ETVR and SVR have different meanings in virological responses. SVR evaluates the long-term efficacy of antiviral drugs, which is also a desirable end point of CHB therapy.
In order to completely and objectively evaluating the efficacy of antivirus therapy, ETVR and SVR are necessary. The definitions of virological responses vary according to the timing (on or after therapy) and type of therapy.1 Viro-logical responses on Interferon therapy are usually evaluated at the end of therapy and at least 24 weeks after the end of therapy.
However, treatment of chronic HBV infection is a complex task; together with a lack of awareness of the disease among patients, high cost and lack of reimbursement are obstacles to measuring SVR in China’s clinical study. Fortunately, some recent studies have reported SVR.
In this study, we have summarized the available evidence from RCTs comparing interferon therapies with interferon plus OMTR therapies for the treatment of CHB. Our results suggest that combination therapies of interferon plus OMTR may achieve significantly higher SVR than interferon therapies alone. Combination therapies of interferon plus OMTR have also shown superior ETVRs, ALT normalizations, HBeAg loss and HBeAg seroconversion. Our meta-analysis data suggested that combined therapy of interferon plus OMTR therapy may be beneficial for treating CHB patients, with higher SVR, and do not result in any additional safety problems. However, we were unable to make firm conclusions because the cases we found were of generally poor quality. Published studies from China were found to be more highly condensed than typical articles published in the Western literature, with key details of study design omitted, especially details concerning random assignment.
From this, we learned that oxymatrine combined with interferon may augment the efficacy of interferon. However, a better understanding of drug synergism between oxymatrine and interferon is needed. Sophora alopecuraides L. has been widely used for the treatment of liver disease in China. Oxymatrine is extracted from Sophora alopecuraides L., one of the most pharmacologically active components in Sophora alopecuraides L.21 Oxymatrine has anti-HBV effect and its effect be determined in cell lines, in animals and in double-blind, randomized, multicenter clinical trials.4–6,22,23 It should be mentioned here that as the anti-HBV effect of OMTR is mediated through Heat-stress cognate 70 (Hsc70) down-regulations (an indirect effect). Hsc70 is a host protein which supports HBV DNA replication.22 OMTR is a selective inhibitor of Hsc70 expressions.22,23 OMTR significantly suppressed HBV de novo synthesis at the reverse transcription staging from pgRNA to DNA.22 The anti-HBV effect of OMTR was mediated through destabilizing Hsc70 mRNA; Hsc70 mRNA 3’UTR sequence was the element responsible for the destabilization effect of OMTR.22 Beside the inhibition of viral replication, OMTR can also enhance specific immune responses against HBV. OMTR influences Toll-Like Receptor 9 (TLR9) signaling transduction, and synergistically improve the immune efficacy of the TLR9 ligand against CHB.24 And OMTR has also been revealed to have anti-fibrotic, antiinflammatory and protecting hepatocytes.7,25–27 These initial studies and their positive findings suggested that OMTR is associated with higher SVR in the treatment of CHB patients when combined with interferon. Moreover, the price for OMTR is lower. The low cost of OMTR is one of the most important reasons for its wider use in China. The most critical challenge and obstacle is the high cost of medical care and antiviral drugs for CHB. Interferon plus OMTR therapies may be a good choice for interferon users especially in low-income countries. Although the quality of included trials in study was poor, based on the above results, we believed that that further trials of oxymatrine and its combination therapy in CHB are justified.
It must be noted that this meta-analysis had some limitations. Firstly, the methodological quality of the trials was not high. Secondly, the asymmetric funnel plot implied that publication biases may occur. Thirdly, the diversity of treatment dose and the small sample number and the lack of long-term follow-ups degraded the validity of the evidence of the clinical trials. We believe that it is possible that further investigation in well-designed trials may help answer the question of whether oxymatrine combined with interferon are more effective than interferon alone for treating CHB.
The Guideline of Prevention and Treatment of Chronic Hepatitis B in China recommended duration of interferon treatment was 24 ~ 48 weeks for CHB. Patients in nine trials included were treated for 24 weeks and in the remaining trials included were treated for 48 weeks. The recommended duration of OMTR treatment was 24 weeks for CHB according to a randomized double blind and placebo-controlled multicenter trial of OMTR therapy for CHB. So duration of OMTR treatment is 24 weeks in ten included trials.
ConclusionsCombined therapy of interferon plus OMTR may yield a higher SVR than interferon monotherapy. Considering that this meta-analysis had the limitations in some ways, the firm conclusions need to perform rigorously designed, multicenter, and large randomized controlled trials.
Conflict of InterestNone declared.
AcknowledgementsThis study was supported by the National Key New Drugs Creation Project, Innovative Drug Research and Development Technology Platform (No. 2012ZX09303009-001), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20123107120002) and the National Natural Science Foundation of China (No. 81403168).
Abbreviations- •
ALT: alanine transaminase.
- •
CHB: chronic hepatitis B.
- •
CI: confidence interval.
- •
ETVR: end-of-treatment viral response.
- •
HBeAg: hepatitis B e antigen.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
Hsc70: heat-stress cognate 70.
- •
INF: interferon.
- •
OMTR: oxymatrine.
- •
RCTs: randomized controlled trials.
- •
RR: relative risk.
- •
SVR: sustained virological response.
- •
TLR9: toll-like receptor 9.