Liver transplantation (LT) provides durable survival for hepatocellular carcinoma (HCC). However, there is continuing debate concerning the impact of wait time and acceptable tumor burden on outcomes after LT. We sought to review outcomes of LT for HCC at a single, large U.S. center, examining the influence of wait time on post-LT outcomes.
Material and MethodsWe reviewed LT for HCC at Mayo Clinic in Florida from 1/1/2003 until 6/30/2014. Follow up was updated through 8/1/ 2015.
ResultsFrom 2003-2014, 978 patients were referred for management of HCC. 376 patients were transplanted for presumed HCC within Milan criteria, and the results of these 376 cases were analyzed. The median diagnosis to LT time was 183 days (8 - 4,337), and median transplant list wait time was 62 days (0 - 1815). There was no statistical difference in recurrence-free or overall survival for those with wait time of less than or greater than 180 days from diagnosis of HCC to LT. The most important predictor of long term survival after LT was HCC recurrence (HR: 18.61, p < 0.001). Recurrences of HCC as well as survival were predicted by factors related to tumor biology, including histopathological grade, vascular invasion, and pre-LT serum alpha-fetoprotein levels. Disease recurrence occurred in 13%. The overall 5-year patient survival was 65.8%, while the probability of 5-year recurrence-free survival was 62.2%.
ConclusionsIn this large, single-center experience with long-term data, factors of tumor biology, but not a longer wait time, were associated with recurrence-free and overall survival.
Liver transplantation (LT) remains the best treatment for carefully selected patients with hepatocellular carcinoma (HCC). In 1996, Mazzaferro, et al. established the Milan Criteria, which demonstrated that LT recipients with early HCC (as defined by having either a single lesion no > 5 cm or < 3 lesions each ≤ 3 cm) can achieve favorable long term patient survival. In 2001, the UCSF group suggested that these guidelines could be extended to include patients with a single lesion ≤ 6.5 cm or no more than 3 lesions each ≤ 4.5 cm and a total sum of these lesions each ≤ 8 cm. Following the introduction of the Model for End-Stage Liver Disease (MELD) system for liver allocation in February of 2002, there has been considerable debate regarding LT wait time in patients with cirrhosis and HCC.1-3 Patients were initially assigned a MELD exception score of 29 when they had HCC within Milan criteria. The intent was to avoid waitlist mortality or dropout due to tumor progression, as this score was thought to represent a 30% probability of three-month mortality. Subsequent studies suggested this adjusted score provided an unfair advantage to patients listed with HCC.4-6 HCC patients continued to demonstrate survival advantages over waitlist patients with non-malignant disease, despite incremental reductions in MELD exception scores being grant-ed.7 A rise in mortality in patients without HCC on the waitlist was observed due to MELD inflation from HCC exception points combined with inadequate liver donor availability.8,9 Consequently, HCC MELD exception scores have been blamed for an exacerbation of the geographical disparity in access to LT. Further, recent reports have suggested expedited LT for HCC may lead to inferior outcomes in these patients.1,2,10 Authors compared HCC patients in different UNOS regions with varying average wait times concluding that wait time rather than transplant center and regional practice variation could account for the differences in post-LT mortality.2,10 Unfortunately, most of these data were reliant on registry studies which are limited by incomplete reporting of post-LT HCC recurrence, varied HCC transplant center behavior and listing practices, as well as regional differences in HCC referral and management.11 Despite significant study limitations, UNOS recently implemented a policy of 6-month wait time before HCC MELD exception scores are assigned to reduce any unfair advantage.12
Large single-center experiences with long-term outcomes data are lacking, yet are necessary to exclude the potential confounding transplant center effect and to clarify the role of wait time on post-LT outcome. The Mayo Clinic in Florida (MCF) experience provides a unique opportunity to examine the impact of short and long wait times for patients with HCC via a large single-center cohort with detailed clinical data, along with uniform listing and transplant behavior.13,14 We have observed a steady increase in wait times from HCC diagnosis to LT over the seventeen year history of our transplant program, and this increase in wait time allows for a more controlled analysis to examine the influence of time from HCC diagnosis to transplant, waitlist drop off, and post-LT outcome. The purpose of this study was to review survival outcomes and HCC recurrence events in a large consecutive series of patients who underwent LT for HCC at our center, and to determine the role wait time played in the outcomes of HCC patients with an intention-to-treat analysis.
Material and MethodsCohort selectionPatients undergoing LT for HCC at MCF from January 1, 2003 to June 30, 2014 meeting pre-LT Milan criteria were considered for analysis, with follow up maintained through August 1, 2015. Data on pre and post-LT variables were manually extracted from the patients’ records. All patients were considered for listing based on pre-LT imaging that met UNOS class 5 criteria, and/or pathologic diagnosis on biopsy. Patients with recognized metastases or macrovascular invasion on pre transplant imaging studies were excluded from LT. Pre-LT imaging modalities included ultrasound, along with intravenous (IV) contrast-enhanced magnetic resonance (MR) and/or IV contrast-enhanced computerized tomography (CT).14 Pre-LT locoregional treatments were performed in all patients if the timeline permitted, unless contraindicated. Locore-gional treatments included transarterial chemoemboliza-tion (TACE), transarterial yttrium-90 radioembolization (TARE) and/or radiofrequency ablation (RFA). TACE was performed as part of the clinical protocol as previously re-ported.15 TARE and RFA was selectively performed when indicated. Those patients with HCC outside of Milan criteria on pre-LT imaging were excluded from analyses; unless they were successfully downstaged using approved regional protocols. Patients with incidentally found HCC at explant but no pre-LT evidence of HCC were excluded from the study.
LT was performed using the piggyback technique as described previously.16 All patients in the study were within the MELD era to allow for consistency of analysis. Standard immunosuppression included tacrolimus, myco-phenolate mofetil, and prednisone, with discontinuation of mycophenolate mofetil in the early post-LT period where possible, and attempted discontinuation of pred-nisone within six months of transplant. Alternative agents such as sirolimus and cyclosporin were chosen in select patients who had contraindications to the standard protocol. Immunosuppressive induction included basiliximab in cases with significant renal impairment.
Post-LT recurrence monitoring involved serum alpha-fetoprotein (AFP) measurements, along with bone scans and cross-sectional imaging at 4, 8, 12, 24, and 36 months after LT. Standard clinical follow up occurred at 4, 8, and 12 months, and then annually thereafter. Phone and email inquiries were made into outcomes of those patients who transferred to other facilities or moved to distant locations.
Definition of HCC groups by pathologic reviewAll liver explants were examined by experienced liver histopathologists. The tumor stage was defined by the ex-plant pathology according to the protocol provided by the College of American Pathologists17 or by pre-LT clinical criteria in treated patients with no viable HCC on explant. Tumor grade was determined according to the 7th edition American Joint Committee on Cancer staging with grade 1 as well differentiated, grade 2 as moderately differentiated and grade 3 as poorly differentiated. Vascular invasion was classified as microvascular invasion when found histologi-cally and as macrovascular invasion when detected on gross evaluation of the explants. Patients with HCC at ex-plant were categorized as within MC, beyond MC but within University of California at San Francisco (UCSF) criteria,18 and beyond UCSF criteria regardless of tumor grade or vascular involvement. Tumor-Node-Metastasis (TNM) staging was calculated at both pre-LT imaging and explant based on National Comprehensive Cancer Network guidelines.17
Wait time was defined as the time from diagnosis to transplant date. Day of diagnosis was defined as the date when the lesion was found on imaging. These lesions either met UNOS class 5a or 5b criteria or were confirmed on pathology by biopsy. A UNOS class 5a lesion met criteria if a given nodule of 1-2 cm in size and meets all qualitative criteria for HCC on cross-sectional imaging.19 A UNOS class 5b lesion included any lesion between 2-5cm in size and meeting qualitative criteria for HCC on cross-sectional imaging. Focusing on the date of HCC diagnosis to LT time allowed us to account for the time spent on patient referral, relocation, transplant evaluation, and subsequent listing at our center. This figure potentially limits bias associated with center specific listing practice and referral patterns.
Recurrence-free survival was assigned if the patient died or had a documented HCC recurrence was censored on death without recurrence or last follow-up date. Comparisons of these groups for overall survival and recurrence-free survival, as well as risk factors for HCC recurrence, were subsequently undertaken.
Data analysisAll numeric data were reported as median and range, and mean. Comparison between groups was performed using the χ2 test for categorical variables. Continuous variables comparisons were made using the Wilcoxon rank-sum test. Survival time started with the day of LT. Overall and recurrence-free survivals in each group were estimated using the Kaplan-Meier method and groups were compared using the log-rank test. Potential risk factors for each of the endpoints were investigated using Cox Proportional Hazards models. These relationships were illustrated with hazard ratios, and 95% confidence intervals for those hazard ratios. Multivariate models were selected using a stepwise selection technique with a retention p-value < 0.1. The patient parameters that were included in the analysis included: age, gender, ethnicity, primary etiology of cirrhosis, hepatitis C (HCV) diagnosis, biologic and listing MELD score, body mass index (BMI), serum AFP, loco-regional treatment prior to LT, and time from HCC diagnosis to LT. Recurrence was related to overall survival by using it as a time-dependent covariate in the Cox proportional hazards model. Parameters associated with the donor organ included: age, gender, donor liver mass or weight (DLM), donor risk index (DRI),20 donation after cardiac death (DCD) or donation after brain death (DBD). Operative data included: warm ischemic time (WIT) in minutes, cold ischemic time (CIT) in hours, transfused packed red blood cells (PRBC), duration of the operation from skin incision to closure. Pre-LT and ex-plant tumor characteristics included: HCC TNM stag-ing,17 tumor number, tumor grade (for those with HCC at explant), largest tumor size, and presence of macro or micro vascular invasion. Statistical analysis was performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC) and STATA (StataCorp, College Station, TX). A statistical significance was assigned at p value < 0.05.
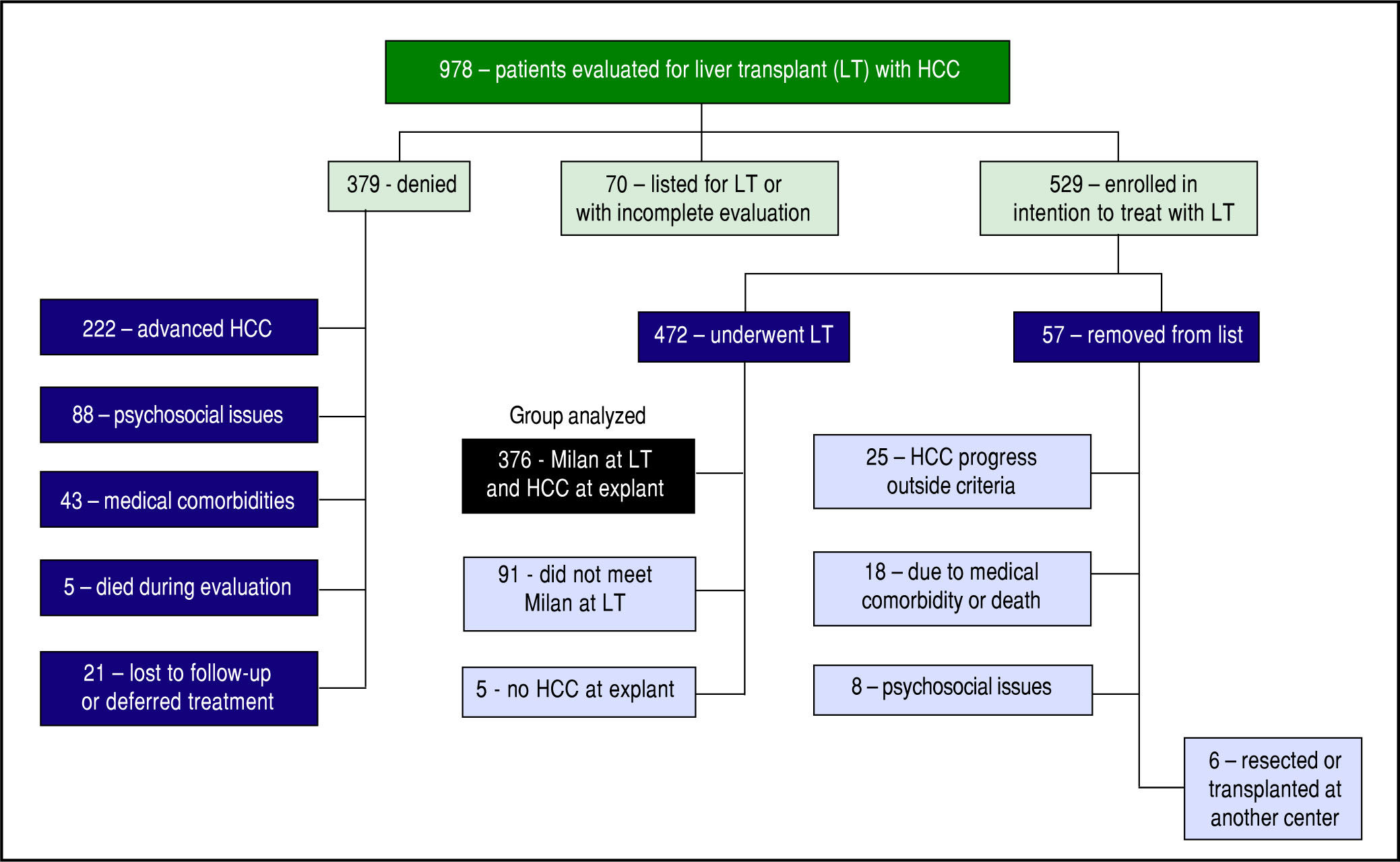
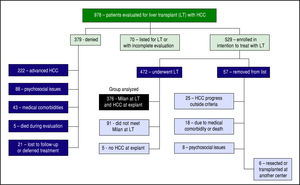
ResultsFrom January 1, 2003 until June 30, 2014, 978 patients with HCC were evaluated for potential LT of HCC (Figure 1). At the time of our analysis, 70 patients were pending listing for LT and had incomplete evaluations. 379 were denied listing for LT: 222 had advanced HCC, 43 had significant medical comorbidities, 88 had psychoso-cial issues (refractory chemical dependency, poor social support, and/or financial constraints), 21 deferred treatment or were lost to follow up, and 5 died during the evaluation.
A total of 529 patients (54.1%) were listed for LT. With a waitlist dropout rate of 10.8%, 57 patients were subsequently removed from listing for the following reasons: 25 developed HCC progression (4.7%), 18 developed medical issues that precluded LT, 6 patients elected to be removed (1 had hepatic resection, 5 were transplanted at other centers), and 8 denied for psycho-social reasons. The remaining 472 patients were transplanted. 96 of these were excluded from our analyses because 5 had no tumor found at explant without previous ablation therapy, and 91 patients had small tumors that did not meet MELD exception points but were transplanted based on MELD score due to advanced liver disease.
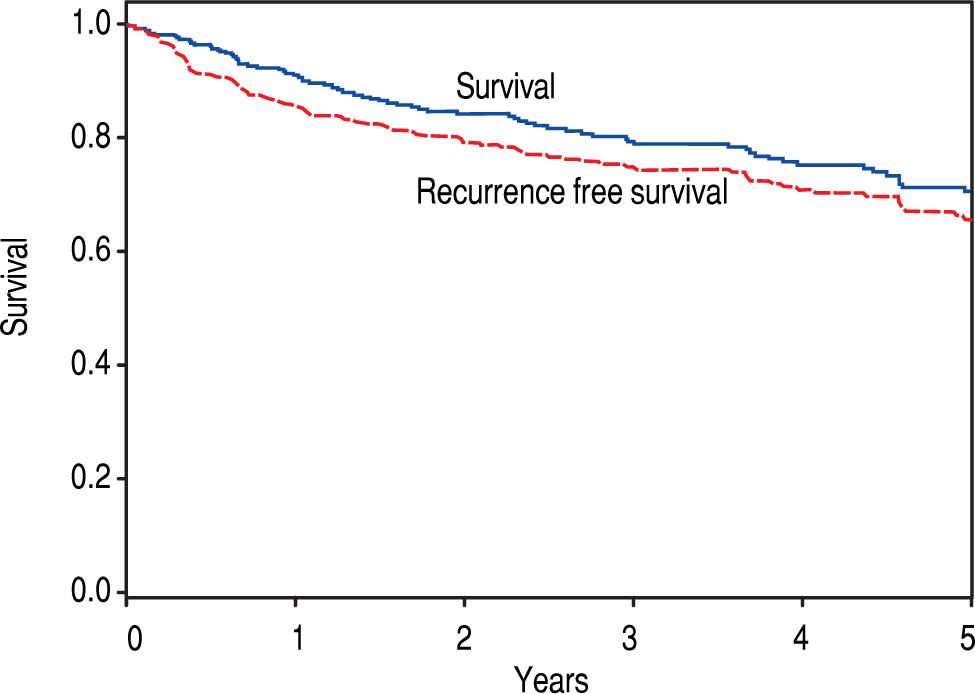
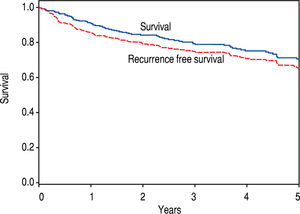
The remaining 376 patients were analyzed. Overall, 75.3% were male and 74.2% were Caucasian, with an average age of 60.2 years at LT. BMI was an average of 29.7, and a history of HCV was noted in 62.5%. The median diagnosis to LT time was 183 days (8 - 4337), and median transplant list wait time was 62 days (0 – 1,815). On initial pre-LT imaging, 333 patients met MC, 38 more were within UCSF, and 5 had HCC beyond MC and UCSF criteria. Those patients who had HCC beyond MC were subsequently downstaged with either TACE and/or RFA and were not listed until they were within MC. On ex-plant review, 327 were T-stage 2 or less (94.2%). Of note, 20 patients were incidentally found to have cholangiocar-cinoma (CCA) or mixed HCC/CCA at explant - these patients were included in the analysis as their diagnosis was unknown prior to transplant. Overall, patient survival at 1, 3, and 5 years was 92.4%, 79.2%, and 65.8%, respectively. HCC recurrence occurred in 38 patients. Recurrence-free patient survival over 1, 3, and 5 years was 88.2%, 76.6%, and 62.2%, respectively (Figure 2).
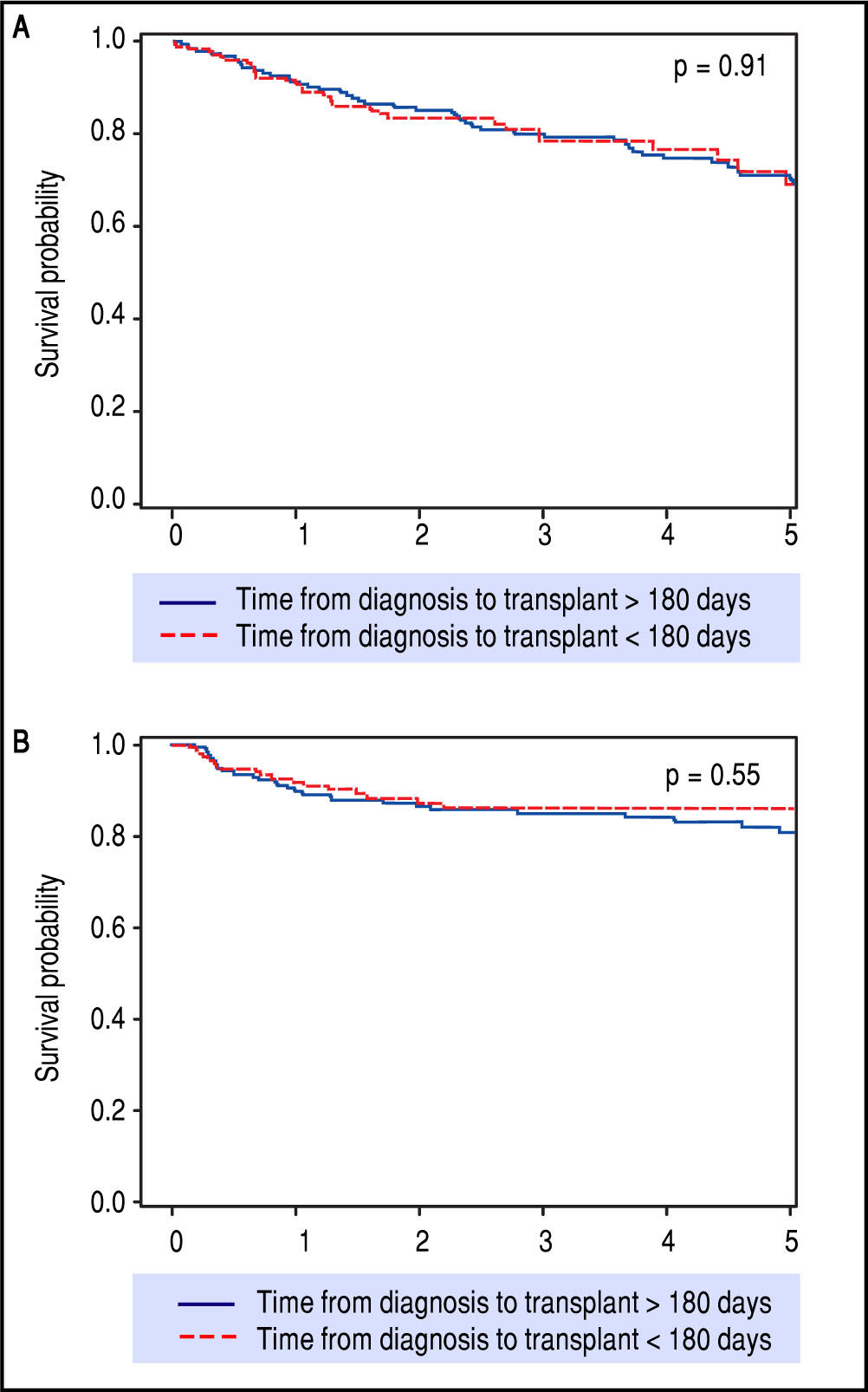
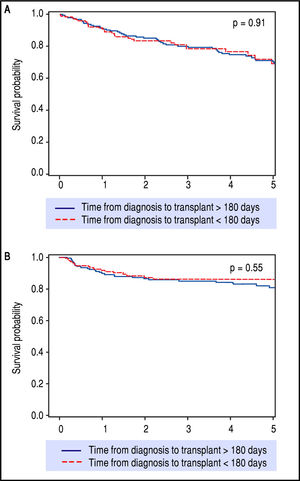
After preliminary analyses, 180 days from HCC diagnosis until LT was selected as our time-point for statistical analysis, to compare short wait times with longer wait times (Table 1). This not only allowed for nearly equal groups of comparison, but also seemed most relevant in light of the recent UNOS policy change. These two groups had similar patient demographics and etiologies for underlying liver disease. The group waiting less than 180 days had lower MELD at LT, larger average tumor size, but had no difference in overall or recurrence-free survival rates over five years of follow-up (Figure 3).
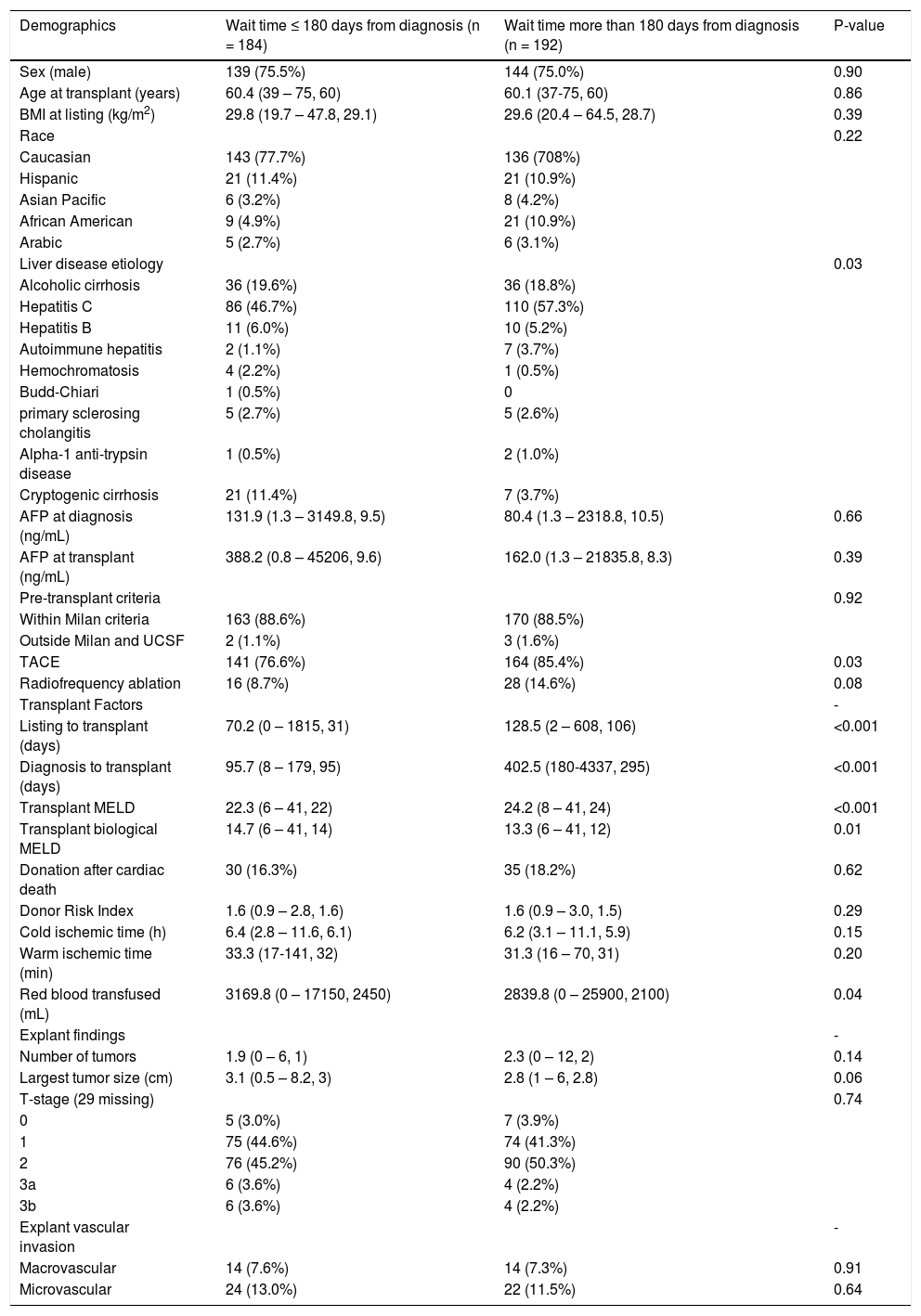
Cohort demographics related to wait time (n = 376).
| Demographics | Wait time ≤ 180 days from diagnosis (n = 184) | Wait time more than 180 days from diagnosis (n = 192) | P-value |
|---|---|---|---|
| Sex (male) | 139 (75.5%) | 144 (75.0%) | 0.90 |
| Age at transplant (years) | 60.4 (39 – 75, 60) | 60.1 (37-75, 60) | 0.86 |
| BMI at listing (kg/m2) | 29.8 (19.7 – 47.8, 29.1) | 29.6 (20.4 – 64.5, 28.7) | 0.39 |
| Race | 0.22 | ||
| Caucasian | 143 (77.7%) | 136 (708%) | |
| Hispanic | 21 (11.4%) | 21 (10.9%) | |
| Asian Pacific | 6 (3.2%) | 8 (4.2%) | |
| African American | 9 (4.9%) | 21 (10.9%) | |
| Arabic | 5 (2.7%) | 6 (3.1%) | |
| Liver disease etiology | 0.03 | ||
| Alcoholic cirrhosis | 36 (19.6%) | 36 (18.8%) | |
| Hepatitis C | 86 (46.7%) | 110 (57.3%) | |
| Hepatitis B | 11 (6.0%) | 10 (5.2%) | |
| Autoimmune hepatitis | 2 (1.1%) | 7 (3.7%) | |
| Hemochromatosis | 4 (2.2%) | 1 (0.5%) | |
| Budd-Chiari | 1 (0.5%) | 0 | |
| primary sclerosing cholangitis | 5 (2.7%) | 5 (2.6%) | |
| Alpha-1 anti-trypsin disease | 1 (0.5%) | 2 (1.0%) | |
| Cryptogenic cirrhosis | 21 (11.4%) | 7 (3.7%) | |
| AFP at diagnosis (ng/mL) | 131.9 (1.3 – 3149.8, 9.5) | 80.4 (1.3 – 2318.8, 10.5) | 0.66 |
| AFP at transplant (ng/mL) | 388.2 (0.8 – 45206, 9.6) | 162.0 (1.3 – 21835.8, 8.3) | 0.39 |
| Pre-transplant criteria | 0.92 | ||
| Within Milan criteria | 163 (88.6%) | 170 (88.5%) | |
| Outside Milan and UCSF | 2 (1.1%) | 3 (1.6%) | |
| TACE | 141 (76.6%) | 164 (85.4%) | 0.03 |
| Radiofrequency ablation | 16 (8.7%) | 28 (14.6%) | 0.08 |
| Transplant Factors | - | ||
| Listing to transplant (days) | 70.2 (0 – 1815, 31) | 128.5 (2 – 608, 106) | <0.001 |
| Diagnosis to transplant (days) | 95.7 (8 – 179, 95) | 402.5 (180-4337, 295) | <0.001 |
| Transplant MELD | 22.3 (6 – 41, 22) | 24.2 (8 – 41, 24) | <0.001 |
| Transplant biological MELD | 14.7 (6 – 41, 14) | 13.3 (6 – 41, 12) | 0.01 |
| Donation after cardiac death | 30 (16.3%) | 35 (18.2%) | 0.62 |
| Donor Risk Index | 1.6 (0.9 – 2.8, 1.6) | 1.6 (0.9 – 3.0, 1.5) | 0.29 |
| Cold ischemic time (h) | 6.4 (2.8 – 11.6, 6.1) | 6.2 (3.1 – 11.1, 5.9) | 0.15 |
| Warm ischemic time (min) | 33.3 (17-141, 32) | 31.3 (16 – 70, 31) | 0.20 |
| Red blood transfused (mL) | 3169.8 (0 – 17150, 2450) | 2839.8 (0 – 25900, 2100) | 0.04 |
| Explant findings | - | ||
| Number of tumors | 1.9 (0 – 6, 1) | 2.3 (0 – 12, 2) | 0.14 |
| Largest tumor size (cm) | 3.1 (0.5 – 8.2, 3) | 2.8 (1 – 6, 2.8) | 0.06 |
| T-stage (29 missing) | 0.74 | ||
| 0 | 5 (3.0%) | 7 (3.9%) | |
| 1 | 75 (44.6%) | 74 (41.3%) | |
| 2 | 76 (45.2%) | 90 (50.3%) | |
| 3a | 6 (3.6%) | 4 (2.2%) | |
| 3b | 6 (3.6%) | 4 (2.2%) | |
| Explant vascular invasion | - | ||
| Macrovascular | 14 (7.6%) | 14 (7.3%) | 0.91 |
| Microvascular | 24 (13.0%) | 22 (11.5%) | 0.64 |
Wait time is defined as HCC diagnosis date until date of liver transplantation. Age and Body Mass Index listed as mean (range, median). Values listed as mean (range, median). AFP: Alpha-feto protein. BMI: Body Mass Index. UCSF: University of California in San Francisco. TACE: Transarterial chemoemboli-zation.
Kaplan-Meier survival (p = 0.91) and recurrence-free survival (p = 0.55) analyses depicting no difference in outcomes after LT when stratifying for diagnosis-to-transplant wait time of greater or less than 180 days. Survival (A) and recurrence-free survival (B) for > 180 days and < 180 days wait time.
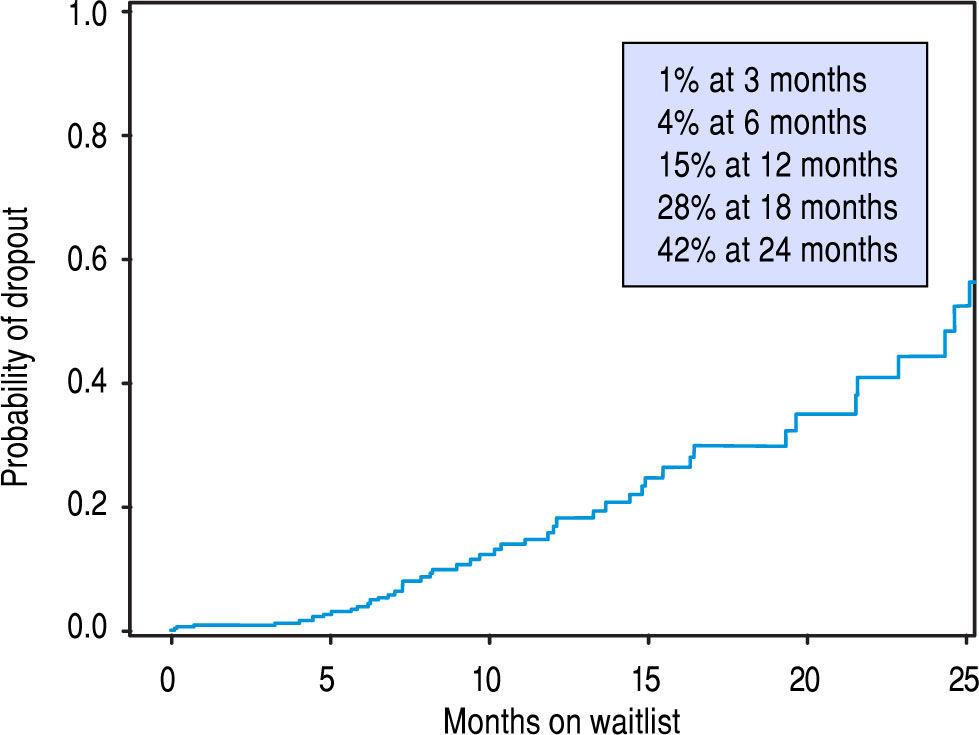
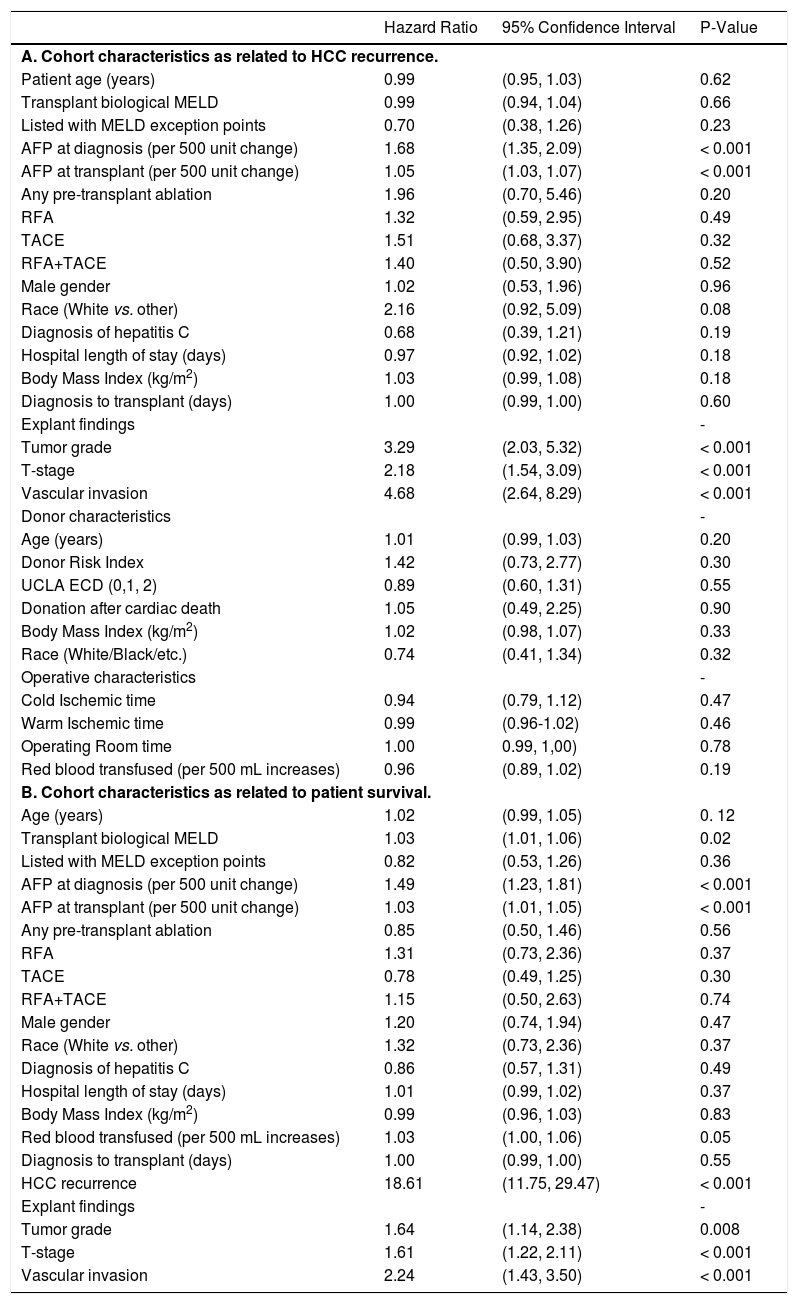
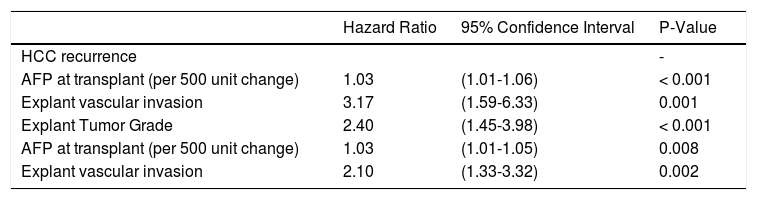
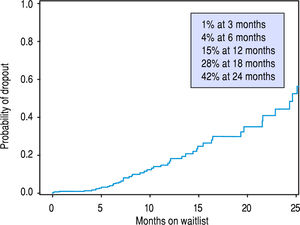
Univariate Cox proportional hazard analyses for variables related to HCC recurrence and survival were performed. Serum AFP at diagnosis and LT, and explant findings of TNM staging, histopathological tumor grade, and vascular invasion all statistically predicted HCC recurrence (Table 2A) and overall survival after LT (Table 2B) on univariate analysis. The only predictive pre-LT variable for recurrence was AFP. Using an AFP of > 400 ng/mL at any time prior to LT, we found that recurrence was 43.5% vs. 9.9% in those who had an AFP < 400 ng/mL. Using a multivariate backwards stepwise Cox proportional hazard model for HCC recurrence and patient survival, we found similar associations (Table 3). AFP at Transplant (HR: 1.03, CI: 1.01-1.06, p < 0.001), explant vascular invasion (HR: 3.17, CI: 1.59-6.33, p = 0.001), and explant tumor grade (HR: 2.40, CI: 1.45-3.98, p < 0.001) were all independently associated with increased risk for recurrence. Only AFP at transplant (HR: 1.03, CI: 1.01-1.05, p = 0.008) and explant vascular invasion (HF: 2.10, CI: 1.33-3.32, p = 0.002) were associated with decreased survival. Figure 4 displays the rate of waitlist drop-out over time, which is found to be 1% at 3 months, 4% at 6 months and 15% at 1 year. The median time to dropout for patients who dropped out was 9.6 months.
Univariate model of HCC recurrence and patient survival.
| Hazard Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| A. Cohort characteristics as related to HCC recurrence. | |||
| Patient age (years) | 0.99 | (0.95, 1.03) | 0.62 |
| Transplant biological MELD | 0.99 | (0.94, 1.04) | 0.66 |
| Listed with MELD exception points | 0.70 | (0.38, 1.26) | 0.23 |
| AFP at diagnosis (per 500 unit change) | 1.68 | (1.35, 2.09) | < 0.001 |
| AFP at transplant (per 500 unit change) | 1.05 | (1.03, 1.07) | < 0.001 |
| Any pre-transplant ablation | 1.96 | (0.70, 5.46) | 0.20 |
| RFA | 1.32 | (0.59, 2.95) | 0.49 |
| TACE | 1.51 | (0.68, 3.37) | 0.32 |
| RFA+TACE | 1.40 | (0.50, 3.90) | 0.52 |
| Male gender | 1.02 | (0.53, 1.96) | 0.96 |
| Race (White vs. other) | 2.16 | (0.92, 5.09) | 0.08 |
| Diagnosis of hepatitis C | 0.68 | (0.39, 1.21) | 0.19 |
| Hospital length of stay (days) | 0.97 | (0.92, 1.02) | 0.18 |
| Body Mass Index (kg/m2) | 1.03 | (0.99, 1.08) | 0.18 |
| Diagnosis to transplant (days) | 1.00 | (0.99, 1.00) | 0.60 |
| Explant findings | - | ||
| Tumor grade | 3.29 | (2.03, 5.32) | < 0.001 |
| T-stage | 2.18 | (1.54, 3.09) | < 0.001 |
| Vascular invasion | 4.68 | (2.64, 8.29) | < 0.001 |
| Donor characteristics | - | ||
| Age (years) | 1.01 | (0.99, 1.03) | 0.20 |
| Donor Risk Index | 1.42 | (0.73, 2.77) | 0.30 |
| UCLA ECD (0,1, 2) | 0.89 | (0.60, 1.31) | 0.55 |
| Donation after cardiac death | 1.05 | (0.49, 2.25) | 0.90 |
| Body Mass Index (kg/m2) | 1.02 | (0.98, 1.07) | 0.33 |
| Race (White/Black/etc.) | 0.74 | (0.41, 1.34) | 0.32 |
| Operative characteristics | - | ||
| Cold Ischemic time | 0.94 | (0.79, 1.12) | 0.47 |
| Warm Ischemic time | 0.99 | (0.96-1.02) | 0.46 |
| Operating Room time | 1.00 | 0.99, 1,00) | 0.78 |
| Red blood transfused (per 500 mL increases) | 0.96 | (0.89, 1.02) | 0.19 |
| B. Cohort characteristics as related to patient survival. | |||
| Age (years) | 1.02 | (0.99, 1.05) | 0. 12 |
| Transplant biological MELD | 1.03 | (1.01, 1.06) | 0.02 |
| Listed with MELD exception points | 0.82 | (0.53, 1.26) | 0.36 |
| AFP at diagnosis (per 500 unit change) | 1.49 | (1.23, 1.81) | < 0.001 |
| AFP at transplant (per 500 unit change) | 1.03 | (1.01, 1.05) | < 0.001 |
| Any pre-transplant ablation | 0.85 | (0.50, 1.46) | 0.56 |
| RFA | 1.31 | (0.73, 2.36) | 0.37 |
| TACE | 0.78 | (0.49, 1.25) | 0.30 |
| RFA+TACE | 1.15 | (0.50, 2.63) | 0.74 |
| Male gender | 1.20 | (0.74, 1.94) | 0.47 |
| Race (White vs. other) | 1.32 | (0.73, 2.36) | 0.37 |
| Diagnosis of hepatitis C | 0.86 | (0.57, 1.31) | 0.49 |
| Hospital length of stay (days) | 1.01 | (0.99, 1.02) | 0.37 |
| Body Mass Index (kg/m2) | 0.99 | (0.96, 1.03) | 0.83 |
| Red blood transfused (per 500 mL increases) | 1.03 | (1.00, 1.06) | 0.05 |
| Diagnosis to transplant (days) | 1.00 | (0.99, 1.00) | 0.55 |
| HCC recurrence | 18.61 | (11.75, 29.47) | < 0.001 |
| Explant findings | - | ||
| Tumor grade | 1.64 | (1.14, 2.38) | 0.008 |
| T-stage | 1.61 | (1.22, 2.11) | < 0.001 |
| Vascular invasion | 2.24 | (1.43, 3.50) | < 0.001 |
RFA: radiofrequency ablation. TACE: transarterial chemoembolization. UCLA ECD: Extended Criteria Donor.
Multivariate model of HCC recurrence and patient survival. Analysis of variables related to patient survival.
| Hazard Ratio | 95% Confidence Interval | P-Value | |
|---|---|---|---|
| HCC recurrence | - | ||
| AFP at transplant (per 500 unit change) | 1.03 | (1.01-1.06) | < 0.001 |
| Explant vascular invasion | 3.17 | (1.59-6.33) | 0.001 |
| Explant Tumor Grade | 2.40 | (1.45-3.98) | < 0.001 |
| AFP at transplant (per 500 unit change) | 1.03 | (1.01-1.05) | 0.008 |
| Explant vascular invasion | 2.10 | (1.33-3.32) | 0.002 |
AFP: alpha-feto protein.
LT provides the most robust survival for selected patients with non-metastatic HCC.21-23 The decision to list a patient for LT must balance the individual's desire for potentially curative surgery with the need to provide the maximal survival benefit for all patients on the waiting list with considerations for appropriate organ utilization.24
Herein, we present a large single center experience of 529 patients who enrolled with an intention-to-treat analysis with LT for HCC, with 472 successfully undergoing LT (89%). Of these 472 patients, 376 met the study's inclusion criteria. The median time from diagnosis to transplant was 183 days while the median time on the UNOS waitlist was 62 days. We chose time from diagnosis to transplant as a reflection of “wait” time, because we felt that this time would more accurately indicate the real world response to the diagnosis of HCC as well as be more universally applicable by eliminating the confound-ers of transplant center wait-listing behavior. Thus, this analysis has a unique perspective compared to the registry reports which are limited to UNOS wait time only.
In our experience, overall survival and recurrence-free survival at 5 years were 65.8% and 62.2%, respectively. 47 of our 376 patients (12.5%) developed HCC recurrence which is comparable to similar studies.25-33 HCC recurrence played a key role in determining overall survival with a strong statistical association (HR 18.61, p < 0.001), making pre-LT identification of patients at risk for recurrence critical to improve future outcomes. Our data highlight similar findings to previously published single and multi-center analyses of LT for HCC that describe significant associations between outcomes and factors related to tumor biology, including AFP kinetics, macro and micro-vascular invasion, and tumor grade.34-36 Staging systems that incorporated vascular invasion have demonstrated superior prediction of outcomes.31 In our study, we found that elevated serum AFP was highly predictive for HCC recurrence and reduced survival after LT. An initially elevated or quickly rising serum AFP level during LT evaluation should be considered a concerning feature for potential disease recurrence. A serum AFP level > 400 ng/ mL at any time prior to LT predicted post-LT disease recurrence and reduced survival.
Regarding wait-time, we demonstrated no difference in survival or HCC recurrence for patients who were transplanted either more or less than 180 days from diagnosis, which is consistent with a previously published experi-ence.37 Several recent studies have hypothesized that there may be intrinsic value in increased wait-times for certain HCC patients by selecting for HCC disease that is less likely to recur post-LT. Controversy around HCC exception scores continue, as the transplant community balances the risk of waitlist drop out from tumor progression vs. complications with unacceptable post-LT outcomes. Some groups have demonstrated improved outcomes in those patients waiting longer for LT by reviewing national registry data. Delaying LT with a longer wait-time is thought to allow patients with more aggressive HCC biology to self-select, along with improving organ allocation equity.1,38 However, the applicability of these conclusions is limited by the challenges that face all registry data: heterogeneous data sampling, underreporting of tumor recurrence, the lack of uniform programs for patient selection, pre-LT ablation, imaging standards, and inconsistent follow up. Thorough analysis of patients who dropped off the wait list was also lacking. Across our cohort, wait time did not impact disease recurrence or long-term outcomes. We believe that an aggressive approach to proceed with LT in patients with HCC can be justified and may even be superior to longer wait times. It is clear that better understanding of tumor biology prior to LT will be critical to identifying patients most likely to do well after LT for HCC. Regardless, prolonged wait time does not appear to be universally beneficial. Our analysis of patient dropout while on the transplant list (Figure 4) demonstrates the probability of dropout increases rapidly as a patient remains listed, in particular 12 to 24 months after diagnosis. In light of the recent changes to UNOS policy, patients are likely to wait longer for LT, allowing for increasing wait list dropout.
Our study strengths include the large and comprehensive data set from a single center and extended length of follow-up. A high percentage of patients enrolled in the intention-to-treat arm provide evidence for the applicability of the findings across populations. Every attempt was made to collect complete and accurate outcome data, even from those patients transferred to other centers after LT. Patients found to have cholangiocarcinoma (CCA) or mixed HCC/CCA at explant were included, as they had an empiric diagnosis of HCC pre-LT based on radiography. The decision to use a diagnosis to LT interval for stratifying our cohort likely limits bias and produces more translatable findings. Limitations in our study include lack of randomization, the inherent deficiencies of a retrospective review, and the unmeasured changes in pre-LT imaging and ablative therapies over the long period of study. Of note, HCC recurrence was not included in the multivari-ate analysis as related to survival as it is confounded by being a time-dependent covariate.
In conclusion, we demonstrate through a large single center experience that post-LT survival and HCC recurrence is not impacted if a patient is transplanted within 6 months after diagnosis or after 6 months after diagnosis; and yet waitlist drop off worsens significantly as time on the waitlist increases. More importantly, we found that tumor biology, not wait time, was the primary factor for both recurrence and survival, and should likely be the focus of any future adjustments to regional and national algorithms in allocating liver allografts for HCC.
Abbreviations- •
AFP: Alpha-fetoprotein.
- •
HCC: Hepatocellular carcinoma.
- •
HCV: Hepatitis C.
- •
LT: Liver transplantation.
- •
MC: Milan Criteria.
- •
MELD: Model of End Stage Liver Disease.
- •
RFA: Radiofrequency ablation.
- •
TACE: Transarterial Chemoembolization.
- •
UCSF: University of California in San Francisco Criteria.
No study funding. The authors declare no conflicts of interest.
AcknowledgementThe authors acknowledge the contributions from our allied health staffs and the colleagues from various subspe-cialties including anesthesiology, infectious disease, blood bank, pulmonary, cardiology, hematology/oncology, ne-phrology, critical care, and radiation oncology at MCF. Specifically, the authors express the gratitude to our previous and current surgical and Hepatology colleagues including Jeffery L. Steers, Lena Sibulesky, James Spivey, C. Burcin Taner and Darren Willingham. We thank Jefree Shalev for managing the transplant database.