Introduction. Among the available nucleos(t)ide analogues adefovir dipivoxil (ADV) is relatively cheap and widely used in rural area in China. However, there are insufficient data on recommendation for patients with suboptimal response to ADV after 48 weeks of treatment in order to reduce the resistance rate in the long term. The aim of this study was to compare the efficacy and safety of LAM add-on combination therapy versus ETV monotherapy for patients with suboptimal response to ADV.
Material and methods. 136 patients with suboptimal response to ADV were randomly assigned to the add-on LAM with ADV combination therapy (68 patients) group and the ETV monotherapy (68 patients) group. Patients in the add-on group were prescribed 100 mg LAM and 10 mg ADV per day, while the monotherapy group received 0.5 mg ETV per day for 48 weeks. Tests for liver and kidney function, HBV serum markers, HBV DNA load, were performed every 3 months.
Results. The mean patient age in LAM add-on group and ETV monotherapy was 38.59 ± 7.65 and 37.56 ± 8.67 years respectively. The HBV DNA undetectable rate in the LAM add-on group and the ETV group were not significant difference at week 4, 12 and 24 (P > 0.05). However, the HBV undetectable rate in the ETV group was higher than that in the LAM add-on group at week 36 and 48 (P = 0.043 for week 36 and P = 0.038 for week 48). There was no significant difference both for HBeAg loss and HBeAg seroconversion between two groups (P > 0.05) at 48 weeks. Meanwhile, our study also demonstrated that the mean eGFR levels in LAM add-on group was decreased from 99.6 ± 8.71 at baseline to 86.4 ± 9.83 at the end of 48 weeks, which was significantly higher than that in the ETV monotherapy group (P < 0.05). 8.8% of patients in LAM add-on group experienced eGFR reduction by 20-30% from baseline at 48 weeks. No patients developed hyposphosphatemia in our study.
Conclusion. Our study clearly showed that switch to ETV monotherapy was the more effective and more safe than that of LAM add-on combination therapy for patients with suboptimal response to ADV.
Hepatitis B virus (HBV) infects more than 350 million people worldwide.1 Hepatitis B is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). High level of HBV DNA is an independent factor associated with disease progression.2 Therefore, the main goal of treatment is complete suppression of HBV replication to limit progressive liver damage and improve natural history of chronic HBV infection (CHB). Currently, oral nucleos(t)ide analogues (NA) have demonstrated success in suppressing virus replication with few side effects. Evidence-based medicine has demonstrated that a slow virologic response after initiation of nucleos(t)ide analogues treatment is associated with high rates of drug resistance in the long-term.3,4
Among the available nucleos(t)ide analogues adefovir dipivoxil (ADV) is a phosphonate acyclic nucleotide analogue of adenosine monophosphate.5 It is a potent inhibitor of HBV reverse transcriptase and is effective both for patients with HBeAg-positive and HBeAg-negative chronic HBV infection.6,7 ADV has also the character of no cross-resistance with other nucleoside analogues such as lamivudine(LAM), telbivudine (LdT) and entecavir (ETV). So it was widely used in rescue therapy for LAM, LdT and ETV resistance.8,9 However, ADV demonstrates a relatively high rate of primary nonresponse in the 12 weeks and high rate of suboptimal response in the 48 week,10,11 probably due to its suboptimal dosage. Meanwhile the rate of ADV resistance in HBeAg positive CHB patients has been reported to be 29% after 5 years of treatment.12 ETV is a cyclopentyl guanosine analogue, and a potent and selective inhibitor of HBV replication. ETV is also a drug with a high genetic barrier to resistance and a very low rate of resistance in nucleoside naïve CHB patients.13,14
The guideline for CHB patients with ADV resistance is use add-on therapy with a nucleotide without cross-resistance (e.g. LAM, Ldt, ETV) or switch to ETV or tenofovir (TDF).15,16 However, there are insufficient data on recommendation for patients with suboptimal response to ADV after 48 weeks of treatment in order to reduce the resistance rate in the long term.
ObjectiveThe aim of this study was to compare the efficacy and safety of LAM add-on combination therapy vs. ETV monotherapy for patients with suboptimal response to ADV.
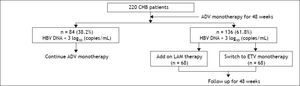
Material and MethodsStudy patients and designFrom January 2009 to March 2010, 255 patients diagnosed with chronic hepatitis B (CHB) at the First Affiliated Hospital of Zhejiang University School of medicine (Hangzhou, China) were treated initially with ADV for 48 weeks. Thirty-five patients were defined as primary nonresponders to ADV treatment, who were switched to ETV treatment. After 48 weeks, 136 patients whose HBV DNA remained > 103 copies/mL were recruited in this study. Patients with hepatitis delta virus, hepatitis C virus, or had HIV co-infection were excluded. Patients with HCC, autoimmune hepatitis, alcoholic liver cirrhosis, severe heart, renal, and brain diseases were also excluded. All patients who participated in this study provided informed consent and were aware of the procedures to be conducted. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University.
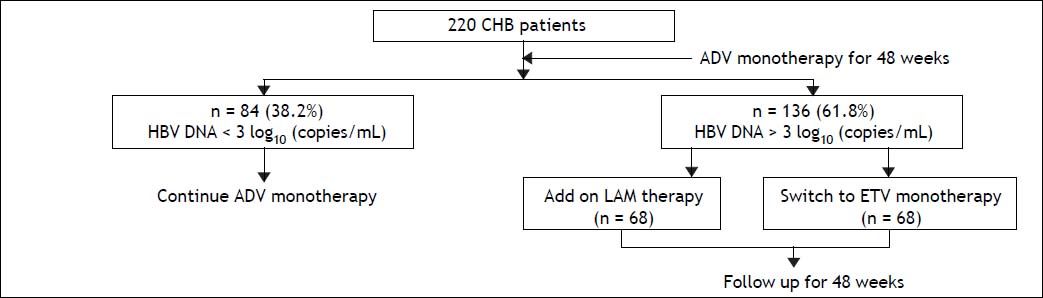
The study was designed as a prospective case-control study. The patients were randomly assigned to the ETV monotherapy (68 patients) group and the add-on LAM and ADV combination therapy (68 patients) group. Baseline data of the two groups were compared to ensure comparability. Patients in the add-on group were prescribed 100 mg LAM and 10 mg ADV per day, while the monotherapy group received 0.5 mg ETV per day for 48 weeks as indicated in figure 1.
Follow-up studiesSerum hepatitis B viral markers, including HB-sAg, anti-HBs, HBeAg, anti-HBe and anti-HBc, were detected by commercially available enzyme immunoassays (Abbott Laboratories, Chicago, IL, USA). Serum HBV DNA was measured by polymerase chain reaction with a linear range between 1 x 103 and 5 x 108 copies/mL (Shanghai ZJ Bio-Tech Co., Ltd., China).
Follow-up observations in the two groups were performed at the start and during 4, 12, 24, 36, 48 weeks. Follow-up clinical assessments included physical examination, HBeAg and HBeAb, quantitative HBV DNA, serum biochemistry, alpha-fetoprotein, renal function, and ultrasonography or CT scan. The lower limit of detection of DNA used in this study was 1.0 x 103 copies/mL (Shanghai ZJ Bio-Tec Co., Ltd, China). The reverse transcriptase region of HBV isolates was directly sequenced from a cohort of all 136 CHB patients at baseline and in 48 weeks of ADV treatment. Replication-competent HBV constructs containing ADV-resistant (rtA181T/ V+N236T and rtN236T) mutations were detected, and compared with wild-type (WT). The eGFR (mL/min/1.73 m2) was calculated by the Chinese equation: eGFR = 175 * Pcr -1.234 * age -0.179 (female * 0.79)
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Measurements were presented as mean ± standard deviation (SD) and comparisons were conducted following analysis of the results using the Student’s t test. Proportions were presented as percentage (%). Rate comparisons were performed using the χ2 test. A P value < 0.05 was considered significant.
ResultsBaseline characteristicsA total of 255 CHB patients received ADV monotherapy as initial antiviral treatment for 48 weeks. At week 12, 35 (13.7%) of patients were defined as primary nonresponder (decline less than 1 log of HBV DNA levels at week 12 of treatment) to ADV treatment. All these patients were switched to ETV monotherapy. Other 220 patients continued to receive ADV treatment. At week 48, 38.2% (84/220) of them had HBV DNA < 3 log10 copies/mL and still received ADV monotherapy. However, 61.8% (136/ 220) of them still had HBV DNA > 3 log10 copies/ mL, all 136 patients were recruited in this study. Sixty-eight patients received LAM add-on ADV combination therapy and other 68 patients switched to ETV monotherapy (Figure 1). No ADV-resistance mutations (rtA181T/V + N236T and rtN236T) were detected in all 136 patients. All the patients have good compliance during the follow up period.
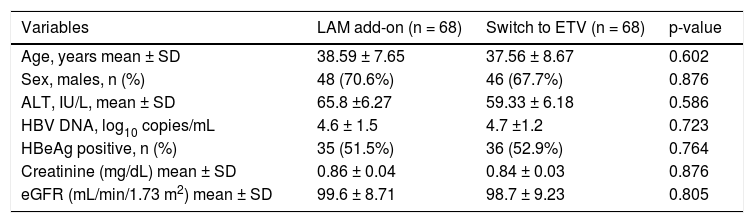
The baseline characteristics of these 136 patients are listed in table 1. The treatment groups were well matched at baseline and no statistically significant differences were observed. The mean patient age in LAM add-on group and ETV monotherapy was 38.59 ± 7.65 and 37.56 ±8.67 years respectively. Thirty-five patients in add-on group and 36 patients in ETV monotherapy group were HBeAg positive. The mean patient creatinine level in add-on group and ETV group was 0.86 ±0.04 mg/dL and 0.84 ± 0.03 mg/dL respectively and mean eGRF was 99.6 ± 8.71 and 98.7 ± 9.23 respectively.
Baseline characteristic of CHB patients with suboptimal response to ADV.
| Variables | LAM add-on (n = 68) | Switch to ETV (n = 68) | p-value |
|---|---|---|---|
| Age, years mean ± SD | 38.59 ± 7.65 | 37.56 ± 8.67 | 0.602 |
| Sex, males, n (%) | 48 (70.6%) | 46 (67.7%) | 0.876 |
| ALT, IU/L, mean ± SD | 65.8 ±6.27 | 59.33 ± 6.18 | 0.586 |
| HBV DNA, log10 copies/mL | 4.6 ± 1.5 | 4.7 ±1.2 | 0.723 |
| HBeAg positive, n (%) | 35 (51.5%) | 36 (52.9%) | 0.764 |
| Creatinine (mg/dL) mean ± SD | 0.86 ± 0.04 | 0.84 ± 0.03 | 0.876 |
| eGFR (mL/min/1.73 m2) mean ± SD | 99.6 ± 8.71 | 98.7 ± 9.23 | 0.805 |
ADV: adefovir dipivoxil. LAM: lamivudine. ETV: entecavir. HBeAg: hepatitis B e antigen. HBV: hepatitis B virus. eGFR: estimated glomerular filtration rate.
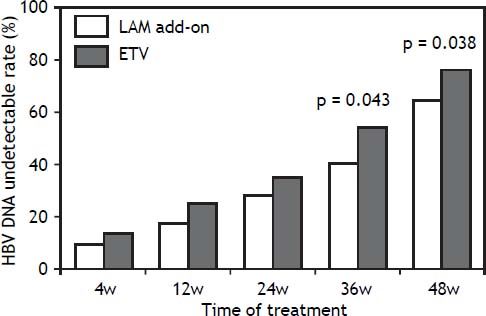
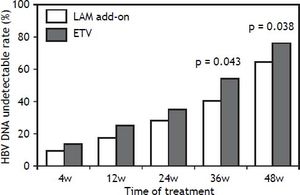
In the add-on group, the proportion of patients with a virological response (HBV DNA < 3 log10 copies/mL) at week 4, 12, 24, 36 and 48 of treatment 8.8% (6/68), 17.6% (12/68), 27.9% (19/68), 39.7% (27/ 68) and 64.7% (44/68), respectively. In ETV group, the proportion of patients with a virological response at week 4, 12, 24, 36 and 48 of treatment was 13.2% (9/68), 25.0% (17/68), 33.8% (23/68), 54.4% (37/68) and 76.5% (52/68) respectively. The HBV DNA undetectable rate in the LAM add-on group and the ETV group were not significantly different at week 4, 12 and 24 respectively (P > 0.05). However, the difference in HBV undetectable rate between two groups was significant at week 36 and 48 (P = 0.043 for week 36 and P= 0.038 for week 48) as indicated in figure 2.
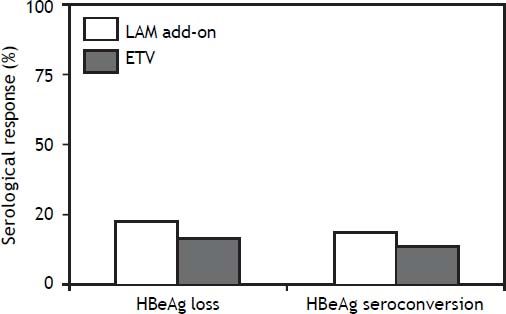
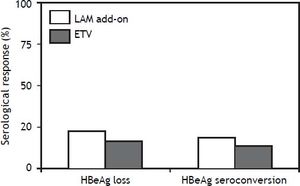
Serological responseAmong the 35 HBeAg positive CHB patients with in LAM add-on group, 22.9% (8/35) of them developed HBeAg loss and 17.1% (6/35) developed HBeAg seroconversion at week 48. Among the 36 patients in ETV monotherapy group, 19.4% (7/36) of them developed HBeAg loss and 13.9% (5/36) developed HBeAg seroconversion at week 48 as indicated in figure 3. There was no significant difference both for HBeAg loss and HBeAg seroconversion between two groups (P > 0.05). After HBeAg seroconversion or undetectable HBV DNA, all the patients still been treated with ETV or LAM plus ADV combination therapy for consolidation.
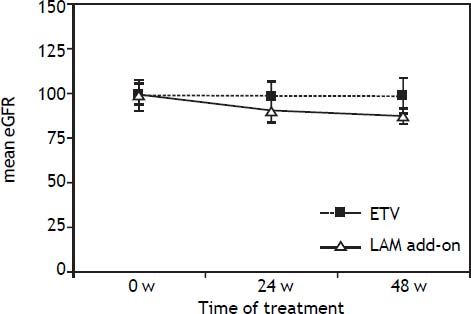
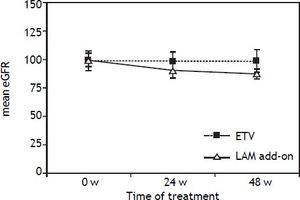
Resistance and safetyAt week 48, no resistance mutations (rtA181T/ V+N236T and rtN236T) were detected in both groups. The creatinine levels of all the 136 patients remain normal at the end of 48 weeks treatment. However, mean patient creatinine level in add-on group increased from 0.86 ± 0.04 at the baseline to 0.97 ±0.06 at the 48 weeks. The mean eGFR levels in LAM add-on group was decreased from 99.6 ± 8.71 at baseline to 86.4 ± 9.83 at the end of 48 weeks treatment, which was significantly higher than that in the ETV monotherapy group (P < 0.05), as indicated in figure 4. 8.8% (6/68) of patients in LAM add-on group experienced eGFR reduction by 20-30% from baseline at 48 weeks, and no patients experienced reduction of eGFR by ≥ 30% after 48 weeks of add-on treatment.
DiscussionThis is the first head to head study to describe the efficacy and safety of LAM add-on combination therapy and ETV monotherapy for patients with suboptimal response to ADV for 48 weeks. Our results showed that HBV undetectable rate in the ETV monotherapy groups was significantly higher than that in LAM add-on group at week 36 and 48 (P = 0.043 for week 36 and P = 0.038 for week 48). Furthermore, 8.8% (6/68) of patients in LAM add-on group experienced eGFR reduction by 20-30% from baseline at 48 weeks, and no patients experienced reduction of eGFR by ≥ 30% after 48 weeks after add-on treatment. No resistance mutations were detected in both groups in the 48 weeks.
In China, four types of nucleos(t)ide analogues (LAM, ADV, Ldt and ETV) are available. Among them ADV is relatively cheap and widely used in the rural area in China. However, some patients have not responded well to ADV monotherapy even after 48 weeks of treatment, as manifested by a low decrease in HBV DNA level. In accordance with previous studies, our results indicated that after 48 weeks of ADV monotherapy, 61.8% (136/220) of patients still had HBV DNA > 103 copies/mL. Several reports have demonstrated that a slow virologic response after initiation of nucleos(t)ide analogues treatment is associated with high rates of drug resistance in the long-term. Thus, there is urgent need for a rescue therapeutic strategy that provides a greater efficacy and a reduced rate of drug resistance. Wang, et al. reported that compared with continues ADV monotherapy, LAM add-on combination had a improved rate of virologic and biochemical response at week 12 and 24 for patients with poor response to ADV.17 But the follow-up time is relative short in their study. Our results demonstrated that after LAM add-on combination therapy, 8.8% (6/68), 17.6% (12/68), 27.9% (19/68), 39.7% (27/68) and 64.7% (44/68) of patients got a virological response at week 4, 12, 24, 36 and 48 of treatment respectively for the patients with suboptimal response to ADV. There results suggested that LAM add-on combination therapy is a one of the good choice for patients with suboptimal response to ADV.
Recent guideline for CHB patients with ADV resistance suggests switch to ETV or tenofovir (TDF) therapy if the patient was neucleos(t)ide analogues naive before ADV treatment, ETV may be preferred in such patients with high viraemia.15 Our results clearly indicated that after 36 and 48 weeks of ETV rescue therapy, 54.4% (37/68) and 76.5% (52/68) of patients with suboptimal response to ADV got HBV DNA undetectable, which was significant higher than that in LAM add-on group.
However, Reijnders, et al. previously reported that ETV showed a limited efficacy in HBeAg- positive CHB patients with a partial virologic response to ADV.18 It should be noted that only 14 patients were recruited in their study and most of them were LAM-experienced. All the CHB patients in our study were NA naïve before ADV treatment and no resistance mutation was detected in all patients before rescue therapy. This may be the possible explanation of discrepancy between two studies.
Furthermore, renal impairment is one of the most serious side effects of ADV. Nephrotoxicity associated ADV is dose-dependent. Although, in phase III clinical trial significant renal toxicity was not observed during 64 weeks follow-up in patients with ADV at 10 mg/day.19 But, renal dysfunction associated with long-term use of low-dose ADV has been demonstrated in many reports published recently. Tanaka, et al. reported that during a median treatment duration of 64 months, 9.6% of patients developed renal impairment (defined as eGFR < 50 mL/min/1.73 m2) and 27.1% of them developed hyposphosphatemia. The cumulative incidences of renal impairment at 1, 3, 5 years were 1.4, 7.5 and 10.5% respectively.20 In consistence with these results, our study also demonstrated that the mean eGFR levels in LAM add-on group was decreased from 99.6 ± 8.71 at baseline to 86.4 ± 9.83 at the end of 48 weeks treatment, which was significantly higher than that in the ETV monotherapy group. 8.8% (6/68) of patients in LAM add-on group experienced eGFR reduction by 20-30% from baseline at 48 weeks. No patients developed hyposphosphatemia in our study because of short duration of observation time.
ConclusionsIn conclusion, our study clearly showed that switch to ETV monotherapy was the more effective and more safe than that of LAM add-on combination therapy for patients with suboptimal response to ADV.
Our study clearly showed that switch to ETV monotherapy was the more effective and more safe than that of LAM add-on combination therapy for patients with suboptimal response to ADV.
Abbreviations- •
ADV: adefovir dipivoxil.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
CHB: chronic hepatitis B.
- •
eGFR: estimated glomerular filtration rate.
- •
ETV: entecavir.
- •
HBeAg: hepatitis B e antigen.
- •
HBV: hepatitis B virus.
- •
LAM: lamivudine.
This work was supported by the National Key Program for Infectious Diseases of China to YD Yang (2013ZX10002001), 12th Five-Year Significant New Drugs Creation Plan of the Ministry of Science and Technology of China to YD Yang (2011ZX09302-003-03) and National Nature and Science Fund to HY Jia (81100286).