Liver transplantation candidates are among the most comorbid patients awaiting lifesaving intervention. Health related quality of life (HRQOL) measured by instruments that incorporate dynamic computerized adaptive testing, could improve their assessment. We aimed to determine the feasibility of administration of the Patient-Reported Outcomes Measurement Information System (PROMIS-CAT) in liver transplant candidates.
Materials and methodsLiver transplantation candidates were prospectively enrolled following a review of their available medical history. Subjects were given a tablet computer (iPad) to access the pre-loaded PROMIS CAT.
Results109 candidates with mean age 55.6±8.6 years were enrolled in this pilot study. Mean MELD-Na score was 16.3±6.3; 92.6% had decompensated liver disease. Leading etiologies of cirrhosis included hepatitis C (34.8%), nonalcoholic steatohepatitis (25.7%) and alcohol (21.1%). Subjects with MELD-Na score>20 had the most significant impairment in HRQOL (anxiety/fear+5.9±2.7, p=0.0289, depression+5.1±2.5, p=0.0428, fatigue+4.3±2.6, p=0.0973) and physical impairment (−7.8±2.5, p=0.0022). Stage of cirrhosis and decompensated liver disease were predictive of impaired HRQOL but Child–Pugh Turcotte score was not. Hepatic encephalopathy was the strongest independent predictor of impaired HRQOL, with significant impairment across all domains of health.
ConclusionsLiver transplant candidates have significantly impaired HRQOL across multiple domains of health as measured by PROMIS-CAT. HRQOL impairment parallels disease severity. Future study is needed to determine how best HRQOL could be systematically included in liver transplantation listing policy, especially in those candidates with hepatic encephalopathy.
Patients with chronic liver disease have impaired health-related quality of life (HRQOL), with far reaching effects that extend beyond individual patients to family members and caretakers [1–6]. Impaired HRQOL in patients with cirrhosis places extreme burdens on both individual patients and their support networks, as well as on society with greater costs to the healthcare system [1,7]. Patients with cirrhosis have more impaired quality of life when compared to those with chronic liver disease in the absence of cirrhosis [8]. Age and female gender have also been shown to predictively impair HRQOL in patients with chronic liver disease [8]. Patients with cirrhosis have worsening HRQOL as their cognition becomes more impaired, yet they retain good insight into their issues with HRQOL regardless of hepatic encephalopathy [1,9]. HRQOL has been shown to predict all-cause mortality in patients with cirrhosis independent of MELD score when assessed by the Short Form Liver Disease Quality of Life instrument [7]. Additionally, MELD-Sodium score has been shown to predict decreased HRQOL prior to liver transplantation [6]. Despite this, healthcare providers rarely assess HRQOL in clinical practice [7]. Liver transplantation waiting list candidates are a highly selected subgroup of patients with chronic liver disease. They are among the most comorbid patients all the while awaiting life-saving transplantation. Current consensus opinion states that liver transplantation leads to increased life span with the capability to return patients to a high functional status [10]. However, more recent studies have challenged this thinking, demonstrating that HRQOL can remain significantly impaired post-transplantation as measured by legacy fixed-length instruments such as the short form thirty-six (SF-36), Beck's Depression Index, and PROMIS-HAQ [2,11,12]. In fact, clinicians often underestimate the magnitude of negative effects of the transplantation event and short-term post-transplantation clinical setbacks on HRQOL following transplantation [13].
Patient-reported outcomes (PROs) are increasingly recognized as important aspects of the overall results of treatment. The National Institutes of Health (NIH) and the National Cancer Institute have taken the lead in bringing PROs to the forefront of both research and clinical practice in order to objectively measure a patient's perceived well-being and disease burden. Highly accurate measurement of multiple health domains can be obtained through the combined use of PRO measures and computerized adaptive testing (CAT) software, which yield efficient measurements with hierarchically structured question selection and reduced ceiling and floor effects, allowing for an individual approach tailored to the health status of each patient. The Patient-Reported Outcomes Measurement Information System (PROMIS) offers significant improvements over the existing fixed length questionnaire evaluations of HRQOL, as CAT software utilizes an underlying framework of hierarchical health domains that are presented to patients in relationship to previous responses in a dynamic environment [14]. This presents an individually tailored, comprehensive assessment that can automatically shorten the lengthy, one-size-fits-all static questionnaires that have been used almost exclusively by previous HRQOL studies in patients with liver disease [2,4,5,7,8,10–13,15–17]. PROMIS is available at http://www.nihpromis.org and includes gender- and age-matched normative data obtained from extensive testing of the general U.S. population for research purposes.
HRQOL measured by instruments that incorporate dynamic CAT software such as PROMIS could improve assessment in liver transplantation. PROMIS CAT based assessments have previously been validated in patients with cirrhosis and paralleled the findings of the more cumbersome, less flexible static instruments [1]. Administration of PROMIS CAT based assessments to liver transplantation candidates has not been exclusively undertaken and for this reason we performed this novel pilot study. To our knowledge, the only transplant patient population evaluated is in heart failure patients undergoing heart transplantation [18]. Our primary hypothesis is that HRQOL is significantly impaired in liver transplantation candidates as measured by PROMIS CAT. We also speculate that the degree of impairment in HRQOL is significantly related to disease severity as measured by MELD-Na score [19,20], stage of cirrhosis [21] and Child–Pugh Turcotte score, and that more advanced disease is associated with lower HRQOL.
2Materials and methodsConsecutive liver transplantation candidates, evaluated at the University of Virginia Charles O. Strickler Transplant Center outpatient clinic, were prospectively enrolled following a review of their available medical history and/or laboratory values. No hospitalized subjects were enrolled. Subjects were excluded if they could not provide informed consent (including the presence of ≥grade 2 encephalopathy which precluded accurate completion of PROMIS CAT in our previous experience), were deemed to not be liver transplant candidates after full assessment by the liver transplant team due to the presence of uncontrolled psychiatric disease presenting a barrier to transplantation as assessed by standard psychosocial evaluation, were younger than 18 years, or were non-English speaking. Prisoners were also excluded. Stage of cirrhosis was defined as follows: stage 1 (no history of esophageal varices or ascites), stage 2 (non-bleeding esophageal varices but no history of ascites), stage 3 (bleeding esophageal varices but no history of ascites), stage 4 (ascites±non-bleeding esophageal varices) or stage 5 (bleeding esophageal varices and a history of ascites) [21].
After informed consent was obtained, subjects were given a tablet computer (iPad) to access the pre-loaded PROMIS CAT website from the NIH through a web browser via a secure wireless Internet connection. Subjects were asked to report their experience over the preceding week for each of the nine health domains. A standardized protocol was used to describe the assessment process to the subjects as well as to provide direct assistance with any technical difficulties with the tablet computer. This was completed by trained study sub-investigators or the principal investigator. We have previously demonstrated both the validity and security of this methodology using tablet computers in medical patients with advanced chronic disease, many of whom have impairments comparable to patients with cirrhosis [22]. For patients with a history of alcohol use (including active use), no sobriety period was mandated prior to completing the PROMIS CAT assessment.
The following nine health domains were assessed: anxiety/fear, cognitive function, depression/sadness, fatigue, instrumental support, pain interference, physical function, sleep disturbance and social roles (Table 1). The set of PRO measures were selected based on the transplant team (transplant hepatologist, transplant surgeon, social worker, nutritionist, nurse coordinator, financial coordinator) and the patient stakeholder review.
Nine health domains assessed by PROMIS CAT imperative to liver transplantation candidacy [29].
| Domain | Description |
|---|---|
| Anxiety/fear | Fear (fearfulness, panic), anxious misery (worry, dread), hyperarousal (tension, nervousness, restlessness) and somatic symptoms related to arousal (racing heart, dizziness) |
| Cognitive function | Mental acuity, concentration, verbal and nonverbal memory, verbal fluency and perceived changes in these cognitive functions and the extent to which cognitive impairments interfere with daily functioning, whether other people observe cognitive impairments and the impact of cognitive dysfunction on QOL |
| Depression/sadness | Negative mood (sadness, guilt), views of self (self-criticism, worthlessness) and social cognition (loneliness, interpersonal alienation), as well as decreased positive affect and engagement (loss of interest, meaning and purpose) |
| Fatigue | Range of symptoms, from mild subjective feelings of tiredness to an overwhelming, debilitating and sustained sense of exhaustion that likely decreases one's ability to execute daily activities and function normally in family or social roles |
| Instrumental support | Perceived availability of assistance with material, cognitive or task performance |
| Pain interference | Consequences of pain on relevant aspects of one's life. This includes the extent to which pain hinders engagement with social, cognitive, emotion, physical and recreational activities |
| Physical function | Self-reported capability rather than actual performance of physical activitiesThis includes dexterity, walking/mobility as well as instrumental activities of daily living (e.g. running errands) |
| Sleep disturbance | Perceptions of sleep quality, sleep depth and restoration associated with sleep |
| Social roles | Satisfaction with performing one's usual social roles and activities |
Scores for each health domain were automatically calculated in real time and scaled to the underlying population distribution obtained from responses through the 2000 U.S. Census [23] using the T-score algorithms provided by the PROMIS Assessment Center software. The mean T-score is 50, with one standard deviation equivalent to±10 units. Scores were automatically stored on the secure NIH PROMIS server. It is standard within the PROMIS framework to report descriptive means and standard deviations as formal statistical comparisons are not possible to compute.
2.1Statistical methodsThe difference in T-scores were calculated for individual PROMIS domains among liver transplant patients grouped into categories of MELD-Na scores, as well as by Child–Pugh–Turcotte (CPT) class, and by stage of cirrhosis. Simple linear regression modeling was used to assess the statistical significance of the differences in mean PROMIS domain score measurements by key demographic and clinical characteristics. Reference variables for each general linear regression model were MELD-Na<10, CPT Class A or stage 1 cirrhosis respectively. Data management and statistical programming were conducted using SAS 9.4 (Cary, N.C) and R statistical software, version 2.13 (R foundation for Statistical Computing, Vienna, Austria). Graph generation was performed using GraphPad Prism version 7.03 for Windows, GraphPad Software (La Jolla, California, USA). A p-value of ≤0.05 was considered statistically significant. Institutional review board approval was obtained from the University of Virginia Institutional Review Board for Health Sciences Research.
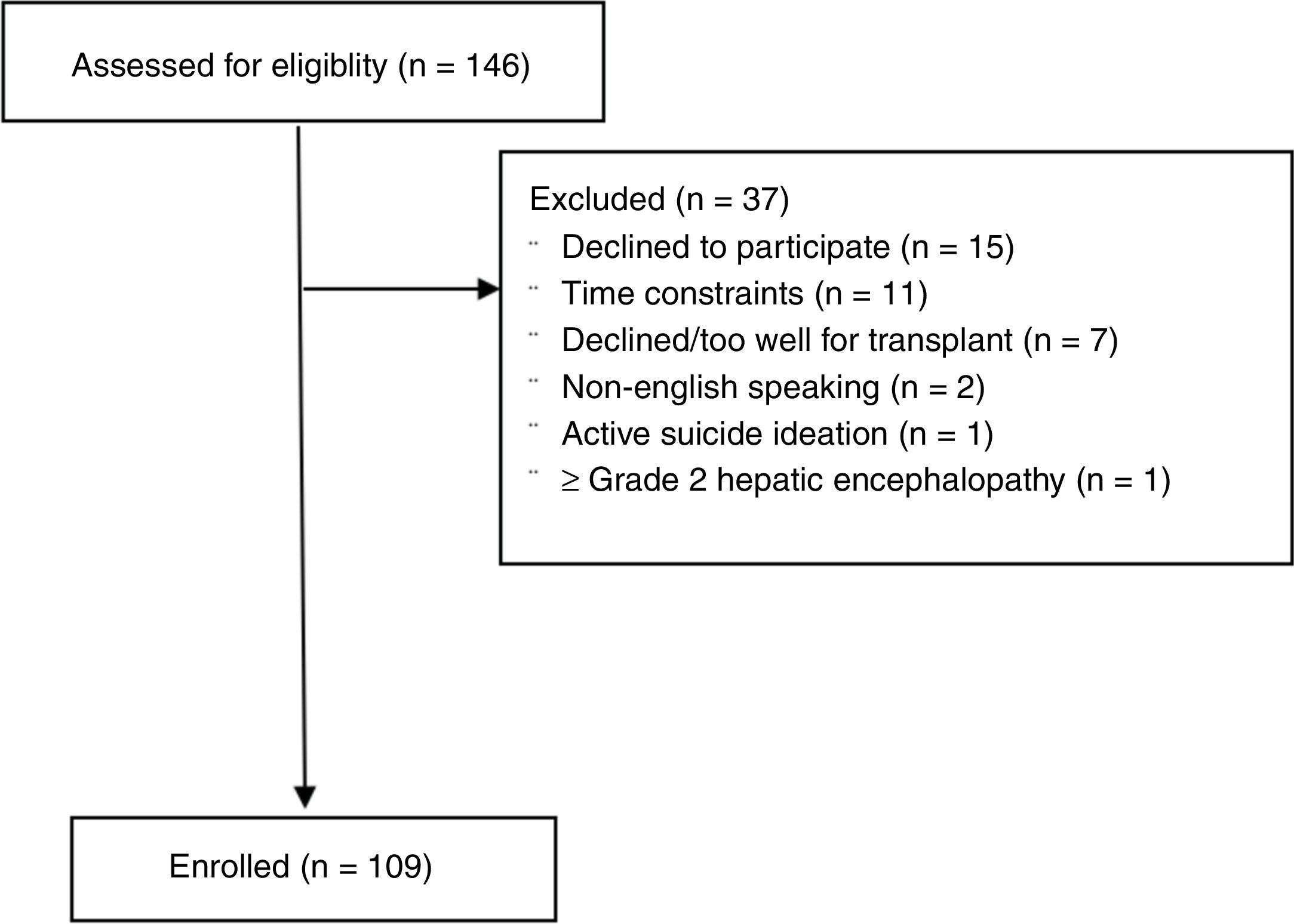
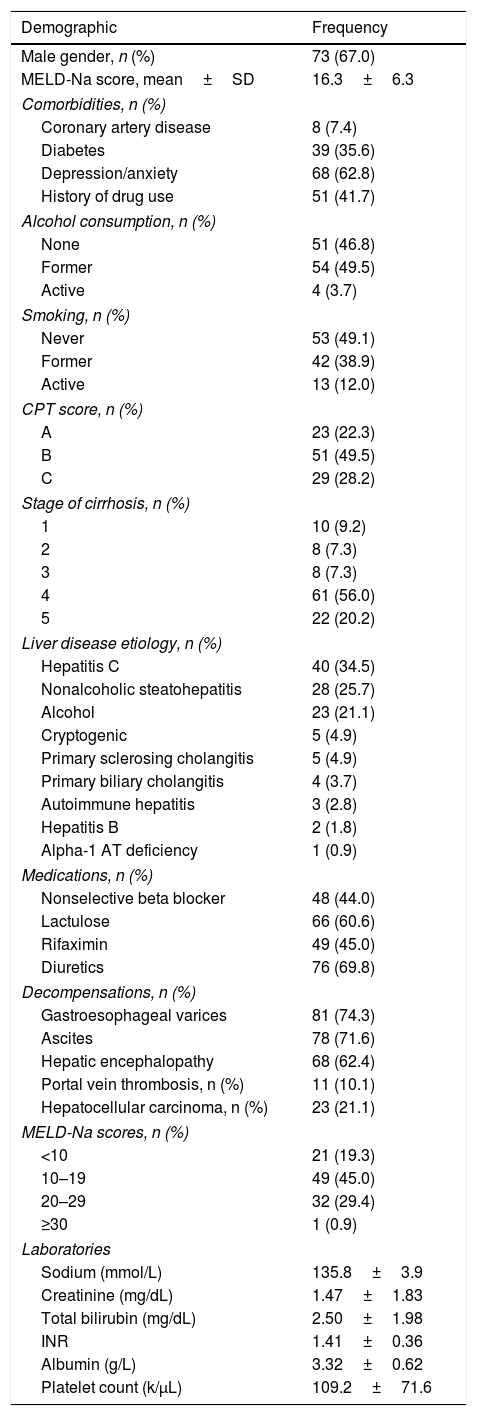
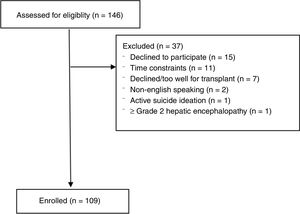
3ResultsOne hundred forty-six potential liver transplant candidates were screened for participation from July 1, 2016 to September 30, 2016. Among all screened patients, 109 were consented and enrolled (Fig. 1). Thirty-seven subjects were screened but not enrolled [declined based on patient preference (n=15), time constraints (n=9), declined/too well for liver transplantation (n=7), non-English speaking (n=2), active suicidal ideation (n=1) and uncontrolled grade II–III hepatic encephalopathy (n=1)]. Baseline characteristics of the cohort are described in Table 2. The mean age was 55.6±8.6 years. The cohort was predominantly male (67.0%) and mean MELD-Na score was 16.3±6.3 (range 7–30). 77.7% of the study cohort had CPT Class B or C disease, and 76.1% had stage 4 or 5 cirrhosis. Mean body mass index was 30.3±6.2kg/m2. The leading etiologies of liver disease were chronic hepatitis C (34.8%), nonalcoholic steatohepatitis (25.7%) and alcoholic liver disease (21.1%). The most common cardiovascular comorbidities included hypertension (51.3%), diabetes (35.8%) and coronary artery disease (7.3%). Portal hypertensive decompensating events included gastroesophageal varices (74.3%), ascites (71.6%), hepatic encephalopathy (62.4%), hepatocellular carcinoma (21.1%, all Barcelona Clinic Liver Cancer Stage A) and portal vein thrombosis (10.1%). Almost all (92.6%) subjects had decompensated liver disease. Medical management of hepatic encephalopathy included 60.6% of subjects being prescribed lactulose and 45.0% rifaximin. 69.8% of subjects were prescribed diuretics for moderate-severe ascites. 62.8% of the population had a history of underlying anxiety, depression or both and 41.2% were being treated with psychoactive medications. 18.6% reported uncontrolled symptoms despite treatment. 58.9% reported a history of alcohol use at one point in their life (3.7% were actively drinking at the time of enrollment) and 47.1% had a history of drug use at one point in their past.
Demographics of the 109 liver transplant candidates in whom PROMIS CAT was administered.
| Demographic | Frequency |
|---|---|
| Male gender, n (%) | 73 (67.0) |
| MELD-Na score, mean±SD | 16.3±6.3 |
| Comorbidities, n (%) | |
| Coronary artery disease | 8 (7.4) |
| Diabetes | 39 (35.6) |
| Depression/anxiety | 68 (62.8) |
| History of drug use | 51 (41.7) |
| Alcohol consumption, n (%) | |
| None | 51 (46.8) |
| Former | 54 (49.5) |
| Active | 4 (3.7) |
| Smoking, n (%) | |
| Never | 53 (49.1) |
| Former | 42 (38.9) |
| Active | 13 (12.0) |
| CPT score, n (%) | |
| A | 23 (22.3) |
| B | 51 (49.5) |
| C | 29 (28.2) |
| Stage of cirrhosis, n (%) | |
| 1 | 10 (9.2) |
| 2 | 8 (7.3) |
| 3 | 8 (7.3) |
| 4 | 61 (56.0) |
| 5 | 22 (20.2) |
| Liver disease etiology, n (%) | |
| Hepatitis C | 40 (34.5) |
| Nonalcoholic steatohepatitis | 28 (25.7) |
| Alcohol | 23 (21.1) |
| Cryptogenic | 5 (4.9) |
| Primary sclerosing cholangitis | 5 (4.9) |
| Primary biliary cholangitis | 4 (3.7) |
| Autoimmune hepatitis | 3 (2.8) |
| Hepatitis B | 2 (1.8) |
| Alpha-1 AT deficiency | 1 (0.9) |
| Medications, n (%) | |
| Nonselective beta blocker | 48 (44.0) |
| Lactulose | 66 (60.6) |
| Rifaximin | 49 (45.0) |
| Diuretics | 76 (69.8) |
| Decompensations, n (%) | |
| Gastroesophageal varices | 81 (74.3) |
| Ascites | 78 (71.6) |
| Hepatic encephalopathy | 68 (62.4) |
| Portal vein thrombosis, n (%) | 11 (10.1) |
| Hepatocellular carcinoma, n (%) | 23 (21.1) |
| MELD-Na scores, n (%) | |
| <10 | 21 (19.3) |
| 10–19 | 49 (45.0) |
| 20–29 | 32 (29.4) |
| ≥30 | 1 (0.9) |
| Laboratories | |
| Sodium (mmol/L) | 135.8±3.9 |
| Creatinine (mg/dL) | 1.47±1.83 |
| Total bilirubin (mg/dL) | 2.50±1.98 |
| INR | 1.41±0.36 |
| Albumin (g/L) | 3.32±0.62 |
| Platelet count (k/μL) | 109.2±71.6 |
CPT=child–pugh-turcotte score; INR=international normalized ratio; MELD=model for end-stage liver disease; Na=sodium.
In general, the patient population was decompensated with advanced liver disease and significant portal hypertension.
Compared to the general population, liver transplant candidates reported lower physical function (42.2±9.3), cognitive function (46.4±9.6) and social roles (47.6±10.3). They also reported higher levels of fatigue (56.5±9.3), pain interference (56.4±10.3), sleep disturbance (54.6±9.5), and instrumental support (58.8±8.2) than the general population. Lastly, they reported anxiety/fear (51.4±9.8) and depression/sadness (50.7±9.0) scores near the population mean (Table 3).
Total health domain scores for 109 liver transplant candidates administered PROMIS CAT.
| Anxiety/fear | Cognitive function | Depression/sadness | Fatigue | Instrumental Support | Pain interference | Physical function | Sleep disturbance | Social roles | |
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | 51.4±9.7 | 46.4±9.6 | 50.7±9.0 | 56.4±9.3 | 58.8±8.1 | 56.4±10.3 | 42.2±9.3 | 54.6±9.5 | 47.6±10.3 |
| Minimum | 40.3 | 27.0 | 41.0 | 33.7 | 34.1 | 41.6 | 22.9 | 32.0 | 27.5 |
| Maximum | 77.8 | 68.0 | 73.1 | 75.8 | 66.2 | 75.6 | 56.9 | 73.3 | 64.2 |
Impairments in health domains in liver transplant candidates were reported for physical function, cognitive function, social roles, fatigue, pain interference and sleep disturbance.
In terms of the severity of liver disease, several significant differences in domain scores were demonstrated across different MELD-Na score cohorts (MELD-Na<10, 10–19, 20–29, >30) (Supplementary Table 1). MELD-Na score was associated with statistically different levels of physical function (p=0.0023) and anxiety/fear (p=0.0311), and differences reported for fatigue approached statistical significance (p=0.0983). MELD-Na score was not associated with differences in cognitive function, depression/sadness, instrumental support, pain interference, sleep disturbance, or social roles.
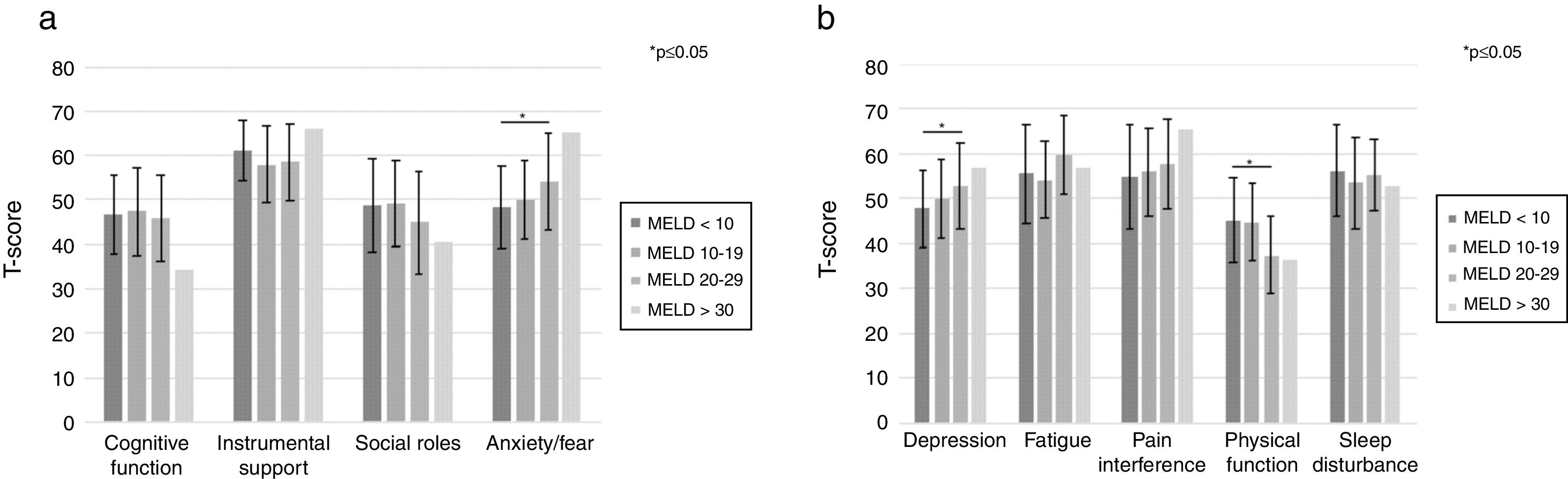
Several statistically significant differences were also noted between MELD-Na groups, specifically when comparing those with MELD-Na<10 to those with MELD-Na 20–29. Subjects with MELD-Na score 20–29 had significantly higher levels of anxiety/fear (+5.9±2.7, p=0.0289), depression (+5.1±2.5, p=0.0428), fatigue (+4.3±2.6, p=0.0973) and physical impairment (−7.8±2.5, p=0.0022) than those with MELD-Na<10. Only one candidate in the pilot study had a MELD-Na score≥30, limiting any conclusions for this subgroup of study (Fig. 2).
PROMIS CAT results stratified by MELD-Na score. Utilizing simple linear regression modeling comparing different groupings of MELD-Na score to those subjects with MELD-Na<10 (reference group), physical function and anxiety/fear were significantly associated with MELD-Na score. The most severe impairment in physical function paralleled the most advanced liver disease as defined by MELD score.
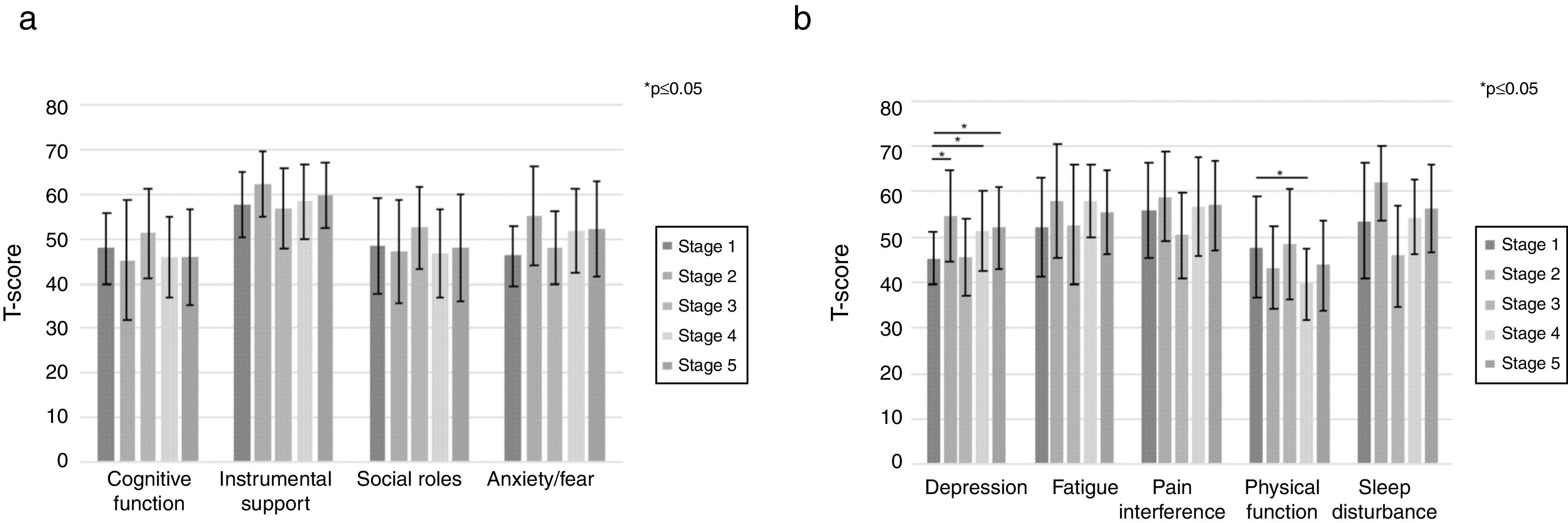
When examining subjects by stage of cirrhosis, higher stages of cirrhosis were associated with lower physical function (p=0.0140), increased sleep disturbance (p=0.0123) and increased depression/sadness (p=0.0663). When compared to the reference group of stage 1 cirrhosis, subjects with stage 2 (+9.3±4.1, p=0.0271), stage 4 (+6.2±3.0, p=0.0324) and stage 5 (+6.8±3.3, p=0.0454) cirrhosis had greater levels of depression/sadness (Fig. 3). Subjects with stage 4 cirrhosis had the worst physical impairment (−8.0±3.1, p=0.0101). CPT class was not associated with differences in HRQOL in any domain with the exception of impaired physical function (p=0.0078). Please refer to Supplementary Table 2.
PROMIS CAT results stratified by stage of cirrhosis. Utilizing simple linear regression modeling comparing different groupings of stage of cirrhosis to those subjects with stage 1 disease (reference group), physical function and sleep disturbance were significantly associated with stage of cirrhosis; however, unlike MELD-Na score, the most severe impairments were not seen in the most advanced stages of cirrhosis.
In general, decompensated cirrhosis was associated with greater impairment in HRQOL across multiple domains of health. Hepatic encephalopathy was the strongest independent predictor of lower HRQOL across each of the measured nine domains of health. Impairments were seen for cognition (p=0.0036), instrumental support (p=0.0211), social roles (p=0.0798), anxiety/fear (p=0.0869), depression/sadness (p=0.0122), fatigue (p=0.0011), pain interference (p=0.0388), physical function (p=0.0023) and sleep disturbance (p=0.0133). Other significant associations between hepatic decompensations and HRQOL included subjects with ascites, whom had significantly impaired physical function (p=0.0152). Hepatocellular carcinoma subjects had more depression/sadness (p=0.0831) and lower physical function (p=0.0039) when compared to subjects without hepatocellular carcinoma. Subjects with portal vein thrombosis had more pain that interfered with HRQOL (p=0.0235).
3.5HRQOL impairment in liver transplant candidates with mental health and substance abuse disordersSubjects with a history of anxiety or depression had significant impairments in HRQOL. A history of anxiety/depression was associated with more anxiety/fear (p=0.0454) and trended toward statistical significance for depression/sadness (0.0641). Active anxiety or depressive symptoms were associated with higher pain interference scores (p=0.0451). A history of past alcohol use was associated with lower cognition (p=0.0152) and there was a trend toward statistical significance for physical function (p=0.0556) but not depression/sadness or anxiety/fear. Past drug use (excluding alcohol) was not associated with a decreased HRQOL.
4DiscussionLiver transplant candidates have significantly impaired HRQOL across multiple domains of health. This data also shows that HRQOL domains largely parallel cirrhosis severity as measured by MELD-Na, stage of cirrhosis and hepatic decompensation but are largely independent of Child–Pugh Turcotte score. Our findings build on the work of Bajaj et al. [1] who found that the PROMIS CAT can be successfully administered to patients with cirrhosis. These patients were generally healthier than our included cohort of liver transplant candidates (lower MELD-Na scores, absence of significant comorbidities or hepatocellular carcinoma).
HRQOL is one of the most important features of liver transplantation facing clinicians on a daily basis. The natural history of cirrhosis leads patients to suffer from debilitating systemic symptoms, negatively disrupting their daily routine and lifestyle [16]. Furthermore, liver transplant candidates are limited by dietary, time, physical and psychosocial restrictions at the discretion of their medical providers [16]. Liver transplantation candidates are assessed loosely across domains of health at most transplant centers; however, most evaluations are subjective, at the discretion of the evaluating clinician, and only began to be implemented within the last five years [24,25]. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT) is the tool currently used most widely by clinicians to identify areas of psychosocial vulnerability conferring increased risk for poor post-transplant medical and psychosocial outcomes. However, this assessment is limited to psychosocial domains of health including social support, psychopathology and neurocognitive impairment, and therefore ignores many of the additional important domains of health evaluated by PROMIS [24,25]. Furthermore, each liver transplant center has different thresholds and listing policies, introducing more subjectivity into the assessment and influence of HRQOL on liver transplantation. While measurement of PROs is inherently subjective, interpretation of PROMIS values in comparison to validated population means provides an alternative, more objective measure of HRQOL with the potential to be incorporated into the standard assessment of liver transplant candidates.
Despite the fact that HRQOL has been shown to predict mortality in cirrhosis patents independent of MELD score [7], current liver transplantation allocation policies ignore HRQOL and rely solely on MELD-Na score and MELD exceptions, the majority of which are not granted for impaired HRQOL. While HRQOL largely mirrored the severity of cirrhosis as assessed by MELD-Na score, stage of cirrhosis or presence or absence of hepatic decompensation, on an individual level this is not always the case. Given this association and the predictive value of impaired HRQOL on patient survival pre-liver transplantation, an objective measurement of HRQOL via PROMIS CAT should be considered to augment both assessment of liver transplant candidates and prognostication of their clinical course prior to transplantation. While the vehicle for implementation would need to be prospectively validated with advanced modeling, our findings lend credence to the importance of this consideration and suggest that standardized incorporation of HRQOL into organ allocation may have additional value in assessing appropriateness of liver transplantation waiting list candidacy. An argument counter to the introduction of HRQOL into organ allocation policy focuses on a lack of patient insight, especially in the presence of hepatic encephalopathy. However, cirrhosis patients with hepatic encephalopathy have been shown to have good insight into their HRQOL impairment as demonstrated in a recent study by Bajaj et al. [9]. Furthermore, only 1/4 of evaluated patients overestimated their degree of HRQOL impairment when compared to objective PROMIS scores. Other concerns related to including HRQOL in organ allocation policy include the prospect of “gaming the system”, where medical providers encourage their patients to report low HRQOL given the subjectivity of PRO measurement. However, the same concerns were raised with MELD and MELD-Na where MELD inflation remains problematic and region dependent utilization of MELD exceptions continues to increase [26].
In general, hepatic decompensation is known to be associated with poor HRQOL, namely with more pain interference and sleep disturbance and less physical function and ability to perform social roles [1]. When examining each decompensation individually, hepatic encephalopathy was the strongest independent predictor of impaired HRQOL in liver transplant candidates despite the majority receiving medical treatment with lactulose and/or rifaximin as encephalopathy was associated with impairment across all nine measured domains of health. In comparison, the presence of ascites was only associated with impairments in two domains and gastroesophageal varices (including a history of bleeding) with none. Furthermore, while our study excluded subjects with active moderate-severe (grade≥2) encephalopathy, it can be inferred that a higher grade of encephalopathy was present at baseline prior to PROMIS CAT administration as the majority of subjects were prescribed medications for the medical management of encephalopathy. This may lead to bias toward the null and under-reporting of the detriment of encephalopathy on HRQOL. The striking association between hepatic encephalopathy and impaired HRQOL suggests a need to reframe our focus on this decompensation, given that overt encephalopathy affects 30–45% of patients with cirrhosis and subclinical/minimal encephalopathy has been reported to affect up to 80 percent [27]. Furthermore, subclinical/minimal encephalopathy is difficult to diagnose as there is often an overlap between this and other medical or psychiatric illnesses [27,28]. Our findings do not support that the impairment in HRQOL in patients with hepatic encephalopathy is solely due to psychiatric illness as the impairment was significantly greater in the hepatic encephalopathy population across all measured domains of health in comparison to those with psychiatric illness whom had increased depressive and anxiety symptoms. This is despite 61.6% of the hepatic encephalopathy subjects having a diagnosed concurrent mood disorder. Unfortunately, the best studied psychometric testing for hepatic encephalopathy is not routinely utilized in clinical practice due to time intensity of the test, need for interpretation by a trained psychologist and complicated statistical methods [27,28]. Serial PROMIS CAT assessments may offer a feasible, readily available solution in both the diagnosis and monitoring of clinical response to treatment of hepatic encephalopathy with good reproducibility and minimal effect on clinic work-flow. Future study validating this premise would be of interest in order to further this proposition.
Our study has several limitations as it was intended to be a three-month feasibility pilot study to confirm that the PROMIS-CAT instrument could successfully be administered to liver transplantation candidates. Namely, we did not capture longitudinal outcomes such as wait-list mortality or post-transplantation outcomes. Future study investigating these end points is currently underway. Another limitation of our work is that comorbid mental health disorders were not controlled for and patients with mental health disorders were included in our analysis, albeit in small numbers. Our study also included few subjects with MELD-Na>30 and the validity of our findings in this patient population who is arguably the sickest of those awaiting lifesaving liver transplantation remains unknown. This offers and intriguing extension of our pilot work into future prospective study.
In conclusion, liver transplant candidates have significantly impaired HRQOL across multiple domains of health as measured with the dynamic PROMIS CAT instrument. HRQOL impairment parallels disease severity. While it has been established that HRQOL remains affected post transplantation [2,11,12], the magnitude of the effect of liver transplantation intervention itself in improving pre-transplantation HRQOL impairment remains unknown. Additional longitudinal analysis is underway to determine if liver transplantation ultimately leads to improved HRQOL across multiple domains of health, in order to best provide liver transplant candidates with both realistic expectations of the benefit of the lifesaving transplantation event on the quality of their life and over what time period it will take for HRQOL to improve above their pre-transplantation impairment.AbbreviationsCAT computerized adaptive testing health-related quality of life Patient-Reported Outcomes Measurement Information System model for end stage liver disease National Institutes of Health patient reported outcomes The Stanford Integrated Psychosocial Assessment for Transplantation
Institutional review board approval was obtained from the University of Virginia's Institutional Review Board for Health Sciences Research.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
AcknowledgmentsWe would like to thank the University of Virginia Transplant Center Team for their assistance with data collection.
Authors contributionsJS, GS, CA designed research; JS, JW, AA, BN, AZ performed research; JS and GS analyzed data; JS, GS, JW, AA, BN, AZ, CA wrote the paper. All authors approved the final version of this paper.
Financial supportThis study was funded by grant funding from the National Institutes of Health (Grant 5T32DK007769-15).
This work was also supported by a Transplant Hepatology Fellowship Award from the American Association for the Study of Liver Diseases (AASLD).
PROMIS was funded with cooperative agreements from the National Institutes of Health (NIH) Common Fund Initiative (U54AR057951, U01 AR052177, U54AR057943, U54R057926, U01AR057948, U01AR052170, U01AR057954, U01AR052171, U01AR052181, U01AR057956, U01AR052158, U01AR057929, U01AR057936, U01AR052155, U01AR057971, U01AR057940, U01AR057967, U01AR052186). The contents of this article uses data developed under PROMIS. These contents do not necessarily represent an endorsement by the US Federal Government or PROMIS. See www.nihpromis.org for additional information on the PROMIS initiative.
The sources of financial and material support had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Conflict of interestThe authors have no competing interests to report.