Introduction and aim. The association between lysosomal acid lipase (LAL) activity and liver steatosis or fibrosis is poorly studied. The aim of our study was to determine the predictive power of LAL for cryptogenic liver steatosis and cryptogenic significant fibrosis/cirrhosis.
Material and methods. Cross-sectional observational study of 101 adult patients with unexplained elevated liver enzymes/hepatomegaly with or without dyslipidemia submitted to the determination of LAL activity and LIPA gene (E8SJM-C.894G^A) mutation. Seventy-one patients underwent liver biopsy or FibroScan®. Patients with an identifiable liver dysfunction cause and well-stablished NAFLD/NASH risk factors were excluded. Predictors for liver steatosis, significant fibrosis (> F2) or cirrhosis (F4) were evaluated.
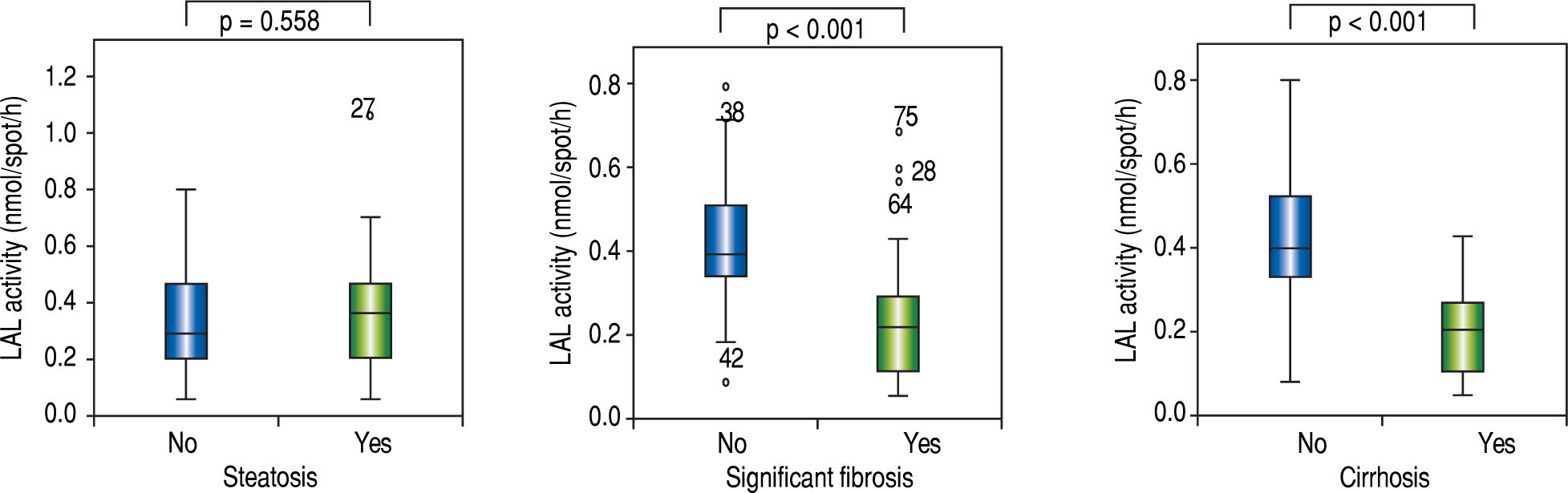
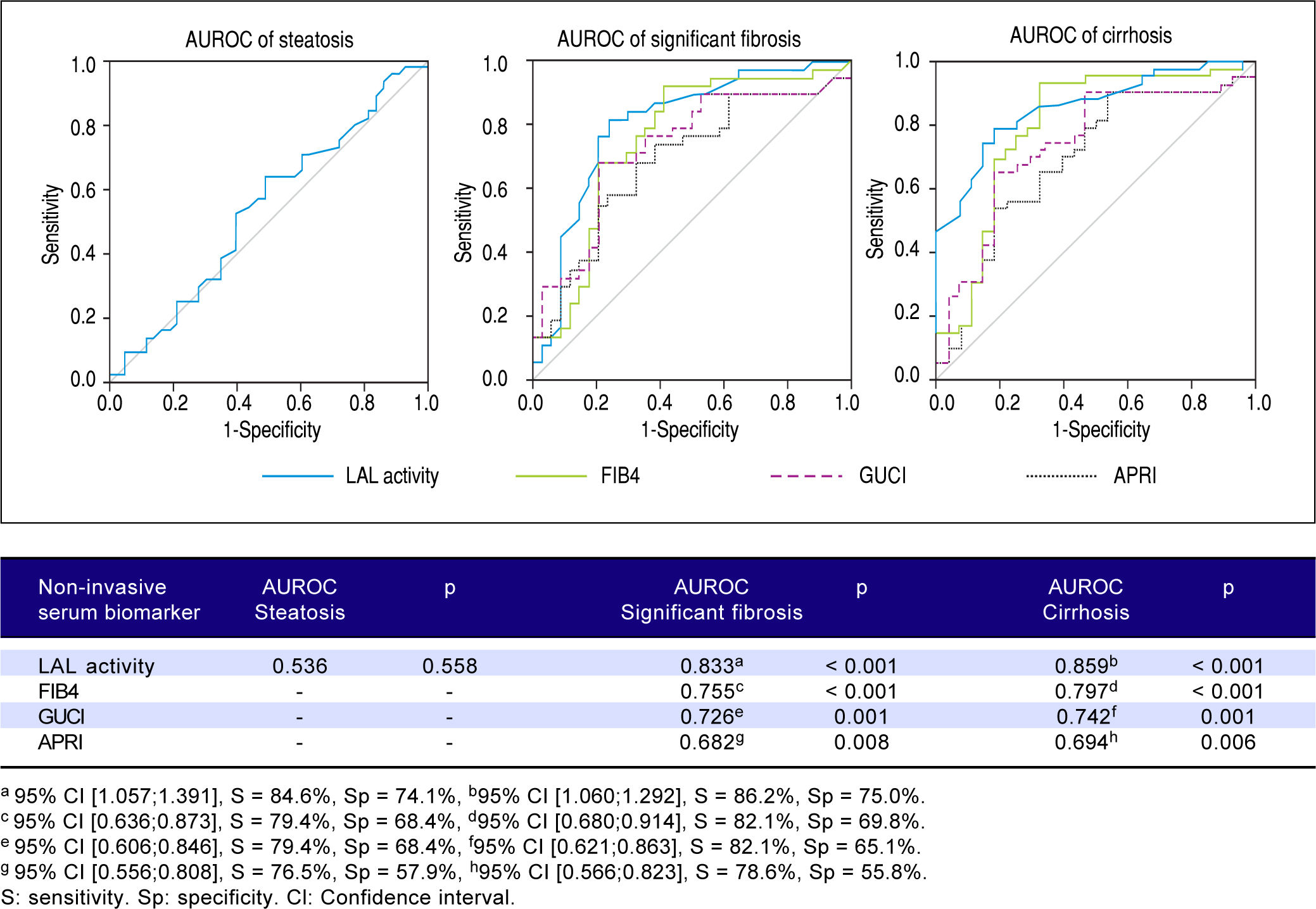
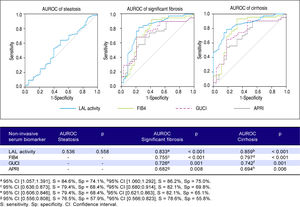
Results. Liver steatosis and fibrosis were mainly assessed by liver biopsy (74.6%; n = 53). Steatosis was present in 62.0% (n = 44), significant fibrosis in 47.9% (n = 34) and cirrhosis in 39.4% (n = 28). The median LAL was 0.36 (0.21-0.46)nmol/spot/h (vs. 0.29 (0.20-0.47); p = 0.558) for liver steatosis, 0.22 (0.11-0.29) nmol/spot/h (vs. 0.40 (0.34-0.51); p <0.001) for significant fibrosis and 0.21 (0.11-0.27) nmol/spot/h (vs. 0.40 (0.32-0.52); p < 0.001) for cirrhosis. No LIPA gene mutations were found. LAL activity was the strongest predictor of significant fibrosis (AUROC: 0.833; p < 0.001) with a cut-off of 0.265 (sensitivity: 85.9%; specificity: 75.0%) and cirrhosis (AUROC: 0.859; p < 0.001) with a cut-off of 0.235 (sensitivity: 86.2%; specificity: 75.0%), being higher than FIB4, GUCI or APRI. However, LAL activity was not associated with liver steatosis (AUROC: 0.536; p =0.558).
Conclusion. LAL activity can be considered a non-invasive new marker of cryptogenic liver fibrosis with higher accuracy than other known biomarkers. LAL activity < 0.265 nmol/spot/h was strongly associated with cryptogenic significant fibrosis and <0.235 nmol/spot/h with cryptogenic cirrhosis. LAL activity was not associated with cryptogenic liver steatosis.
Steatosis and fibrosis are a reflection of liver injury triggered by multiple factors. Despite the diagnosis of most liver diseases be possible through blood tests alone, liver biopsy remains the “gold standard” for assessing liver fibrosis and its severity degree, which is crucial for deciding when to start therapy and establishing prognosis. However, liver biopsy is being performed less and less due to its invasiveness, potential complications, poor patient acceptance, considerable costs and shortcomings related to sampling error and sample quality from needle biopsy specimens.1 As an alternative, FibroScan® currently represents a well-stablished and validated non-invasive method for assessing both fibrosis and steatosis, with high accuracy when compared to liver biopsy.2,3 Several noninvasive serum biomarkers/scores have been emerging to evaluate the presence of significant fibrosis, some of them involving multiple variables and expensive markers (a2-macroglobulin, hyaluronic acid, etc.).4
Lysosomal acid lipase (LAL) is an enzyme that plays a key role in lipid metabolism, responsible for triglycerides and cholesterol esters hydrolysis through the low-density lipoprotein (LDL)-receptor pathway. Reduced LAL activity leads to accumulation of cholesterol esters in lyso-somes. Genetically-determined LAL deficiency with a severe reduction (< 0.03 nmol/spot/h) occurs in Wolman disease and cholesterol ester storage diseases5-8 by LIPA gene mutations encoding LAL, namely the E8SJM variant (50%-70% of cases).9,10 These conditions are associated with a severe liver steatosis and liver failure.5 Current knowledge about the role of LAL activity besides these genetic diseases is very scarce. Recent literature has found an association between reduced LAL activity and non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steato-hepatitis (NASH), possibly related to metabolic syndrome, which is characteristic of most of these patients.5,8,9,11 In addition, some studies have shown an association between reduced LAL activity and liver cirrhosis of any cause.10,11 However, in the presence of a well-known liver disease it is difficult to determine whether reduced LAL activity is the cause or consequence of liver injury itself or whether it is determined by the liver disease-causing agent. Recent studies have also shown that LAL activity is reduced in cryptogenic cirrhosis, although with no clear relationship between them.10,11,13 Despite one study in pediatric population,14 there are no studies concerning LAL activity in adult patients with abnormal liver enzymes and different degrees of fibrosis besides advanced liver disease (cirrhosis).
The aims of this study are:
- •
To determine the association of LAL activity with cryptogenic steatosis and with different degrees of cryptogenic fibrosis in patients with unexplained persistent abnormal liver enzymes.
- •
To assess the presence of the E8SJM mutation in patients with LAL activity below the upper limit of the carrier range.
- •
To evaluate the determinants of reduced LAL activity in these patients.
- •
To determine the predictive power of LAL activity for liver steatosis, significant fibrosis and cirrhosis compared to other non-invasive and non-expensive conventional biomarkers.
A cross-sectional observational study of 101 consecutive adult patients with unexplained abnormal liver enzymes, who attended to the Gastroenterology Department of the Centro Hospitalar e Universitario Coimbra between November 2014 and June 2017. Inclusion criteria were as follows:
- •
All outpatients and inpatients aged > 18 years.
- •
Persistent abnormal liver enzymes (> 6 months) or hepatomegaly of unknown etiology with or without dyslipidemia (hypercholesterolemia and/or hypertriglyceridemia).
- •
An exhaustive liver study workup to rule out the most common causes of abnormal liver enzymes: anti-HCV, anti-HBs, anti-HBc, anti-HAV, anti-HEV antibodies, HBs-Ag; antinuclear (< 1:80), anti-mitochondrial, anti-smooth muscle and anti-liver and kidney microsomal antibodies; ferritin and transferrin saturation (< 40%), serum levels of ceruloplasmin and alpha-1 antitrypsin; serum levels of immunoglobulins IgA, IgG and IgM; anti-tissue transglutaminase IgA and serum protein electrophoresis.
- •
The presence of an abdominal ultrasound with measurement and recording of the highest longitudinal distance between the dome of the liver/spleen and the tip (splenic/liver length, in cm).
Exclusion criteria included well-stablished risk factors for NAFLD/NASH, such as obesity (body mass index > 30 kg/m2), type 2 diabetes and metabolic syndrome, significant alcohol consumption (> 20 g/day if woman and > 30 g/day if man), use of drugs-induced liver injury or hepatocellular carcinoma.
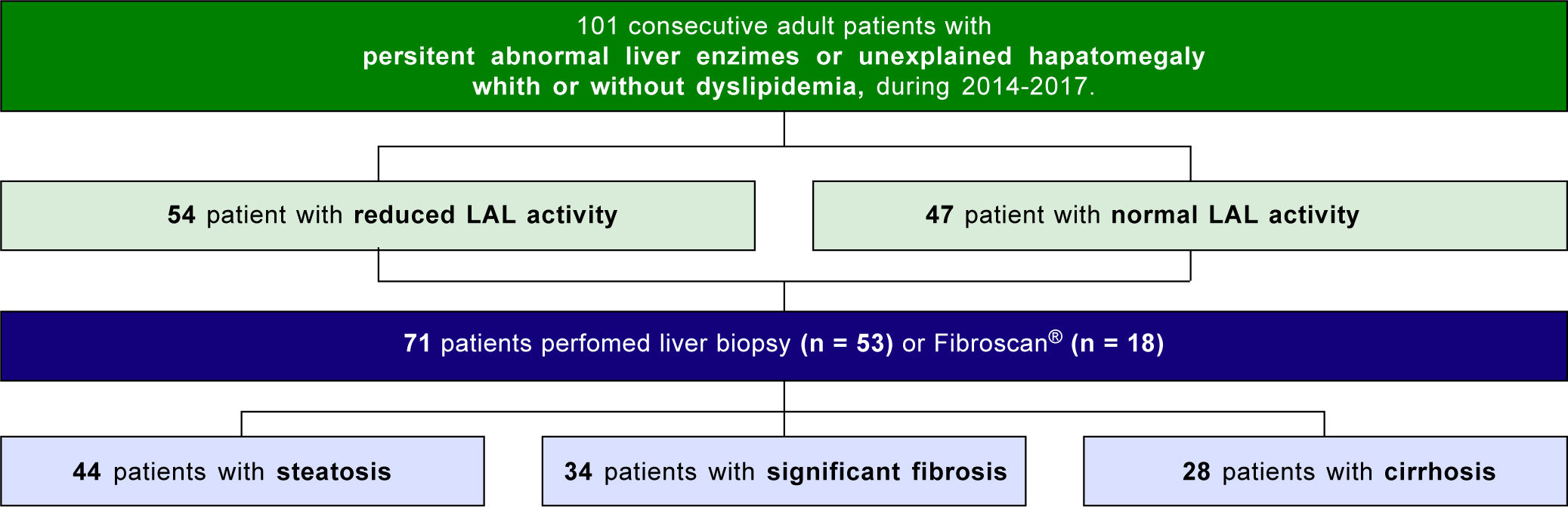
Seventy-one patients underwent liver biopsy or Fibro-Scan® to assess the presence of steatosis and fibrosis, and categorization of fibrosis degree (Figure 1).
Etiological investigation of fibrosis/cirrhosis was based on standard clinical, laboratory and histologic findings. For the purpose of this study, cryptogenic cirrhosis was defined as cirrhosis of unknown etiology in the absence of significant alcohol consumption, well-stablished risk factors for NAFLD/NASH or other known causes of chronic liver disease.
All patients enrolled in this study agreed to participate and signed informed consent. The study was approved by the Ethics Committee of our hospital.
MethodsDemographic, clinical and laboratory data were recorded for each patient. Laboratory parameters included alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, total bilirubin, international normalized ratio, albumin, platelet count and lipid profile (total-cholesterol (C), high-density lipoprotein (HDL)-C, estimated LDL-C, triglycerides). The study population was evaluated according to LAL activity. Additionally, patients were categorized into three subgroups based on the presence of liver steatosis, significant fibrosis and cirrhosis, and the differences between these subgroups were evaluated.
Determination of LAL activityFor all patients, a 5cc fresh venous blood sample was collected into ethylenediaminetetraacetic acid tube, after 12 hours of fasting. The blood sample was distributed to fill all four circles in a filter paper [Whatman #903 card for dried blood spot testing (DBS) by Hamilton J, et al,15 according to the requirements of the National Committee for Clinical Laboratory Standard Protocol. After drying overnight at room temperature, the samples were stored in a resealable plastic ziplock bag with desiccant at -20¯C and analyzed within 1 week after collection. LAL activity was determined using the inhibitor Lalistat-2 (Cheschire, CT, USA) and expressed in nmol/spot/h of 4-Methylumbelliferone. The reference range considered for normal LAL activity was 0.37-2.30 nmol/spot/h, since it was the reference range of the laboratory that analyzed our DBS-LAL activity samples (Biochemistry Department, Queen Elizabeth University Hospitals, Glasgow, UK).15 Heterozygous carriers were considered for LAL activity between 0.15 and 0.40 nmol/spot/h, which corresponds to an intermediate activity.15
E8SLM mutation analysis in LIPA geneIn case of reduced LAL activity, a second sample was collected using DBS and the mean between the two determinations was considered. If LAL activity < 0.40 nmol/ spot/h, an additional collection of fresh venous blood (4x ethylenediaminetetraacetic acid tubes of 2.7 mL) was performed and stored at -80¯C. Sample analysis was then completed using DNA extraction and determination of E8JSM mutation (SNP rs116928232 G>A) (Instituto Nacional de Saude Doutor Ricardo Jorge, Lisboa, Portugal). The cut-off 0.40 nmol/spot/h was chosen to guarantee higher sensitivity by including heterozygous carriers in addition to patients with LAL activity < 0.03 nmol/spot/h (homozygous).
Steatosis and fibrosis assessmentPatients who underwent liver biopsy (74.6%; n = 53) or FibroScan® (25.4%; n = 18) were included. Quality of liver measurements was guaranteed, since all liver samples had at least 11 portal spaces for liver biopsy; and a median value of at least 10 successful measurements with an interquartile range < 30% from the median and success rate > 60% for FibroScan®.1 In liver biopsy, steatosis was considered if was present in > 10% of hepatocytes16 (macrovesicular, microvesicular or mixed) and fibrosis according to the METAVIR classification, categorized as significant (> F2) or cirrhosis (F4). In FibroScan®, steatosis was considered if controlled attenuation parameter > 280dB/m2 and fibrosis for elastography cut-off values of 7.1kPa to > F2 and 12.5kPa to F4.2’3 For cirrhotic patients, liver function scores Child-Turcotte-Pugh (CTP) and Model for end-stage liver disease-sodium (MELD-Na) scores were also evaluated. Risk factors for steatosis, significant fibrosis and cirrhosis were determined by comparison with patients without these conditions. In addition, the predictive power of LAL activity for significant fibrosis/cirrhosis was also assessed by comparison with other common serum biomarkers/tests such as FIB4, APRI and GUCI. These biomarkers were chosen since they are non-invasive, non-expensive and easy to use, as stated in recent EASL guidelines with an accuracy to predict significant fibrosis above 83.3% (the accuracy achieved for LAL activity in our study to predict significant fibrosis).4
Statistical analysisStatistical analysis was carried out using the social package for social sciences, version 22.0 for Windows (Chicago, IL, USA). The level of significance was set at p-value less than 0.05. Continuous data were expressed as mean and SD and compared using Student’s t-test if normality was verified or Mann-Whitney test if no normality. Categorical variables were expressed as percentage and compared using x2-test if normality was confirmed or Fisher’s exact probability test if there was no normality. Multivariate logistic regression analysis was used to study the influence of independent variables on reduced LAL activity. Area under the receiver operating characteristic curve (AUROC) was applied in the evaluation of predictive ability of LAL activity and other known non-invasive biomarkers for steatosis, significant fibrosis and cirrhosis, assigning the best cut-off in terms of sensitivity and specificity.
ResultsThe study was conducted in 101 inpatients and outpatients with persistently abnormal liver enzymes and/or unexplained hepatomegaly with/without dyslipidemia, after common liver diseases have been ruled out.
Characterization of study population and determinants of reduced LAL activityOf all patients, median LAL activity was 0.35(0.21-0.48) mmol/spot/h (minimum: 0.05; maximum: 1.06) with a predominance of female gender [61.4% (n = 62)] and mean age of 58.0 ± 17.3 years old. Concerning liver enzymes, patients presented a slight elevation of transaminases (aspartate aminotransferase: 41.7 ± 27.0IU/L; N < 35IU/L); alanine aminotransferase: 50.2 ± 35.6IU/L; N < 45IU/L) and mean gamma-glutamyl transferase of 158.6 ± 143.2IU/L (N < 55IU/L). Fifty patients (49.5%) showed dyslipidemia with slight elevation of total-C (mean: 200.8 ± 91.5 mg/dL; N < 200 mg/dL) and triglycerides (mean: 156.2 ± 162.0 mg/dL; N < 150mg/dL) (Table 1).
Univariate and multivariate analyses of study population according to LAL activity.
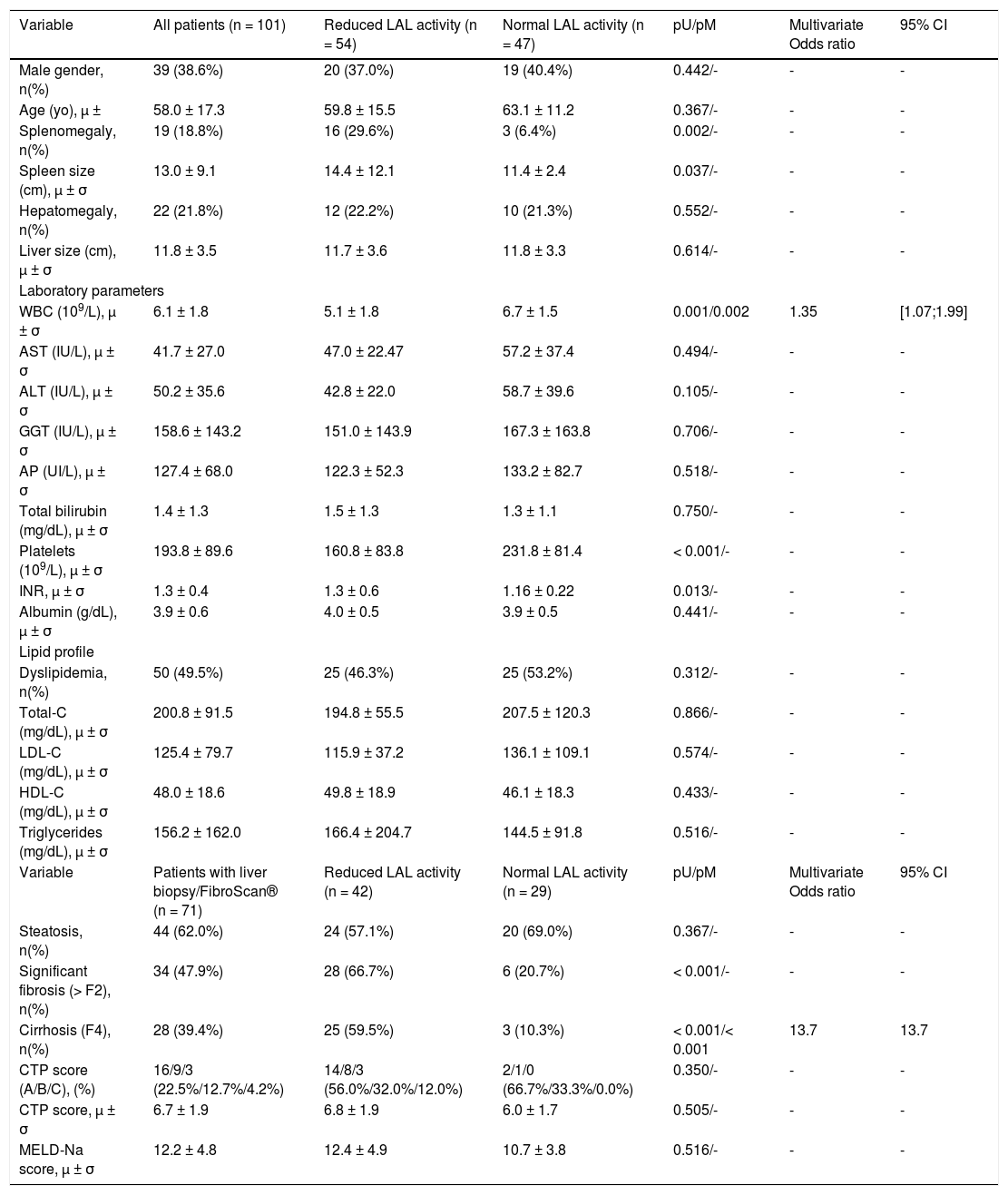
| Variable | All patients (n = 101) | Reduced LAL activity (n = 54) | Normal LAL activity (n = 47) | pU/pM | Multivariate Odds ratio | 95% CI |
|---|---|---|---|---|---|---|
| Male gender, n(%) | 39 (38.6%) | 20 (37.0%) | 19 (40.4%) | 0.442/- | - | - |
| Age (yo), μ ± | 58.0 ± 17.3 | 59.8 ± 15.5 | 63.1 ± 11.2 | 0.367/- | - | - |
| Splenomegaly, n(%) | 19 (18.8%) | 16 (29.6%) | 3 (6.4%) | 0.002/- | - | - |
| Spleen size (cm), μ ± σ | 13.0 ± 9.1 | 14.4 ± 12.1 | 11.4 ± 2.4 | 0.037/- | - | - |
| Hepatomegaly, n(%) | 22 (21.8%) | 12 (22.2%) | 10 (21.3%) | 0.552/- | - | - |
| Liver size (cm), μ ± σ | 11.8 ± 3.5 | 11.7 ± 3.6 | 11.8 ± 3.3 | 0.614/- | - | - |
| Laboratory parameters | ||||||
| WBC (109/L), μ ± σ | 6.1 ± 1.8 | 5.1 ± 1.8 | 6.7 ± 1.5 | 0.001/0.002 | 1.35 | [1.07;1.99] |
| AST (IU/L), μ ± σ | 41.7 ± 27.0 | 47.0 ± 22.47 | 57.2 ± 37.4 | 0.494/- | - | - |
| ALT (IU/L), μ ± σ | 50.2 ± 35.6 | 42.8 ± 22.0 | 58.7 ± 39.6 | 0.105/- | - | - |
| GGT (IU/L), μ ± σ | 158.6 ± 143.2 | 151.0 ± 143.9 | 167.3 ± 163.8 | 0.706/- | - | - |
| AP (UI/L), μ ± σ | 127.4 ± 68.0 | 122.3 ± 52.3 | 133.2 ± 82.7 | 0.518/- | - | - |
| Total bilirubin (mg/dL), μ ± σ | 1.4 ± 1.3 | 1.5 ± 1.3 | 1.3 ± 1.1 | 0.750/- | - | - |
| Platelets (109/L), μ ± σ | 193.8 ± 89.6 | 160.8 ± 83.8 | 231.8 ± 81.4 | < 0.001/- | - | - |
| INR, μ ± σ | 1.3 ± 0.4 | 1.3 ± 0.6 | 1.16 ± 0.22 | 0.013/- | - | - |
| Albumin (g/dL), μ ± σ | 3.9 ± 0.6 | 4.0 ± 0.5 | 3.9 ± 0.5 | 0.441/- | - | - |
| Lipid profile | ||||||
| Dyslipidemia, n(%) | 50 (49.5%) | 25 (46.3%) | 25 (53.2%) | 0.312/- | - | - |
| Total-C (mg/dL), μ ± σ | 200.8 ± 91.5 | 194.8 ± 55.5 | 207.5 ± 120.3 | 0.866/- | - | - |
| LDL-C (mg/dL), μ ± σ | 125.4 ± 79.7 | 115.9 ± 37.2 | 136.1 ± 109.1 | 0.574/- | - | - |
| HDL-C (mg/dL), μ ± σ | 48.0 ± 18.6 | 49.8 ± 18.9 | 46.1 ± 18.3 | 0.433/- | - | - |
| Triglycerides (mg/dL), μ ± σ | 156.2 ± 162.0 | 166.4 ± 204.7 | 144.5 ± 91.8 | 0.516/- | - | - |
| Variable | Patients with liver biopsy/FibroScan® (n = 71) | Reduced LAL activity (n = 42) | Normal LAL activity (n = 29) | pU/pM | Multivariate Odds ratio | 95% CI |
| Steatosis, n(%) | 44 (62.0%) | 24 (57.1%) | 20 (69.0%) | 0.367/- | - | - |
| Significant fibrosis (> F2), n(%) | 34 (47.9%) | 28 (66.7%) | 6 (20.7%) | < 0.001/- | - | - |
| Cirrhosis (F4), n(%) | 28 (39.4%) | 25 (59.5%) | 3 (10.3%) | < 0.001/< 0.001 | 13.7 | 13.7 |
| CTP score (A/B/C), (%) | 16/9/3 (22.5%/12.7%/4.2%) | 14/8/3 (56.0%/32.0%/12.0%) | 2/1/0 (66.7%/33.3%/0.0%) | 0.350/- | - | - |
| CTP score, μ ± σ | 6.7 ± 1.9 | 6.8 ± 1.9 | 6.0 ± 1.7 | 0.505/- | - | - |
| MELD-Na score, μ ± σ | 12.2 ± 4.8 | 12.4 ± 4.9 | 10.7 ± 3.8 | 0.516/- | - | - |
Fifty-four patients (53.5%) had reduced LAL activity by DBS, who were compared with patients with normal LAL activity. The two groups were comparable in terms of age and gender. After univariate analysis, risk factors related to reduced LAL activity were the presence of significant liver fibrosis (66.7% vs. 20.7%; p < 0.001), cirrhosis (59.5% vs. 10.3%; p < 0.001) and splenomegaly (29.6% vs. 6.4%; p = 0.002) with 1.26-fold increased spleen size (14.4 ± 12.1 vs. 11.4 ± 2.4; p = 0.037). Regarding biochemical parameters, patients with reduced LAL activity had a higher international normalized ratio (1.34 ± 0.56 vs. 1.16 ± 0.22; p = 0.013), lower white blood cell count (WBC) (5.5 ± 1.8 vs. 6.7 ± 1.5; p = 0.001; N:4-10*109/L) and platelet count (160.8 ± 83.8 vs. 231.8 ± 81.4; p < 0.001; N:150-400*109/L). After a multivariate analysis of significant variables in univariate analysis, risk factors associated with reduced LAL activity were cirrhosis (OR 13.7; p < 0.001) and low WBC (OR 1.35; p = 0.002). No significant differences were found between two groups relatively to liver size, steatosis, dyslipidemia or lipid profile. Among cirrhotic patients, those with reduced LAL activity did not present more severe liver dysfunction determined by CTP and MELD-Na scores, when compared with cirrhotic patients with normal LAL activity (Table 1).
Risk factors for steatosis, significant fibrosis and cirrhosisAmong the patients who underwent liver biopsy or FibroScan®, 62.0% (n = 44) presented steatosis, 47.9% (n = 34) significant fibrosis and 39.4% (n = 28) cirrhosis. Median LAL activity was reduced in the three subgroups. Moreover, median LAL activity was lower among patients with significant fibrosis (82.4%; n = 28; median LAL activity: 0.22 (0.11-0.29) vs. 0.40 (0.34-0.51); p < 0.001) and cirrhosis (89.3%; n = 25; median LAL activity: 0.21(0.11-0.27) vs. 0.40 (0.32-0.52); p < 0.001) than patients without these conditions. However, there was no association between LAL activity and steatosis (0.36 (0.21-0.46) vs. 0.29 (0.20-0.47); p = 0.558) (Table 2 and Figure 2).
Univariate and multivariate analyses of study population in relation to the three study subgroups (liver steatosis, significant fibrosis and cirrhosis).
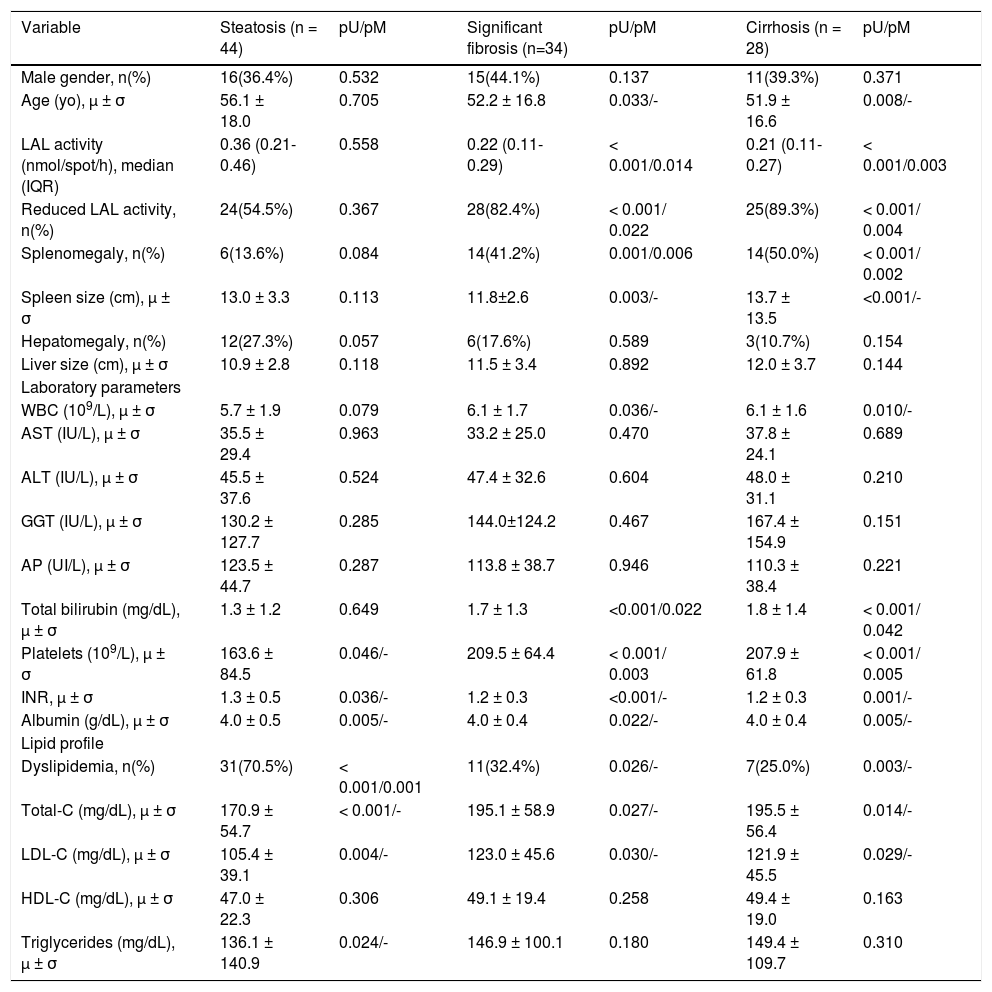
| Variable | Steatosis (n = 44) | pU/pM | Significant fibrosis (n=34) | pU/pM | Cirrhosis (n = 28) | pU/pM |
|---|---|---|---|---|---|---|
| Male gender, n(%) | 16(36.4%) | 0.532 | 15(44.1%) | 0.137 | 11(39.3%) | 0.371 |
| Age (yo), μ ± σ | 56.1 ± 18.0 | 0.705 | 52.2 ± 16.8 | 0.033/- | 51.9 ± 16.6 | 0.008/- |
| LAL activity (nmol/spot/h), median (IQR) | 0.36 (0.21-0.46) | 0.558 | 0.22 (0.11-0.29) | < 0.001/0.014 | 0.21 (0.11-0.27) | < 0.001/0.003 |
| Reduced LAL activity, n(%) | 24(54.5%) | 0.367 | 28(82.4%) | < 0.001/ 0.022 | 25(89.3%) | < 0.001/ 0.004 |
| Splenomegaly, n(%) | 6(13.6%) | 0.084 | 14(41.2%) | 0.001/0.006 | 14(50.0%) | < 0.001/ 0.002 |
| Spleen size (cm), μ ± σ | 13.0 ± 3.3 | 0.113 | 11.8±2.6 | 0.003/- | 13.7 ± 13.5 | <0.001/- |
| Hepatomegaly, n(%) | 12(27.3%) | 0.057 | 6(17.6%) | 0.589 | 3(10.7%) | 0.154 |
| Liver size (cm), μ ± σ | 10.9 ± 2.8 | 0.118 | 11.5 ± 3.4 | 0.892 | 12.0 ± 3.7 | 0.144 |
| Laboratory parameters | ||||||
| WBC (109/L), μ ± σ | 5.7 ± 1.9 | 0.079 | 6.1 ± 1.7 | 0.036/- | 6.1 ± 1.6 | 0.010/- |
| AST (IU/L), μ ± σ | 35.5 ± 29.4 | 0.963 | 33.2 ± 25.0 | 0.470 | 37.8 ± 24.1 | 0.689 |
| ALT (IU/L), μ ± σ | 45.5 ± 37.6 | 0.524 | 47.4 ± 32.6 | 0.604 | 48.0 ± 31.1 | 0.210 |
| GGT (IU/L), μ ± σ | 130.2 ± 127.7 | 0.285 | 144.0±124.2 | 0.467 | 167.4 ± 154.9 | 0.151 |
| AP (UI/L), μ ± σ | 123.5 ± 44.7 | 0.287 | 113.8 ± 38.7 | 0.946 | 110.3 ± 38.4 | 0.221 |
| Total bilirubin (mg/dL), μ ± σ | 1.3 ± 1.2 | 0.649 | 1.7 ± 1.3 | <0.001/0.022 | 1.8 ± 1.4 | < 0.001/ 0.042 |
| Platelets (109/L), μ ± σ | 163.6 ± 84.5 | 0.046/- | 209.5 ± 64.4 | < 0.001/ 0.003 | 207.9 ± 61.8 | < 0.001/ 0.005 |
| INR, μ ± σ | 1.3 ± 0.5 | 0.036/- | 1.2 ± 0.3 | <0.001/- | 1.2 ± 0.3 | 0.001/- |
| Albumin (g/dL), μ ± σ | 4.0 ± 0.5 | 0.005/- | 4.0 ± 0.4 | 0.022/- | 4.0 ± 0.4 | 0.005/- |
| Lipid profile | ||||||
| Dyslipidemia, n(%) | 31(70.5%) | < 0.001/0.001 | 11(32.4%) | 0.026/- | 7(25.0%) | 0.003/- |
| Total-C (mg/dL), μ ± σ | 170.9 ± 54.7 | < 0.001/- | 195.1 ± 58.9 | 0.027/- | 195.5 ± 56.4 | 0.014/- |
| LDL-C (mg/dL), μ ± σ | 105.4 ± 39.1 | 0.004/- | 123.0 ± 45.6 | 0.030/- | 121.9 ± 45.5 | 0.029/- |
| HDL-C (mg/dL), μ ± σ | 47.0 ± 22.3 | 0.306 | 49.1 ± 19.4 | 0.258 | 49.4 ± 19.0 | 0.163 |
| Triglycerides (mg/dL), μ ± σ | 136.1 ± 140.9 | 0.024/- | 146.9 ± 100.1 | 0.180 | 149.4 ± 109.7 | 0.310 |
As reported in Table 2, after a multivariate analysis of the statistically significant variables in univariate analysis, a positive association was found between liver steatosis and dyslipidemia (OR = 5.0; p = 0.001). In addition, significant fibrosis was positively associated with splenomegaly (OR11.0; p = 0.006), total bilirubin (OR = 7.2; p = 0.022) and reduced LAL activity (OR = 4.1; p = 0.022); and inversely with platelet count (OR = 1.013;p = 0.003) and LAL activity (OR = 1.006; p = 0.014). Among patients with cirrhosis, a positive association was verified with splenomegaly (OR = 13.3; p = 0.002), reduced LAL activity (OR = 12.6; p = 0.004) and total bilirubin (OR = 7.2; p = 0.042), and a negative association with platelet count (OR = 1.010; p = 0.005) and LAL activity (OR = 1.005; p = 0.003).
E8SJM mutation in LIPA geneSixty-five patients (64.4%) had LAL activity < 0.40 nmol/spot/h. These patients were tested to E8SJM mutation in LIPA gene. However, none of them was homozygous or heterozygous for this mutation.
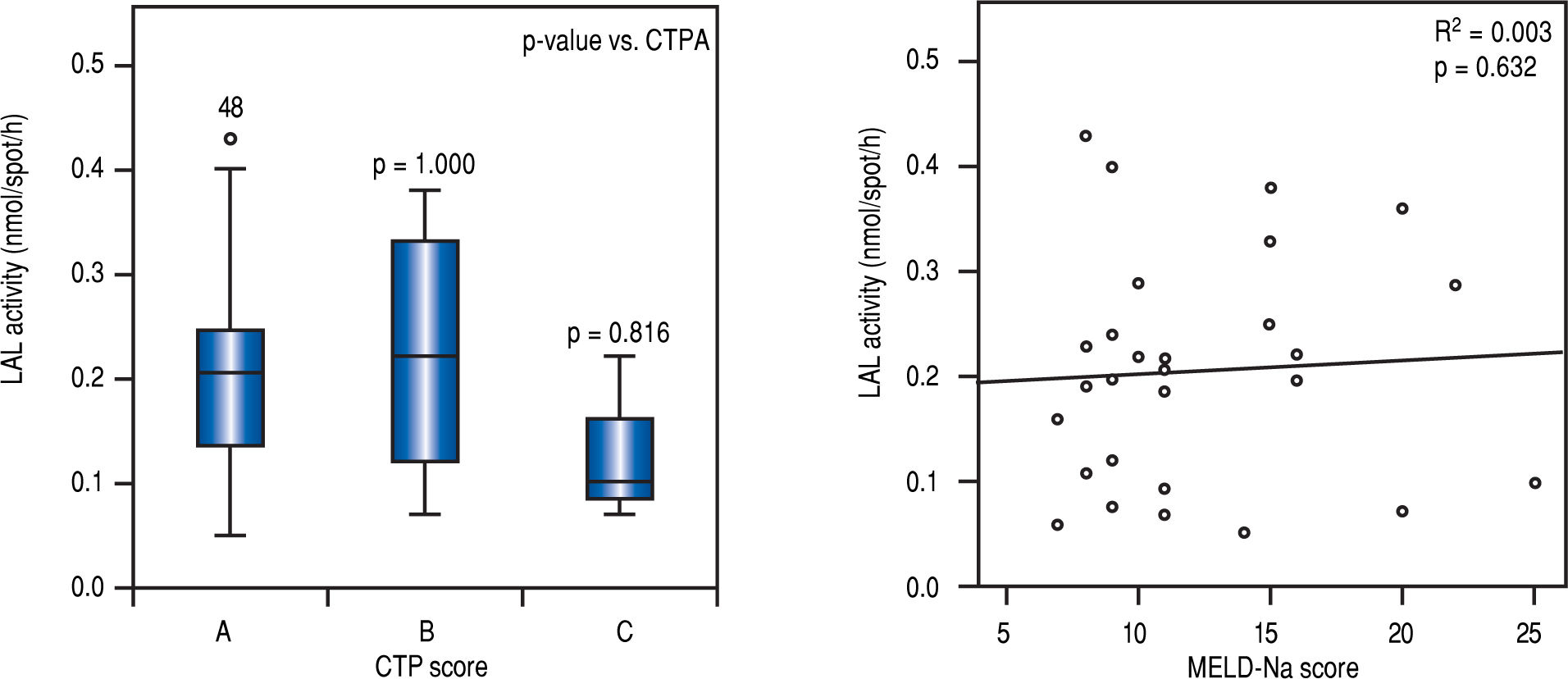
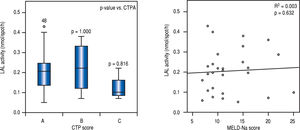
LAL activity among patients with cirrhosis and severity of liver diseaseIn multivariate analysis, LAL activity as a categorical or continuous variable was independently associated with cirrhosis (Table 2). Despite of median LAL activity be lower in cirrhotic patients than in patients with significant fibrosis, there was no association between LAL activity and liver dysfunction, assessed by CTP (p > 0.05 for CTP B or C compared with CTP A) and MELD-Na (r2 = 0.003; p = 0.632) (Figure 3).
Predictive power of LAL activity for steatosis, significant fibrosis and cirrhosisFigure 4 shows a lack of association between LAL activity and steatosis (AUROC = 0.536; p = 0.558). However, LAL activity showed a strongly positive association with significant fibrosis (AUROC = 0.833; p < 0.001) for a cut-off of 0.265 (sensitivity = 84.6%; specificity = 74.1%) and with cirrhosis (AUROC = 0.859;p = < 0.001) for a cut-off of 0.235 (sensitivity = 86.2%; specificity = 75.0%). LAL activity was superior to other non-expensive conventional biomarkers in predicting significant fibrosis and cirrhosis (FIB4: > F2-AUROC = 0.797; p < 0.001, F4-AUROC = 0.755; p < 0.001; GUCI: > F2-AUROC = 0.742; p = 0.001, F4-AUROC = 0.726; p = 0.001; APRI: > F2-AUROC = 0.694; p = 0.006; F4-AUROC = 0.682;p = 0.008).
DiscussionOur study expands knowledge about the influence of LAL activity on liver disease by adding paramount findings about this non-genetic test, as an independent non-invasive serum biomarker of significant fibrosis and cirrhosis in patients with persistent abnormal liver enzymes of unknown etiology with higher accuracy than other non-expensive conventional biomarkers. There was no correlation between LAL activity and cryptogenic steatosis after well-stablished risk factors for NAFLD/NASH have been ruled out.
For the first time, our study determined independent factors associated with reduced LAL activity in an adult population with persistently abnormal liver enzymes of unknown etiology with different degrees of fibrosis beyond cirrhosis. A positive and independent correlation was observed between reduced LAL activity and cryptogenic liver cirrhosis, also verified by other works,10,11 probably in relation to others factors transverse to cirrhosis of any etiology not yet recognized. However, our median LAL activity for cryptogenic cirrhosis was lower than in other works (0.21 vs. 0.49-0.62), which was comprised within heterozygous carrier range.10,11 Like other studies, low WBC was also a determinant of DBS-determined LAL activity in multivariate analysis, probably because LAL mainly comes from leucocytes and the enzymatic activity is normalized to spot diameter and not to protein concentration in DBS.8,17,18 However, Vespasiani-Gentilucci, et al. only considered cirrhotic patients which may have influenced the low WBC as a consequence of portal hypertension and splenomegaly.7
Since platelets contain lysosomes,19 several studied attempted to evaluate the correlation between LAL activity and platelet count.7,8,18 Some studies suggested that LAL activity could be in relation with platelet count in a cirrhosis context,7,8 and more recently, in healthy subjects.18 However, our study performed in patients with different degrees of fibrosis beyond cirrhosis showed no association between LAL activity and platelet count, suggesting the involvement of other unknown mechanisms in LAL activity reduction for cryptogenic fibrosis/cirrhosis.
Additionally, since steatosis and fibrosis are the major determinants of liver dysfunction progression regardless of liver disease etiology, the authors also determined the factors associated with steatosis and significant fibrosis.
Concerning liver steatosis, there was an association with dyslipidemia. In fact, only studies evaluating the relationship between LAL activity and cirrhosis by NAFLD/NASH have been performed, where the association with steatosis is undoubted. Despite the association of dyslipidemia in patients with NAFLD/NASH and LAL activity, some studies have reported an association with a high total-C and LDL-C,6,20 suggesting that a reduction in lipid droplet clearance by lysosomal pathway could lead to accumulation of both esterified and non-esterified cholesterol in hepatocytes.21 However, it remains unknown whether the reduction of circulating LAL activity is susceptible to cause a significant reduction in liver LAL activity.22 Indeed, our study found a correlation between LAL activity and significant fibrosis/ cirrhosis subgroups but not with steatosis subgroup. This fact may be related to the exclusion of patients with well-stablished risk factors to NAFLD/NASH, including obesity, type 2 diabetes and metabolic syndrome. In NAFLD/NASH background, LAL activity can be modulated by these risk factors and not by NAFLD/ NASH condition per se. Even in studies with a large number of patients with cryptogenic cirrhosis, most patients had obesity (mean body mass index of 30.9 ± 7.8 Kg/m2) and type 2 diabetes (68%),7,11 both conditions strongly associated with the presence and progression of NAFLD/NASH.4,5 For the first time, authors evaluated the role of LAL activity in patients without well-stablished risk factors for NAFLD/NASH, and no association was found with dyslipidemia or lipid profile. This suggests that only non-atherogenic form of dyslipidemia may be related to reduced LAL activity, contrarily to atherogenic dyslipidemia, which was associated with NAFLD/NASH, characterized by hypertriglyceridemia, low HDL-C and high LDL-C.12,23 In order to evaluate the predictive power of LAL activity in liver steatosis subgroup, no association was evidenced through multivariate and AUROC analyses, suggesting that the association with steatosis in other studies was a result of NAFLD/NASH etiology of cirrhosis and metabolic syndrome6,7 and not due to steatosis without these features.
Our study also describes, for the first time in adult patients, the role of LAL activity and other factors as determinants of significant fibrosis beyond cirrhosis. A study in pediatric population found a significant lower LAL activity in children with significant fibrosis than those with mild fibrosis. However they did not have cirrhotic children in study population and therefore did not assess differences between significant fibrosis and cirrhosis.14 In addition to LAL activity, other factors such as splenomegaly, high total bilirubin and low platelet count were independently associated with both significant fibrosis and cirrhosis. These parameters represent indirect markers of cirrhosis due to portal hypertension and hypersplenism.7,14 However, these factors were also related to significant fibrosis, suggesting that these factors are already present at an early stage of fibrosis progression, although with higher expression in cirrhosis. Despite a good correlation between reduced LAL activity and fibrosis degree, this correlation was independent of liver function. According to pathophysiology, reduced LAL activity could be a mere consequence of overall decrease of viable hepatocytes and subsequently less protein synthesis.14 Additionally, some studies have shown that lysosomal-associated natural killer cells were crucial in preventing fibrosis progression in liver disease.24,25 However, LAL activity was not correlated with liver dysfunction assessed by CTP and MELD-Na scores, as demonstrated in another study with the evaluation of one of these scores, the CTP score.7,11 This fact suggests that mechanisms related to the reduction of LAL activity are associated with the fibrosis process and not with liver function. Like Vespasiani-Gentilucci, et al, and contrarily to Baratta, et al.,6 no association was found between LAL activity and transaminase levels. Nonetheless, Baratta, et al.6 only evaluated patients with NAFLD/NASH where the inflammatory component plays an important role given its association with insulin resistance, a central feature of metabolic syndrome.26 Since LAL activity was independently related to significant fibrosis and cirrhosis, our study also evaluated the predictive power of LAL activity in their prediction. Currently, there are no specific biomarkers/tests for fibrosis or cirrhosis.4 For the first time, LAL activity was compared to other known non-invasive and non-expensive fibrosis biomarkers/tests. The strongest predictor for significant fibrosis with an AU-ROC of 0.833 (p < 0.001) and most striking for cirrhosis with an AUROC of0.859 (p < 0.001) for a cut-off of 0.265 nmol/spot/h and 0.235 nmol/spot/h respectively, which fits the heterozygous carrier range of LAL activity. This fact suggests that in patients with unexplained abnormal liver enzymes, LAL activity below 0.265 nmol/spot/h is strongly related to the presence of significant fibrosis and when LAL activity falls below 0.235 nmol/spot/h makes the presence of cirrhosis very likely.
Additionally, as Vespasiani-Gentilucci, et al.,7 the reduction of LAL activity in patients with significant fibrosis and cirrhosis was probably acquired and not genetically determined,26 since the major mutation of LIPA gene was not found.27,28 Furthermore, in an adult population with a suspected clinical and laboratory context of cholesterol ester storage diseases, screening for LIPA gene mutation should be performed to a threshold below the upper limit of heterozygous carrier range in case of significant fibrosis or cirrhosis. However, if reduced LAL activity is a cause or consequence of significant fibrosis and cirrhosis remains to be determined.
The limitations of this study included:
- •
The cross-sectional study design, and therefore, the impossibility to establish a cause-effect relationship between reduced LAL activity and the presence of cryptogenic significant fibrosis or cirrhosis.
- •
A single-center study.
- •
Fibrosis and steatosis were evaluated by 2 different techniques (liver biopsy and FibroScan®), but the majority of patients performed liver biopsy (almost 3/4) and FibroScan® is a validated tool to accurately assess fibrosis and steatosis, significantly reducing the heterogeneity of study population.
On the other hand, our study has many strengths:
- •
The exclusion of potential confounders through a multivariate logistic analysis.
- •
The evaluation of LAL activity determinants to properly interpret the results in subgroups of steatosis, significant fibrosis and cirrhosis.
- •
The evaluation of LAL activity determinants for different degrees of liver fibrosis.
- •
The comparison, for the first time, between LAL activity and other conventional biomarkers/tests to assess liver fibrosis.
In conclusion, our study highlights that the reduction of LAL activity appears to occur on an acquired-basis for significant fibrosis and cirrhosis at levels < 0.40 nmol/ spot/h. LAL activity < 0.265 nmol/spot/h was strongly associated with cryptogenic significant fibrosis and < 0.235 nmol/spot/h with cryptogenic cirrhosis. Contrarily, no correlation was found between LAL activity and cryptogenic liver steatosis in the absence of well stablished risk factors for NAFLD/NASH. LAL enzyme seems to be a new non-invasive serum biomarker of cryptogenic liver fibrosis with higher accuracy than conventional known biomarkers/tests.
Further prospective multicenter studies are needed to understand the role of LAL in liver fibrogenesis and its underlying mechanisms related to the progression of cryptogenic fibrosis to cirrhosis.
Abbreviations- •
AUROC: area under receiver operating characteristic curve.
- •
C: cholesterol.
- •
CTP: Child-Turcotte-Pugh.
- •
DBS: dried blood spot testing.
- •
HDL-C: high-density lipoprotein-cholesterol.
- •
LAL: lysosomal acid lipase.
- •
LDL: low density lipoprotein.
- •
LDL-C: low-density lipoprotein-cholesterol.
- •
MELD-Na: model for end-stage liver disease-sodium.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
WBC: white blood cell count.
Protection of human and animal subjects.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of DataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to Privacy and Informed ConsentThe authors declare that no patient data appears in this article.
Conflicts of InterestThe authors declares that there is no conflict of interest regarding the publication of this article.
Source of FundingAuthors have no source of founding to declare.
Author’s ContributionsMarta Gravito-Soares and Elisa Gravito-Soares contributed equally to the patient recruitment, study design, data analysis and interpretation and wrote the manuscript; Dario Gomes and Luis Tome followed up patients, analyzed data, interpreted results and revised the manuscript for the important intellectual content.
Guarantor of the ArticleMarta Gravito-Soares.